Abstract
The effect of Sm on thermally stimulated decomposition of SiOx and the formation of silicon nanocrystals during high-temperature air annealing was studied by measuring the spectra of photoluminescence (PL), infrared (IR) reflection and transmission and Micro-Raman scattering (RS). The SiOx: Sm films were produced by thermal co-evaporation of silicon monoxide and metallic samarium powders in a vacuum onto polished c-Si and silica substrates. The Sm concentration varied from 0.8 to 2.0 wt. %. In the SiOx: Sm films annealed at 970 ºC, an intense PL band of silicon nanocrystals was found in the 850–900 nm region, the position and intensity of which depend on the samarium concentration. The formation of Si nanocrystals in those films is also confirmed by studying their Raman spectra. At the same time, no Si nanocrystals were found in undoped SiOx films annealed under similar conditions. The analysis of these spectra made it possible to determine the sizes of silicon nanocrystals: 3.7 nm from the PL spectra and 3.6 nm from the Raman spectra. A possible mechanism of thermally stimulated interaction of Sm atoms with the SiOx matrix is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thin-film ncs-Si − SiOx structures containing Si nanoclusters (ncs-Si) embedded in a SiOx matrix, both undoped and containing ions of rare-earth elements (lanthanides (Ln)), attract the attention of many researchers due to their promising application in modern photonic devices compatible with CMOS technology (Lisovskyi et al. 2014; Pavesi 2008; Heitmann et al. 2005; Priolo et al. 2014; Polman et al. 2004; Mertens et al. 2005; Jia et al 2006). The Ln3+ ions (Sm3+, Yb3+, Tb3+, Er3+, etc.) display sharp emission bands that span from the ultraviolet to the near-infrared spectral regions. The emission spectrum of ncs-Si takes the form of a broad band with a maximum that can shift from the visible to the near infrared region of the spectrum (650–950 nm) depending on the ncs-Si particles’ size. Since the ncs-Si absorption cross section is almost three orders of magnitude higher than the cross section for direct excitation of Ln ions (Pacifici et al. 2003a, 2003b), the ncs-Si − SiOx structures doped with Ln3+ ions can take advantage of the energy transfer from Si-nanoclusters to dopant ions. Many techniques have been developed for the synthesis of ncs-Si in an oxide matrix. These include thermal evaporation of SiO (Lisovskyi et al. 2014), implantation of Si ions in SiO2 (Shimizu-Iwayama et al. 1994), co-sputtering of Si and SiO2 (Kanzawa et al. 1997), plasma-assisted chemical vapor deposition of silicon suboxide (SiOx) (Iacona et al. 2000) and others. As a result of high-temperature annealing of the obtained SiOx film, silicon nanoparticles are formed in an oxide matrix, the size and concentration of which depend mainly on the temperature, annealing time, and composition of the initial film.
Several methods have also been proposed for doping such structures with lanthanide ions, including their implantation (Franzò et al. 2003) into annealed ncs-Si–SiOx structures. However, the method of introducing an impurity at the stage of film deposition by thermal co-evaporation, or reactive high-frequency co-sputtering, turned out to be simpler and technologically advanced (Vlasenko et al. 2012; Mustafa et al. 2013). In this case, during high-temperature annealing, the dopant interacts with the SiOx matrix, affecting the formation of silicon nanoparticles and, consequently, their size and light-emitting characteristics.
Of the ncs-Si–SiOx structures doped with lanthanides, the most studied are structures doped with erbium, which emits an intense band with a maximum of about 1.54 μm, coinciding with the absorption minimum of the silica optical fibers. This makes it possible to integrate emitters based on such structures not only with the standard telecommunication systems, but also with the many silicon-compatible optical devices. Silicon based nanocomposites doped with samarium are less studied, although their promise for creating solid-state lasers, information recording, nanophosphors for display applications was demonstrated in several works (Farries et al. 1988; Kurita et al. 1994; Mbakaan et al. 2021).
In this work, we study ncs-Si–SiOx structures doped with samarium during thermal co-evaporation in vacuum of SiO and metallic Sm. By measuring the spectra of photoluminescence (PL), IR reflection and Raman scattering, the effect of samarium on thermally stimulated decomposition of SiOx during high-temperature annealing, the formation of ncs-Si and their crystallization was studied. A possible mechanism of thermally stimulated interaction of Sm atoms (ions) with the SiOx matrix and ncs-Si is discussed.
Experimental procedure
The studied SiOx: Sm films were produced using thermal co-evaporation of SiO (Cerac Inc., Milwaukee, WI, USA, purity of 99.9%) and metallic Sm powders in a vacuum ~ 10–3 Pa onto polished c-Si and silica substrates. The ratio of the SiOx and Sm masses in the deposited film was monitored in situ by two quartz-crystal-oscillator monitor systems (KIT-1). Reference SiOx films without Sm were deposited under similar conditions. The thickness of the obtained films was measured on a microinterferometer (MII-4) and was found to be within 300–400 nm. After deposition, the samples were annealed for 30 min in air at temperatures of 600–970 °C. As a result, the phase decomposition of the non-stoichiometric silicon oxide and the growth of ncs-Si in the oxide matrix occur. Typically, such annealing is carried out in a vacuum or in an inert atmosphere to avoid additional oxidation of both the SiOx matrix and ncs-Si. However, it was shown in a number of papers (Vlasenko et al. 2012; Nikolenko et al. 2012; Sopinskyy et al. 2015) that air annealing of thermally deposited SiOx layers doped with erbium fluoride also makes it possible to form structures with inclusions of silicon nanoparticles. This is due to the fact that the phase separation process proceeds much faster than the oxidation process (Indutnyy et al. 2006; Coffin et al. 2006). In addition, in the case of silicon inclusions, the oxidation rate of ncs-Si strongly decreases with a decrease in their size (Biteen et al. 2004; Coffin et al. 2006).
The PL spectra were measured at room temperature in the wavelength range from 500 to 1100 nm using a ZMR-3 monochromator (spectral resolution ~ 1.2 nm for 10 μm slot in the investigated wavelength range) coupled to a photomultiplier (FEU-62 or FEU-51). The PL signal was excited by diode laser 405 nm radiation and recorded by a lock-in 232B nanovoltmeter referenced to the chop frequency of 110 Hz. After the excitation source, a 5 mm thick SS-8 filter with 350–500 nm transmission region was placed, which cut off the LED radiation in the 500–1500 nm range. The obtained PL spectra were corrected with regard to the spectral sensitivity of the measuring setup. Infrared spectra of air-annealed SiOx and SiOx:Sm samples on c-Si substrates were measured using a FTIR Spectrum BXII PerkinElmer spectrometer with a resolution of 4 cm−1 working in the reflective mode at the normal incidence. Raman spectra were excited by light from an Ar+ laser with an excitation wavelength of 488 nm (excitation power on sample surface ~ 1 mW) and registered using a Horiba Jobin–Yvon T-64000 Raman spectrometer at room temperature in the spectral range 100–600 cm−1. An Olympus 50X/0.75 microscope objective was used. Spectral resolution was less than 0.15 cm–1.
Results and discussion
The Sm-doped SiOx PL spectra show emission bands of samarium ions in two charge states, Sm3+ and Sm2+, along with the PL bands of nanosilicon. The spectral position of these bands corresponds to the spectral region 550–750 nm (Kushnirenko et al. 2008; Sakirzanovas et al. 2012).
Figure 1 shows the PL spectra of SiOx:Sm films with the impurity content of 2 wt % before and after annealing at 970º C. In the non-annealed film two bands with maxima at 550 and 725 nm are observed (curve 1). These bands correlate well with the spectral position of the emission bands of Sm3+ and Sm2+ ions in a glassy matrix (Malchukova et al. 2019). After annealing, the position of the bands maxima at 725 nm and at 550 nm does not change. However, the ratio of their intensities changes (comparing curves 1 and 2 on Fig. 1). The observed change in the intensity ratio could be due to a change in the chemical composition of the Sm ions’ environment during annealing (Nogami and Abe 1996; Wang et al. 2011). The purpose of this work is to study the effect of samarium impurity on the formation of silicon nanoparticles in a SiOx matrix. Accordingly, the results of studying the PL of the samples for the spectral range 750–1050 nm are presented below. It is this spectral interval that corresponds to the emission of silicon nanocrystals in our samples.
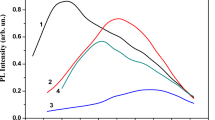
Figure 2 shows the PL spectra of SiOx:Sm films on c-Si substrates with a samarium concentration of 1.5 wt. %, annealed at 600 (1), 800 (2) and 970 ºC (3) for 30 min. It is observed that the PL of a film annealed at 600 ºC covers a wide spectral region, and its intensity increases in the direction from IR towards the visible region. A short-wavelength emission is usually associated with luminescence of amorphous silicon nanoclusters, which are formed in SiOx films at relatively low annealing temperatures (Tan ~ 600 − 900 ºC) and emit in the 700 − 760 nm region (Indutnyi et al. 2010). Additionally, the manifestation of a weak band at 830 nm is noticeable.
Photoluminescence spectra of samples with a SiOx:Sm film deposited on a c-Si substrate after annealing in air for 30 min at 600 (1), 800 (2), and 970 ºC (3). Sm concentration—1.5 wt. %. Excitation by radiation at λ = 405 nm. Curve 4: PL spectrum of non-doped SiOx film annealed under the same conditions at 970 ºC
When the annealing temperature is increased to Tan = 800 ºC, the emission spectrum of SiOx:Sm films changes—emission from amorphous silicon nanoclusters practically disappears, with only a broad band with a maximum at about 850 nm remaining (curve 2). With further increase in Tan to 970 ºC (curve 3), there is a noticeable increase in the intensity of this PL band; its maximum shifts to longer wavelengths and is located at 870 nm. The spectral position of this band allows us to associate it with the appearance of crystalline ncs-Si (Kahler and Hofmeister 2002; Limpens et al. 2015). The observed change in the PL spectrum can be explained by the fact that an increase in Tan leads to an increase in the amount of ncs-Si and their coarsening, which in turn leads to a long-wavelength shift of the PL band maximum in accordance with the quantum size effect. It should be noted that the Gaussian shape of this band and the spectral position of its maximum are similar to the characteristics of the PL band observed in SiOx films annealed at Tan ≥ 1100º C in an argon atmosphere (Lisovskyy et al. 2009).
For comparison, Fig. 2 shows the PL spectrum of a SiOx film without samarium on a c-Si substrate after annealing at 970 ºC in air for 30 min (curve 4). In the SiOx film in the spectral region under study, a weaker PL intensity is observed compared to the radiation of the SiOx:Sm sample annealed both at the same temperature and at 800 ºC. The emission from a SiOx film annealed in air is also significantly weaker than the PL of similar films annealed at 1000 ºC in a nitrogen atmosphere (Dan’ko et al. 2005). Additionally, the PL maximum of SiOx films annealed in an inert atmosphere at 1000 ºC is about 900–920 nm, meaning there was a shift to longer wavelengths compared to SiOx:Sm samples annealed in air. These results are due to some additional oxidation of the silicon nanoparticles being formed during air annealing. In SiOx films, additional oxidation is quite significant, which manifests itself in a significant weakening of PL as compared to annealing in an inert atmosphere, while in SiOx: Sm it manifests itself as a slight short-wavelength shift of the PL maximum. The significantly higher PL intensity of crystalline ncs-Si in annealed SiOx:Sm films as compared to SiOx films (curves 3 and 4 in Fig. 2) indicates the effect of the samarium impurity on the formation of silicon nanoparticles during high-temperature annealing. We posit that the interaction of samarium atoms with oxygen atoms and ncs-Si surface atoms can contribute to the passivation of the nanoparticle surface and inhibits the process of their oxidation by air atmosphere. This is similar to what takes place in layers of porous silicon doped with samarium where formation of Sm–O–Si bonds resulted in enhancement of their photoluminescence intensity (Zarroug et al. 2017).
The average size of nanocrystals can be estimated using empirical dependences that relate the band gap of a nanoparticle (i.e., the energy of a radiation quantum) to the particle diameter. We used the formula Eg = 1.12 + 1.86/D1.39, where Eg and D represent the nc-Si’s band-gap (in eV) and the diameter of Si nanocrystall (in nm), respectively. The feasibility of using this expression for similar samples has been shown using high-resolution TEM images (Limpens et al. 2015). For a SiOx: Sm sample annealed at 970 ºC in air, the average crystalline ncs-Si diameter is 3.7 nm.
The results of studying the dependence of the luminescent properties of SiOx:Sm films annealed in air at 970 ºC for 30 min on the impurity concentration are shown in Fig. 3. The PL spectrum presented by curve 1 corresponds to a SiOx:Sm film with a samarium concentration of 0.8, curve 2—1.5 and 3—2.0 wt. %. Figure 3 shows that, with an increase in the Sm concentration in the initial as-prepared SiOx films, the maxima of their PL bands shift to the long-wavelength region of the spectrum, with the highest integrated PL intensity found in the film with 1.5 wt. % Sm. The revealed dependence of the position and intensity of the PL band of the samarium concentration also indicates its participation in the processes of thermal decomposition of SiOx and formation of crystalline ncs-Si. However, the obtained experimental data do not allow separating samarium’s contribution as a stimulator of nanoparticle formation from its contribution as a passivator of their surface to an increase in the intensity of crystalline ncs-Si photoluminescence.
In the spectral range of 850–1350 cm−1, we studied the IR transmission and reflection spectra of SiOx and SiOx: Sm films, both as deposited and annealed at 970 ºC, on c-Si substrates. Since silicon (nanosilicon) does not absorb in this spectral region, measurements of IR spectra in it enable the studying of structural features of the SiOx oxide matrix before and after thermal annealing. It is known that phase changes in the SiOx composition lead to a shift in the maximum of the main IR absorption band, corresponding to the asymmetric stretching vibrations of the bridging oxygen atoms. Figure 4 shows the IR transmission spectra of SiOx (curve 1) and SiOx:Sm (curve 2) films with a Sm concentration of 1.5 wt. % before annealing. It is seen that for unannealed SiOx films, the minimum of the transmission band (and, accordingly, the maximum of absorption) corresponds to the frequency νm = 1043 cm−1 (Fig. 4, curve 1). If we use the empirical relationship between the frequency of the main vibration band of Si − O − Si and the value of the stoichiometry index of the oxide matrix (x) from (Nakamura et al. 1984), then we find that, for this sample, x = 1.45. For unannealed films doped with samarium, νm = 996 cm −1, which corresponds to x = 1.1. It is known that samarium reacts with oxygen even at room and higher temperatures (Afanas’eva et al. 2014). This explains a difference in the value of x, determined from the IR spectra of unannealed samples. This difference may be due to the interaction of samarium with the oxygen of suboxide matrix during the deposition of doped samples.
Figure 5 shows the IR reflection spectra of the same films after annealing at 970 ºC in air. For such samples (a thin absorbing film with a thickness 20 times less than the probe radiation wavelength on a thick transparent substrate), the position of the reflection minimum correlates with the absorption maximum of the film also.
Comparison of curves 1 and 2 in Fig. 5 shows that, in the SiOx:Sm film, the absorption and half-width of the band are larger than in the film without samarium, and the maximum position νm = 1098 cm −1 is shifted to a higher-frequency region of the spectrum compared to the undoped film (νm = 1090 cm−1). Both of these values are higher than the value νm = 1082 cm−1 expected for stoichiometric SiO2 film from the νm(x) empirical dependence obtained in (Nakamura et al., 1984). Similar results with νm = 1088–1095 cm−1 have been found for ncs-Si − SiOx structures obtained by high temperature annealing of SiOx films in a number of other papers (Hinds et al. 1998; Garrido Fernandez et al. 2002; Lisovskyy et al. 2009, 2016; Sopinskyy et al. 2015). This is to the fact that position of the maximum of the main IR absorption band of SiO2 films dependents on their structure. Specifically, this band in silicon dioxide films consists of the elementary sub-bands corresponding to different kinds of SiO4 tetrahedra rings (Lisovskii et al. 1992, 1995). The greater is the predominance of six-fold rings, the greater is the νm value. In particular, in SiO2 layers obtained by thermal oxidation of silicon, where the six-membered rings of SiO4 tetrahedra predominate, the position of the maximum of the IR absorption band is νm ≈ 1095 cm−1 (Lisovskii et al. 1992). The increase of νm in our samarium doped ncs-Si − SiOx films compared to non-doped ones is similar to results obtained for air-annealed SiOx films doped with ErF3 (Sopinskyy et al. 2015), where it was shown that the oxide matrices of the annealed SiOx and SiOx:ErF3 samples consist of SiO4 tetrahedra interconnected in four- and sixfold rings. However, sixfold rings predominate in the doped sample. In sixfold rings, oxygen atoms oscillate with a slightly higher frequency, which explains the observed high-frequency shift of the band (curve 2, Fig. 5).
To confirm the formation of nanocrystalline silicon in SiOx:Sm films after annealing, we investigated their structure by analyzing the micro-Raman scattering (RS) spectra. Figure 6 presents the Raman spectra of the SiOx: Sm (Sm concentration — 1.5 wt%) and undoped SiOx film of the same thickness and deposited in the same conditions, both after annealing at 970 ºC. The experimental curve for SiOx: Sm film shows bands characteristic of both amorphous and crystalline nanosilicon. The bands characteristic of amorphous silicon can be attributed to transverse acoustic TA (~ 146 cm−1), longitudinal acoustic and optical LA&LO (~ 330 cm−1) and transverse optical TO (~ 485 cm−1) phonons. On the high-frequency side of the TO band, a narrow band with a maximum of 516 cm−1 is clearly visible (the position of the band maximum was determined after decomposition of the spectrum into the Gaussian components). It is known that the maximum of the Raman spectrum of a bulk silicon crystal is at 520 cm−1. The appearance in the Raman spectrum of a line with a maximum at ~ 516 cm–1 indicates the presence of Si nanocrystals in the film. The maximum of the ncs-Si band is shifted to the low-frequency region with respect to the position of the maximum of the Raman band for a bulk silicon crystal, which is characteristic of nanocrystalline silicon structures due to the effect of spatial confinement of optical phonons. Both the presence of a band with a maximum at ~ 516 cm–1 in the Raman spectrum and the presence of a band at about 870 nm in the PL spectrum (curve 3 in Fig. 2) independently indicate that the SiOx:Sm films annealed at 970ºC contain Si nanocrystals. However, in non-doped SiOx samples annealed under the same conditions (curve 1), we did not detect a noticeable Raman signal corresponding to nanocrystalline silicon. The emission of Si nanocrystals for these samples is also practically absent in the PL spectra (curve 4 in Fig. 2). Based on the results of our optical studies, we can conclude that silicon nanocrystals can be formed under the indicated annealing conditions only in doped SiOx films.
By neglecting the size dispersion of crystalline ncs-Si and fitting the measured Raman spectra to those calculated for spherical silicon nanoparticles, one can determine the volume fraction of nanocrystals and their sizes in the studied structures (Maslova et al. 2010). (Ke et al. 2011) considered the influence of the size distribution of silicon nanocrystals on their Raman spectrum. They obtained a simple analytical expression linking the Raman frequency shift of ncs-Si (Δω, in cm−1) with their diameter (D, in nm):
where a = 0.543 nm is the c-Si lattice constant. Therefore, using expression (1) and the experimentally obtained value Δω = 520 cm−1—516 cm−1 = 4 cm−1, we estimated the average diameter of silicon nanocrystalls in the SiOx:Sm film, which was about 3.6 nm. The obtained value of D turned out to be close to the value of their average size (D = 3.7 nm), which we found from the position of the maximum of the PL band.
Several mechanisms have been proposed that describe the effect of metal impurities on the thermally stimulated decomposition of SiOx, the formation and crystallization of silicon nanoparticles in an oxide matrix. In (He et al. 2006) the influence of nickel on the near-infrared luminescence emitting from silicon nanocrystals embedded in SiO2 matrix was investigated, and a thermodynamic model was proposed, according to which nickel can be an additional driving force to the phase separation process. Similar studies of Er-doped SiOx layers (Mustafa et al. 2013) demonstrated a promoting action of Er for SiOx disproportionation. At annealing, the Er centers scavenge mobile oxygen atoms, thereby locally enhancing SiOx disproportionation and consequently promoting Si-nucleation near Er. Upon annealing in air of the films obtained by coevaporation of SiO and ErF3, impurity-stimulated acceleration of the formation of Si nanocrystals was observed, as well as inhibition of the oxidation of SiOx (Vlasenko et al. 2012; Nikolenko et al. 2012; Sopinskyy et al. 2015).
Similar results were obtained in our studies presented above: the doping of SiOx films with metallic samarium stimulates the formation of nanocrystalline silicon in the oxide matrix, which manifests itself in the IR and Raman spectra. However, there are still not enough experimental results to accurately determine the mechanisms of the influence of samarium impurity on the processes of SiOx decomposition, formation, and crystallization of ncs-Si, their passivation and oxidation during annealing in air. Analogous to the results of studies on Er-doped SiOx layers, it can be assumed that the mechanism of samarium-stimulated decomposition of SiOx also involves the oxidation of Sm atoms with the oxygen of the oxide matrix. This leads to a local increase in the Si concentration and stimulates the formation of crystalline ncs-Si. The absence of Si nanocrystals in the undoped film may be partly due to their more efficient oxidation upon annealing. In contrast, the passivation of the ncs-Si surface with samarium atoms in SiOx: Sm films can slows down their additional oxidation with atmospheric oxygen.
Conclusions
In summary, by measuring the spectra of photoluminescence, IR transmission and reflection, Raman scattering, the effect of samarium on thermally stimulated decomposition of SiOx: Sm films deposited by thermal co-evaporation in vacuum of SiO and metallic Sm has been studied. A significant decrease in the frequency of the main vibration band of Si–O–Si in deposited SiOx: Sm samples compared to undoped SiOx films was found. This may be due to the interaction of samarium with oxide matrix oxygen already in the process of deposition of doped samples. In undoped SiOx samples annealed in air, silicon nanocrystals do not manifest themselves in either the Raman or PL spectra. The presence of bands with a ~ 516 cm–1 maximum in the Raman spectrum and a band at ~ 850–900 nm in the PL spectrum of the SiOx:Sm samples annealed in air indicates that Sm promote formation of nanocrystalline silicon in oxide matrix.
References
Afanas’eva EY (2014) Oxidation of a thin films of samarium on iridium. Tech Phys Lett 40:520–522. https://doi.org/10.1134/S1063785014060169
Biteen JS, Lewis NS, Atwater HA, Polman A (2004) Size-dependent oxygen-related electronic states in silicon nanocrystals. Appl Phys Lett 84:5389–5391. https://doi.org/10.1063/1.1765200
Coffin H, Bonafos C, Schamm S, Cherkashin N, Ben Assayag G, Claverie A, Respaud M, Dimitrakis P, Normand P (2006) Oxidation of Si nanocrystals fabricated by ultralow-energy ion implantation in thin SiO2 layers. J Appl Phys 99:044302. https://doi.org/10.1063/1.2171785
Dan’ko VA, Indutnyi IZ, Lysenko VS, Maidanchuk IYu, Min’ko VI, Nazarov AN, Tkachenko AS, Shepelyavyi PE (2005) Kinetics of structural and phase transformations in thin SiOx films in the course of a rapid thermal annealing. Semiconductors 39(10):1197–1203. https://doi.org/10.1134/1.2085270
Farries MC, Morkel PR, Townsend JE (1988) Sm3+-doped glass laser operating at 651 nm. Electron Lett 24:709–711. https://doi.org/10.1049/el:19880479
Franzò G, Boninelli S, Pacifici D, Priolo F, Iacona F, Bongiorno C (2003) Sensitizing properties of amorphous Si clusters on the 1.54-μm luminescence of Er in Si-rich SiO2. Appl Phys Lett 82:3871–3873. https://doi.org/10.1063/1.1579555
Garrido Fernandez B, López M, Garcı́a C, Pérez-Rodrı́guez A, Morante JR, (2002) Influence of average size and interface passivation on the spectral emission of Si nanocrystals embedded in SiO2. J Appl Phys 91:798–807. https://doi.org/10.1063/1.1423768
He Y, Ma K, Bi L, Feng JY, Zhang ZJ (2006) Nickel-induced enhancement of photoluminescence from Si-rich silica films. Appl Phys Lett 88:031905. https://doi.org/10.1063/1.2165292
Heitmann J, Müller F, Zacharias M, Gösele U (2005) Silicon nanocrystals: size matters. Adv Mater 17:795–803. https://doi.org/10.1002/adma.200401126
Hinds BJ, Wang F, Wolfe DM, Hinkle CL, Lucovsky G (1998) Study of SiOx decomposition kinetics and formation of Si nanocrystals in an SiO2 matrix. J Non-Cryst Solids 227–230:507–512. https://doi.org/10.1016/S0022-3093(98)00094-5
Iacona F, Franzò G, Spinella C (2000) Correlation between luminescence and structural properties of Si nanocrystals. J Appl Phys 87:1295–1303. https://doi.org/10.1063/1.372013
Indutnyi IZ, Michailovska EV, Shepeliavyi PE, Dan’ko VA (2010) Visible photoluminescence of selectively etched porous nc-Si–SiOx structures. Semiconductors 44:206–210. https://doi.org/10.1134/S1063782610020120
Indutnyy IZ, Lysenko VS, Maidanchuk IY, Minko VI, Nazarov AN, Tkachenko AS, Shepelyavyi PE, Danko VA (2006) Kinetics of thermally induced structural-phase transformations and formation of silicon nanoparticles in thin SiOx films. Nanosyst Nanomat Nanotechnol 4:145–155
Jia Ch, Xie E, Peng A, Jiang R, Ye F, Lin H, Xu T (2006) Photoluminescence and energy transfer of terbium doped titania film. Thin Solid Films 496:555–559. https://doi.org/10.1016/j.tsf.2005.08.378
Kahler U, Hofmeister H (2002) Size evolution and photoluminescence of silicon nanocrystallites in evaporated SiOx thin films upon thermal processing. Appl Phys A 74:13–17. https://doi.org/10.1007/s003390100993
Kanzawa Y, Kageyama T, Takeoka S, Fujii M, Hayashi S, Yamamoto K (1997) Size-dependent near-infrared photoluminescence spectra of Si nanocrystals embedded in SiO2 matrices. Solid StateCommun 102:533–537. https://doi.org/10.1016/S0038-1098(96)00774-0
Ke W, Feng Y, Huang Y (2011) The effect of Si-nanocrystal size distribution on Raman spectrum. J Appl Phys 109(8):083526. https://doi.org/10.1063/1.3569888
Kurita A, Kushida T, Izumitani T, Matsukawa M (1994) Room-temperature persistent spectral hole burning in Sm2+-doped fluoride glasses. Opt Lett 19(5):314–316. https://doi.org/10.1364/OL.19.000314
Kushnirenko VI, Sopinskyy MV, Manoilov EG, Khomchenko VS (2008) Luminescent spectroscopy of TbF3 and TbF3–SmF3–HoF3–PrF3 crystals. J Alloys Compd 451:209–211. https://doi.org/10.1016/j.jallcom.2007.04.178
Limpens R, Lesage A, Fujii M, Gregorkiewicz T (2015) Size confinement of Si nanocrystals in multinanolayer structures. Sci Rep 5:17289. https://doi.org/10.1038/srep17289
Lisovskii IP, Litovchenko VG, Lozinskii VB, Steblovskii GI (1992) IR spectroscopic investigation of SiO2 film structure. Thin Solid Films 213:164–169. https://doi.org/10.1016/0040-6090(92)90278-J
Lisovskii IP, Litovchenko VG, Lozinskii VG, Frolov SI, Flietner H, Fussel W, Schmidt EG (1995) IR study of short-range and local order in SiO2 and SiOx films. J Non-Cryst Solids 187:91–95. https://doi.org/10.1016/0022-3093(95)00118-2
Lisovskyi IP, Sarikov AV, Sypko MI (2014) Thin film structures with silicon nanoinclusions. Kyiv-Chernivci, Knigi-XXI (in Ukrainian)
Lisovskyy IP, Voitovich MV, Sarikov AV, Litovchenko VG, Romanyuk AB, Melnyk VP, Khatsevich IM, Shepeliavyi PE (2009) Transformation of the structure of silicon oxide during the formation of Si nanoinclusions under thermal annealing. Ukr J Phys 54:383–390
Lisovskyy I, Voitovych M, Litovchenko V, Voitovych V, Nasieka Iu, Bratus V (2016) Radiation induced enhancement of hydrogen influence on luminescent properties of nc-Si/SiO2 structures. Nanoscale Res Lett 11:545. https://doi.org/10.1186/s11671-016-1744-7
Malchukova EV, Boizot B, Trapeznikova IN, Terukov EI (2019) Optical properties and kinetics of the luminescence decay of Sm3+ and Sm2+ ions in aluminoborosilicate glasses. Bull Russ Acad Sci Phys 83:277–281. https://doi.org/10.3103/S1062873819030158
Maslova NE, Antonovsky AA, Zhigunov DM, Timoshenko VYu, Glebov VN, Seminogov VN (2010) Investigation of silicon nanocrystals in silicon suboxide layers by Raman scattering spectroscopy. Semiconductors 44:1040–1043. https://doi.org/10.1134/S1063782610080154
Mbakaan C, Ahemen I, Dejene FB, Onojah AD, Motloung SJ, Ocaya R, Reyes-Rojas A (2021) Structural and optical properties of nano-SiO2 derived from the husk of some rice varieties and the effect of doping with Sm3+ ions. Opt Mater Express 11:965–987. https://doi.org/10.1364/OME.419552
Mertens H, Polman A, Aarts IMP, Kessels WMM (2005) Absence of the enhanced intra-4f transition cross section at 1.5 μm of Er3+in Si-rich SiO2. Appl Phys Lett. Doi 10(1063/1):1949720
Mustafa D, Biggemann D, Martens JA, Kirschhock ChEA, Tessler LR, Breynaert E (2013) Erbium enhanced formation and growth of photoluminescent Er/Si nanocrystals. Thin Solid Films 536:196–201. https://doi.org/10.1016/j.tsf.2013.03.027
Nakamura M, Mochizuki V, Usami K, Itoh Y, Nozaki T (1984) Infrared absorption spectra and compositions of evaporated silicon oxides (SiOx). Solid State Commun 50:1079–1081. https://doi.org/10.1016/0038-1098(84)90292-8
Nikolenko AS, Sopinskyy MV, Strelchuk VV, Veligura LI, Gomonovych VV (2012) Raman study of Si nanoparticles formation in the annealed SiOx and SiOx:Er, F films on sapphire substrate. J Optoelectron Adv Mater 14:120–124
Nogami M, Abe Y (1996) Fluorescence properties of Sm2+ ions in silicate glasses. J Appl Phys 80:409–414. https://doi.org/10.1063/1.362770
Pacifici D, Franzo G, Priollo F, Iacona F, Dal Negro L (2003a) Modeling and perspectives of the Si nanocrystals–Er interaction for optical amplification Phys. Rev B 67:245301. https://doi.org/10.1103/PhysRevB.67.245301
Pacifici D, Irrera A, Franzo G, Miritello M, Iacona F, Priolo F (2003b) Erbium-doped Si nanocrystals: optical properties and electroluminescent devices. Physica E 16:331–340. https://doi.org/10.1016/S1386-9477(02)00615-X
Pavesi L (2008) Silicon-based light sources for silicon integrated circuits. Adv Opt Technol 2008:416926. https://doi.org/10.1155/2008/416926
Polman A, Van Veggel FCJM (2004) Broadband sensitizers for erbium-doped planar optical amplifiers: review. J Opt Soc Am B 21:871–895. https://doi.org/10.1364/JOSAB.21.000871
Priolo F, Gregorkiewicz T, Galli M, Krauss TF (2014) Silicon nanostructures for photonics and photovoltaics. Nat Nanotechnol 9:19–32. https://doi.org/10.1038/nnano.2013.271
Sakirzanovas S, Katelnikovas A, Dutczak D, Kareiva A, Justel T (2012) Concentration influence on temperature-dependent luminescence properties of samarium substituted strontium tetraborate. J Lumin 132:141–146. https://doi.org/10.1016/j.jlumin.2011.08.011
Shimizu-Iwayama T, Fujita K, Nakao S, Saitoh K, Fujita T, Itoh N (1994) Visible photoluminescence in Si+-implanted silica glass. J Appl Phys 75:7779–7783. https://doi.org/10.1063/1.357031
Sopinskyy MV, Vlasenko NA, Lisovskyy IP, Zlobin SO, Tsybrii ZF, Veligura LI (2015) Formation of nanocomposites by oxidizing annealing of SiOx and SiOx<Er, F> films: Ellipsometry and FTIR analysis. Nanoscale Res Lett 10:232. https://doi.org/10.1186/s11671-015-0933-0
Vlasenko NA, Sopinskii NV, Gule EG, Strelchuk VV, Oleksenko PF, Veligura LI, Nikolenko AS, Mukhlyo MA (2012) Effect of erbium fluoride doping on the photoluminescence of SiOx films //. Semiconductors 46:323–329. https://doi.org/10.1134/S1063782612030232
Wang J, Huang Y, Li Y (2011) The reduction and luminescence characteristics of Sm2+ doped in Ba3BP3O12 crystal. J Am CeramSoc 94:1454–1459. https://doi.org/10.1111/j.1551-2916.2010.04250.x
Yu AE (2014) Oxidation of a thin film of samarium on iridium. Tech Phys Lett 40:520–522. https://doi.org/10.1134/S1063785014060169
Zarroug A, Bouznif Z, Hamed ZB, Derbali L, Ezzaouia H (2017) Optoelectronic effect of porous silicon surface treatment with samarium ions for different deposition times and characterizations. Int J Adv Manuf Technol 93:2403–2410. https://doi.org/10.1007/s00170-017-0600-y
Acknowledgements
The authors thank Jeffrey Monastyrsky (USA) for his help with the English edition of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michailovska, K.V., Indutnyi, I.Z., Shepeliavyi, P.E. et al. Samarium-induced enhancement of SiOx decomposition and Si nanocrystals formation. Appl Nanosci 13, 4779–4786 (2023). https://doi.org/10.1007/s13204-022-02617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02617-0