Abstract
In the present study, we report a simple, rapid, cost-effective approach for the white synthesis of silver nanoparticles (AgNPs) using Capra aegagrus hircus milk. The formation of AgNPs was visually examined and further investigated using UV–visible spectrophotometer, transmission electron microscopy, scanning electron microscopy with energy dispersive X-ray, Fourier infrared spectroscopy, and X-ray diffractometer. Crystalline lattice indices of AgNPs were performed using the XRD analysis. The diffraction peaks at 2θ values of 37.7°, 46.1°, 67.4°, and 76.84° corresponding to lattice planes (111), (200), (220), and (311), respectively. The obtained AgNPs were spherical in shape with the size between 5 and 50 nm. The antibacterial activity of AgNPs against Klebsiella sp. (Accession Number: KC899845), and Staphylococcus sp. (Accession Number: KC688883) were evaluated by means of cell growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoparticles are being considered as an eminent component of the widely accelerating field of nanotechnology exemplifying various real-world applications. In the realms of metal nanoparticles, silver, gold, copper, and zinc oxide have been demonstrated as phenomenon alternative therapeutic agents (Khan et al. 2021). Metallic nanoparticles have attracted considerable scientific interest due to their unique optical properties of surface plasmon resonance (SPR) and physicochemical properties (Castro et al. 2014). They are classified into different types such as carbon, metal, ceramic, polymeric, semi-conductor, and lipid-based nanoparticles, based on size, and structure properties (Thomas et al. 2015). Among them, silver nanoparticles (AgNPs) are one of the most sought after and greatly investigated metal nanoparticles.
Silver nanoparticles (AgNPs) are one of the most engineered nanoparticles used in several commercial areas, including medical devices, healthcare products, cleaning agents, food storage, packing, and textile coatings (Rolim et al. 2019). Silver nanoparticles (AgNPs) play a significant role in resolving numerous medical problems due to their chemical biocompatibility, inertness, oxidation resistance, and safe use as antibacterial activity (Carlson et al. 2008) against a variety of microorganisms (Mahmoud et al. 2021). The antimicrobial potency of silver nanoparticles increased the usage of silver nanoparticles in wound dressing, drug carriers, and in artificial implantation (Liao et al. 2019). They are also used for environmental applications because of their potent antimicrobial activity against bacteria, viruses, and fungi (Rolim et al. 2019; Ahluwalia et al. 2018).
To exploit the numerous properties of AgNPs, innovative methods of synthesis are being employed. Chemical and physical modes are widely implemented for the synthesis of AgNPs (Khan et al. 2021; Gurunathan et al. 2015). Eco-friendly green synthesis of nanoparticles is biocompatible, cost effective, less time and energy consuming and it also non toxic compared to the physical and chemical methods (Veeraraghavan et al. 2021). Accordingly, numerous biological resources are being explored for the synthesis of AgNPs including, plants (Aravinthan et al. 2015; Mythili et al. 2018; Sengottaiyan et al. 2016; Ameen et al., 2019; Sampath et al., 2021), bacteria (Ameen et al. 2020), fungi (Mukherjee et al. 2002), algae (Gopu et al., 2021; Valarmathi et al., 2020; Govarthanan et al. 2017) oilcakes (Govarthanan et al. 2016), organic compound (Govarthanan et al. 2014), and even vegetable waste (Mythili et al. 2018). So far, many studies reported for AgNPs as plant extracts, due to medical value owing to the presence of phenolic compounds (polyphenols, tannic acid, flavonoids, terpenoids), amino acids and vitamins (Ebrahiminezhad et al. 2018). But only few studies attempted the biosynthesis of AgNPs using milk and other related bioresources.
Lee et al. (2013) reported that, synthesis of AgNPs using cow milk have potential application in medical and pharmaceutical sciences. Although a wide variety of biological sources have been used to synthesize AgNPs, the use of Capra aegagrus (goat) milk is not common. However, there is no report on synthesis of AgNPs using goat milk. In addition, goat milk has various effects of human health considering the total solid, fat, protein, mineral, vitamins, and lactose (Turkmen et al. 2017). In particular, goat milk contains 13.2% total solids, consisting of 4.5% fat, 3.6% protein, 0.8% minerals, and 4.3% lactose (Üçüncü 2013). It has been reported that the goat milk has maximum number of conjugated linoleic acids which play an important role in stimulation of immunity, growth promotion, and prevention of diseases. The most important effect of goat milk proteins is their healing effect on cow milk allergy, the most common food allergy, which causes many deaths in infants (Turkmen 2017). The present study investigated the biological synthesis of silver nanoparticles using fresh goat milk and evaluate the antibacterial activity of the synthesized AgNPs against the human pathogens.
Materials and methods
Materials and chemicals
Silver nitrate was purchased from Sigma–Aldrich (St. Louis, MO), nutrient broth was purchased from Hi-media Laboratories Pvt. Ltd (Mumbai, India). Capra aegagrus milk was procured from local goat farm in Mallasamudram, Tamil Nadu, India.
Synthesis of AgNPs
AgNPs synthesis was carried out according to Lee et al. (2013). Briefly, 4 ml of goat milk was mixed with 96 ml of 1 mM silver nitrate solution. The mixture was then incubated in a rotary shaking incubator at 37 °C until the color changed to dark brown. The reaction precipitates were then filtered and centrifuged at 10,000 rpm for 15 min. the resulting AgNPs were washed with sterile water. The obtained AgNPs was freeze dried at – 80 °C and used for further characterization and antibacterial studies.
Characterization of AgNPs
The optical absorption spectra of the synthesized AgNPs were observed using UV–visible spectrophotometer (Elico-SL 164) in the range of 200–800 nm. The morphology and size of the biogenic AgNPs were determined by transmission electron microscopy (TEM, FEI Tecnai TF 20 high resolution). The presence of elemental silver in the AgNPs was analyzed by scanning electron micrograph-energy dispersive spectroscopy (SEM–EDS; Jeol JSM 6390). Fourier transform infrared (FT-IR) spectra of the AgNPs were analyzed using Perkin-Elmer FT-IR spectrophotometer (IRAffinity-1S) operated at a resolution of 4 cm−1. The spectra were recorded at wavelength ranges from 500 to 4000 cm 1. The structural characterization was conducted using X-ray powder diffraction (XPERT-Pro diffractometer using Cu–Ka radiation). Sample Scanning was done in the region of 2θ from 20 to 80° at 0.04°/min with a time constant of 2 s.
Antibacterial activity
The antibacterial activity against Klebsiella sp. (Accession Number: KC899845), and Staphylococcus sp. (Accession Number: KC688883) of AgNPs synthesized from goat milk was evaluated according to Govarthanan et al. (2014). Briefly, the strains cultivated in 100 mL of nutrient broth amended with different concentrations (1–5 mM) of AgNPs. The growth of the bacterial strains was indexed by measuring the optical density (at λ = 600 nm) at regular intervals (0–48 h) using spectrophotometer. The growth curve was plotted between optical density and time. The flask without AgNPs was used as a control for this experiment.
Results and discussion
Characterization of AgNPs
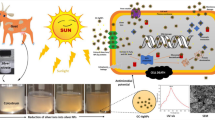
In the present study investigated a white chemistry milk approach-based biosynthesis of AgNPs from Capra aegagrus milk. The development AgNPs through the reduction of Ag+ into Ag0 ions was visually confirmed from colorless to dark brown in the flasks within 5 h. It has been reported that the proteins present in the milk could be responsible for the reduction of Ag+ in the mixture. The process of goat milk mediated AgNPs synthesis is shown in Fig. 1. Our previous study obtained after 8 h of reaction, the mixture turned from milky white to dark brown (Lee et al. 2013). The preliminary conformation of AgNPs synthesis was recorded by UV–Vis spectroscopy. The surface plasmon resonance (SPR) was found to be higher at 420 nm and its absorbance intensity increased with the increase of the incubation period (Fig. 2). The SPR at 420–450 is corresponding to the silver nanoparticles formation, according to the earlier reports (Singh et al. 2015).
The TEM images of biogenic AgNPs are shown in Fig. 3. The size of the AgNPs was in the range of 5–50 nm. The AgNPs were found to be spherical in shape, well dispersed, and homogeneous in nature. Shape is another important parameter that has an influence on the biological activity of nanomaterials. It was stated that the cellular uptake of spherical shaped nanoparticles was higher than rod-shaped (Barabadi et al. 2021). Further validate the formation of AgNPs, samples were characterized by SEM–EDS and the results are presented in Fig. 4. Strong silver peak was observed at 3 keV, which is reasonable absorption peak of AgNPs which could be the crystallites due to SPR. The results are consistent with previous studies reporting strong absorption of AgNPs approximately at 3 keV (Lee et al. 2013). The agglomerations of the particles on the surface are due to the multilayer coating of the particle leading to the formation of a few huge clusters on the surface.
FT-IR analysis involved in identifying the possible functional groups and/or secondary metabolites involved in AgNPs synthesis. Figure 5 shows the FT-IR spectra of AgNPs synthesized from goat milk. The band at 2929 cm−1 indicates the presence of C–H bonds of alkanes. The bands at 1662 and 1536 cm−1 are characteristic of amide I and amide II bands, respectively. Subsequently, intense peaks were identified at 1404 and 1028 cm−1, corresponding to C=C and C–O bonds, respectively. The peak recorded at 1662 cm−1 could be due to the C=C stretching associated with the presence of aromatic rings, while the band around 1404 cm−1 could be attributed to aliphatic and aromatic groups in the plane deformation vibrations of methyl and methylene groups (Memon et al. 2018; Zhao et al. 2016).
Crystalline lattice indices of AgNPs were performed using the XRD analysis (Fig. 6). The diffraction peaks at 2θ values of 37.7°, 46.1°, 67.4°, and 76.84° corresponding to lattice planes (111), (200), (220), and (311), respectively. The observed peaks have been attributed to hexagonal phase of AgNPs (JCPDS no. 41–1402). A lot of unimportant peaks are seen owing to the bioorganic phases of crystallization that are stuck to the synthesized nano particles’ surfaces. The XRD pattern strongly confirms the high crystalline nature of the biosynthesized silver nanoparticles. The similar XRD peaks were also found in the earlier studies have been observed the diffraction peaks of AgNPs at lattice planes of (111), (200), (220), and (311), respectively, (Govarthanan et al. 2016; Aravinthan et al. 2015).
Antibacterial activity
The antibacterial activity of AgNPs against Klebsiella sp. (Accession Number: KC899845), and Staphylococcus sp. (Accession Number: KC688883) was evaluated by means of cell growth. Optical density was measured at 600 nm and plotted as a function of time for 48 h at regular intervals with various concentrations (1–5 mM) of AgNPs (Fig. 7a and b). The results showed that the higher concentration (5 mM) of AgNPs effectively encountered the population of cell growth. As expected, the increasing concentration of AgNPs decreased the growth of bacterial population in the medium (Saxena et al. 2012).
It has previously been reported that nanoparticles with a size smaller than 20 nm can interact more easily with membrane proteins, causing maximum permeability, which leads to the cell death of bacteria (Deshmukh et al. 2019). The mechanism of antibacterial activity of AgNPs remains unclear to date. Meanwhile, the mechanism of action of silver itself is by disrupting the cell membrane, causing ROS, penetration of cell membrane, and will bind to DNA and protein. However, based on existing works of literature, the principle of antibacterial mechanism of AgNPs is divided into oxidative stress, metal ion release, and non-oxidative mechanism (Sukweenadhi et al. 2021).
Conclusion
A simple one step white synthesis of AgNPs is reported along with the bio-reduction as well as biostabilization potentials of Capra aegagrus milk. The elemental analysis confirmed the presence of elemental silver in AgNPs. FT-IR analysis confirmed that the functional groups of goat milk have played a significant role for the bio-reduction of Ag+ ions into AgNPs. The antibacterial activity results clearly demonstrated that AgNPs synthesized by goat milk could be used for the treatment of disease which will cause by Klebsiella sp. and Staphylococcus sp. Thus, the white synthesized AgNPs can be used as a potential antibacterial agent in near future.
References
Ahluwalia V, Elumalai S, Kumar V, Kumar S, Sangwan RS (2018) Nano silver particle synthesis using Swertia paniculata herbal extract and its antimicrobial activity. Microb Pathog 114:402–408. https://doi.org/10.1016/j.micpath.2017.11.052
Ameen F, Srinivasan P, Selvankumar T, Kamala-Kannan S, Al Nadhari S, Almansob A, Govarthanan M (2019) Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg Chem 88:102970
Ameen F, Alyahya S, Govarthanan M, Aljahdali N, Al-Enazi N, Alsamhary K, Alharbi SA (2020) Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J Mol Struct 1202:233
Aravinthan A, Govarthanan M, Selvam K, Praburaman L, Selvankumar T, Balamurugan R, Kamala-Kannan S, Kim JH (2015) Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int J Nanomed 10:1977. https://doi.org/10.2147/IJN.S79106
Barabadi H, Mojab F, Vahidi H, Marashi B, Talank N, Hosseini O, Saravanan O (2021) Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg Chem Commun 129:108647. https://doi.org/10.1016/j.inoche.2021.108647
Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112:13608. https://doi.org/10.1021/jp712087m
Castro L, Blázquez ML, González FG, Ballester FG (2014) Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev Adv Sci Eng 3:199. https://doi.org/10.1166/rase.2014.1064
Deshmukh SP, Patil SM, Mullani SB, Delekar SD (2019) Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C 97:954–965. https://doi.org/10.1016/j.msec.2018.12.102
Ebrahiminezhad A, Zare-Hoseinabadi A, Sarmah AK, Taghizadeh S, Ghasemi Y, Berenjian A (2018) Plant-mediated synthesis and applications of iron nanoparticles. Mol Biotechnol 60:154–168. https://doi.org/10.1007/s12033-017-0053-4
Gopu M, Kumar P, Selvankumar T, Senthilkumar B, Sudhakar C, Govarthanan M, Selvam K (2021) Green biomimetic silver nanoparticles utilizing the red algae Amphiroa rigida and its potent antibacterial, cytotoxicity and larvicidal efficiency. Bioprocess Biosyst Eng 44(2):217–223
Govarthanan M, Selvankumar T, Manoharan K, Rathika R, Shanthi K, Lee KJ, Cho M, Kamala-Kannan S, Oh BT (2014) Biosynthesis and characterization of silver nanoparticles using panchakavya, an Indian traditional farming formulating agent. Int J Nanomed 9:1593–1599. https://doi.org/10.2147/IJN.S58932
Govarthanan M, Seo YS, Lee KJ, Jung IB, Ju HJ, Kim JS, Cho M, Kamala-Kannan S, Oh BT (2016) Low-cost and eco-friendly synthesis of silver nanoparticles using coconut (Cocos nucifera) oil cake extract and its antibacterial activity. Artif Cells Nanomed Biotechnol 44:1878–1882. https://doi.org/10.3109/21691401.2015.1111230
Govarthanan M, Selvankumar T, Mythili R, Sudhakar C, Selvam K (2017) Biosynthesis of silver nanoparticles from Spirulina microalgae and its antibacterial activity. Environ Sci Pollut Res 24:19459–19464. https://doi.org/10.1007/s11356-017-9772-0
Gurunathan S, Park JH, Han JW, Kim JH (2015) Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy. Int J Nanomed 10:4203–14022. https://doi.org/10.2147/IJN.S83953
Khan AA, Alanazi AM, Alsaif N, Wani TA, Bhat MA (2021) Pomegranate peel induced biogenic synthesis of silver nanoparticles and their multifaceted potential against intracellular pathogen and cancer. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2021.06.022
Lee KJ, Park SH, Govarthanan M, Wang PH, Seo YS, Cho M, Lee WH, Lee JY, Kamala-Kannan S, Oh BT (2013) Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater Lett 105:128–131. https://doi.org/10.1016/j.matlet.2013.04.076
Liao C, Li Y, Tjong SC (2019) Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci 20(2):449. https://doi.org/10.3390/ijms20020449
Mahmoud MG, Asker MS, Mohamed SS (2021) Facile green silver nanoparticles synthesis to promote the antibacterial activity of cellulosic fabric. J Ind Eng Chem 99:224–234. https://doi.org/10.1016/j.jiec.2021.04.030
Memon AA, Arbab AA, Patil SA, Mengal N, Sun KC, Sahito IA, Jeong SH, Kim HS (2018) Synthesis of solution processed f-CNT@ Bi2S3 hybrid film coated linen fabric as a free-standing textile structured photo catalyst. Appl Catal A 566:87–95. https://doi.org/10.1016/j.apcata.2018.06.015
Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M (2002) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporium. ChemBioChem 3:461–463. https://doi.org/10.1002/1439-7633(20020503)
Mythili R, Selvankumar T, Kamala-Kannan S, Sudhakar C, Ameen F, Sabri AA, Selvam K, Govarthan M, Kim H (2018) Utilization of vegetable waste for silver nanoparticles synthesis and its antibacterial activity. Mater Lett 225:101–104. https://doi.org/10.1016/j.matlet.2018.04.111
Rolim WR, Pelegrino MT, de Araújo LB, Ferraz LS, Costa FN, Bernardes JS, Rodigues T, Brocchi M, Seabra AB (2019) Green tea extract mediated biogenic synthesis of silver nanoparticles: characterization, cytotoxicity evaluation and antibacterial activity. Appl Surf Sci 463:66–74. https://doi.org/10.1016/j.apsusc.2018.08.203
Sampath G, Govarthanan M, Rameshkumar N, Vo DVN, Krishnan M, Sivasankar P, Kayalvizhi N (2021) Eco-friendly biosynthesis metallic silver nanoparticles using Aeglemarmelos (Indian bael) and its clinical and environmental applications. Appl Nanosci. https://doi.org/10.1007/s13204-021-01883-8
Saxena A, Tripathi RM, Zafar F, Singh P (2012) Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater Lett 67:91–94. https://doi.org/10.1016/j.matlet.2011.09.038
Sengottaiyan A, Mythili R, Selvankumar T et al (2016) Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res Chem Intermed 42:3095–3103
Singh P, Kim YJ, Singh H, Mathiyalagan R, Wang C, Yang DC (2015) Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J Nanomater. https://doi.org/10.1155/2015/234741
Sukweenadhi J, Setiawan KI, Avanti C, Kartini K, Rupa EJ, Yang DC (2021) Scale-up of Green synthesis and characterization of silver nanoparticles using ethanol extract of Plantago major L. leaf and its antibacterial potential. S Afr J Chem Eng. https://doi.org/10.1016/j.sajce.2021.06.008
Thomas SC, Mishra PK, Talegaonkar S (2015) Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des 21(42):6165–6188
Turkmen N (2017) The nutritional value and health benefits of goat milk components. J Funct Foods. https://doi.org/10.1016/B978-0-12-809762-5.00035-8
Üçüncü M (2013) Süt ve Mamülleri Teknolojisi. Meta Basım, İzmir
Valarmathi N, Ameen F, Almansob A, Kumar P, Arunprakash S, Govarthanan M (2020) Utilization of marine seaweed Spyridia filamentosa for silver nanoparticles synthesis and its clinical applications. Mater Lett 263:127244
Veeraraghavan VP, Periadurai ND, Karunakaran T, Hussain S, Surapaneni KM, Jiao X (2021) Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929). Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2021.05.007
Zhao H, Hou L, Lu Y (2016) Electromagnetic interference shielding of layered linen fabric/polypyrrole/nickel (LF/PPy/Ni) composites. Mater Des 95:97–106. https://doi.org/10.1016/j.matdes.2016.01.088
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project Number (RSP-2021/228), King Saud University, Riyadh Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mythili, R., Srinivasan, P., Praburaman, L. et al. Biogenic production of silver nanoparticles from milk of Capra aegagrus hircus and mechanism of antibacterial activity on different bacteria. Appl Nanosci 13, 1611–1618 (2023). https://doi.org/10.1007/s13204-021-02095-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-02095-w