Abstract
The use of biological inoculants in replacement of the application of chemical fertilizers is a desirable strategy taking into account it is more sustainable and economically less costly. Considering that agricultural practices can produce effects on soil microbial communities associated to the plant crops, the objective of this study was to analyze and compare the effect of these two practices on the structure of the rhizobacterial community of peanut and maize plants. For this purpose, microcosm assays were performed in which peanut and maize plants were inoculated individually with native peanut phosphate solubilizing strains or chemical fertilized with phosphorus, nitrogen, zinc and sulphur. At the beginning and at the end of the assays, samples of rhizospheric soil DNA were obtained and the structure of the rhizospheric bacterial community was analyzed by high-throughput sequencing of the 16S rRNA gene by using Illumina MiSeq platform. The results obtained indicated that the structures of the rhizospheric bacterial communities were different depending on plant type. It was possible to observe changes with respect to the initial bacterial structure in all taxonomic levels analyzed of all treatments. The more notorious structural changes of bacterial community were observed in those rhizospheres exposed to chemical fertilizers, mainly in soil samples associated to maize plants. The rhizospheric bacterial community of peanut showed to change mainly with plant growth. In conclusion, the rhizobacterial community structure is highly dynamic and influenced by different factors such as type of plant, the fertilizer input and bio-inoculant applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The constant increase in world population generates a challenge at the level of food production. Early agricultural revolutions in industrialized countries primarily involved expansion of an agricultural area to increase aggregate food production. Such extensification was later followed by intensified use of resources on the same land causing deterioration of soil health (McBratney et al. 2014; Pretty and Bharucha 2014). Agricultural intensification has greatly increased soil nutrient demand of crops which has led to an increase in the application of synthetic fertilizers (Fertilizar 2020). Nitrogen (N), phosphorus (P) and potassium (K) fertilization replenishes some of the nutrients removed by intensive production (Jones et al. 2013). Nevertheless, the use of fertilizers and other agrochemicals is not always the most effective and healthier strategy. A percentage of the fertilizers applied is precipitated and is converted in potential soil pollutants (Sainju et al. 2019). A healthier strategy in replacement of this agricultural practice is the use of soil bacteria with plant beneficial traits designated as plant growth promoting bacteria (PGPB) (Olanrewaju et al. 2017). The plant growth promotion by this group of microorganisms may be attributed by several mechanisms such as nitrogen-fixing, phosphate-solubilizing, hormone production and biological control (Santoyo et al. 2016). Along with the enhancement of plant productivity and yield, the application of biological inoculants with PGPB can allow for significant reductions in the application of chemical fertilizers and pesticides (Singh et al. 2016; Vicario et al. 2016; Timmusk et al. 2017). When selecting PGP bacteria, those that are native of the agricultural soil in which they are going to be applied is an additional benefit since they are better adapted to the ecological soil environment (Trabelsi et al. 2011).
The microbial soil community structure can be affected by a wide range of factors including environmental factors, soil types, plant species and phenological stage, agricultural management regime, inorganic fertilizer application, bacterial inoculation (Sessitsch et al. 2012; Trabelsi and Mhamdi 2013; Ma et al. 2018; Anzuay et al. 2021). The most significant studies that reported the effect of biological inoculants and fertilization on soil microbial communities has been assessed by a range of different techniques. The sequencing of amplicons in 16S rRNA gene hypervariable regions using next-generation sequencing technologies is currently considered to be one of the best approaches for describing microbial communities (Tringe and Hugenholtz 2008; Caporaso et al. 2010).
As mentioned, many soils of the planet have decreased their fertility due the intensive practices and those of the agricultural area of Argentina are not exempt (Sainz Rozas et al. 2013; Agrovoz 2015). One of the main crops of this country is peanut (Arachis hypogaea L.) being Argentina one of the world’s leading peanut exporters and producers (National Institute of Agricultural Technology 2021). In the peanut cultivating area this legume is rotated mainly with maize (Zea mays L.) which is a crop of great economical importance of Argentina since this country is among the four main world maize producers (Food and Agriculture Organization of the United Nations 2018; Pedelini and Monetti 2021). In addition to the importance of these crops in Argentina, peanuts and maize are of great relevance in international food markets (Food and Agriculture Organization of the United Nations 2022).
In these Argentine agricultural soils, values below the critical levels of P were reported for peanut and maize (6.6–8.5 mg kg−1) (Sainz Rozas et al. 2012; Anzuay et al. 2015, 2017). The critical values of P for plants peanut and maize plants are 10 and 12–20 mg kg−1, respectively (Gudelj et al. 2016). Considering the importance of peanut and maize crops and the need to replace the high chemical fertilizer application, it is necessary to deeply analyze new healthier management practices. Within the alternative strategies, the use of native soil PGPB is a friendlier option, not only to increase productivity but also to reduce the disturbances generated by agrochemicals. Nevertheless, this exogenous bacterial application, although a more sustainable solution, would generate an impact on the microbial soil community. Thus, it is important to know the possible effect of the application of biological inoculants on the rhizobacterial community associated to these plants. The objective of this study was to analyze and compare the effect of bacterial inoculation and chemical fertilizer application on the structure of the rhizobacterial community of peanut and maize plants using next-generation sequencing technologies. For this, under microcosm conditions, peanut and maize plants were grown and treated with PGPB or chemical fertilizers and rhizospheric soil samples were taken at the beginning and at the end of the assays to explore the potential bacterial community shifts caused by these agricultural practices.
2 Materials and methods
2.1 Bacteria and culture media
Two native phosphate solubilizing bacteria isolated from nodules of peanut plants cultivated in central and southern region of Córdoba, Argentina (latitude, 32° to 34°, longitude, 63° to 65°) were employed in this study: Enterobacter sp. J49 and Serratia sp. J260 (Taurian et al. 2010; Anzuay et al. 2013). In addition to tricalcium phosphate solubilizing ability, these native strains presented also the ability to solubilize other P insoluble sources such as Fe- and Al-P, phosphatase activity and siderophore production (Anzuay et al. 2017). Bradyrhizobium sp. SEMIA 6144, recommended as peanut microsymbiont inoculant for Instituto de Pesquisas Agronómicas, IPAGRO, was used on peanut plants. Nodumax®Azo (Laboratorio López S.R.L.) is a commercial inoculant based on the diazotrophic microorganism Azospirillum brasilense and RIZOFOS® (RIZOBACTER) that contained the phosphate solubilizing bacteria Pseudomonas fluorescens PMT1 were the commercial inoculants used on maize plants. The phosphate solubilizing strains and A. brasilense were grown on LB (Luria–Bertani) (Miller 1972) or TY (tryptone yeast) (Beringer 1974) media at 28 °C. Bradyrhizobium sp. SEMIA 6144 was grown in YEMA (yeast extract mannitol agar) (Vincent 1970) at 28 °C. All the bacteria were maintained in 20% glycerol (v/v) at − 80◦C in the respective medium.
2.2 Microcosm assays
Seeds of peanut (cv. granoleico) or maize (NK 910 TD Max hybrid, Syngenta) were superficially disinfected according to Taurian et al. (2002) and Pereira et al. (2011), respectively and were inoculated with bacteria when required. Subsequently, 1 seed per pot was placed on plastic pots (35 cm diameter, 40 cm height) containing approximately 7 kg of unsterilized sieved soil per pot. The soil used as plant growth was obtained from the peanut cultivation area of Córdoba (organic matter: 1.92% (Walkley Black method), pH: 7.3 (Potenciometry 1:2.5), moisture: 14.71% (100–105 °C), N: 12.9 µgg−1soil (phenolsulfonic acid) and P: 13.6 µgg−1 (Kurtz and Bray I method)). The following treatments were used in peanut microcosm assays: (1) seeds inoculated with Enterobacter sp. J49 (P_IJ49); (2) seeds inoculated with Serratia sp. J260 (P_IJ260); (3) seeds inoculated with Bradyrhizobium sp. SEMIA 6144 (P_IB); (4) uninoculated seeds grown on fertilized soil (P_Fert); (5) uninoculated and unfertilized seeds (P_C). For maize microcosm assays the treatments used were: (I) seeds inoculated with Enterobacter sp. J49 (M_IJ49); (II) seeds inoculated with Serratia sp. J260 (M_IJ260); (III) seeds inoculated with Nodumax® Azo maíz and RIZOFOS® (M_IP.A); (IV) uninoculated seeds grown on fertilized soil (M_Fert); (V) uninoculated and unfertilized seeds (M_C). In fertilized treatments, MicroEssentials® SZ ™ (Mosaic) granules (12% Nitrogen, 40% Phosphorous Pentoxide, 10% Sulfur and 1% Zinc) was used in the recommended doses.
For preparation of bacterial inoculum, native bacteria were grown in TY liquid medium until stationary phase and stored at 4 °C until use. The number of viable cells, colony forming units (CFU) ml−1 was determined following the method described by Somasegaran and Hoben (1994). Bacterial inoculants prepared with the native strains were mixed with the disinfected seeds and a 20% sucrose solution was used as adherent. On the other hand, the commercial inoculants were prepared according to the technical specifications of the maker and disinfected seeds were mixed with the commercial adherent. The initial doses of the bacterial inoculum were 109 CFU ml−1 at the time of being placed in the seeds, being 108 CFU ml−1 the number of bacterial cells adhered to them.
Peanut and maize plants were maintained under controlled environmental conditions (light intensity of 200 μR.m−2 s−1 16 h day/8 h night cycle, at a constant temperature of 28 °C and a relative humidity of 50%) and watered regularly with tap water. Rhizospheric soil samples were obtained at the beginning of the assays (designated as ES-experimental soil) and at plants harvest. Peanut and maize were harvested at 120 and 100 days postinoculation, respectively. Approximately 5 g of rhizospheric soil was sampled of each pot and at harvest plants were removed. Two independent microcosm assays were performed (n = 8–10).
2.3 Analysis of rhizobacterial community structure of peanut and maize plants using next-generation sequencing technologies from soil samples
Rhizobacterial community structure, using Illumina MiSeq sequencing of 16S rRNA gene, of rhizospheric soil DNA associated to peanut and maize plants was analyzed. For this, four to five rhizospheric soil samples from each treatment were collected, pooled and homogenized. Two replicate DNA extractions were used per treatment on both microcosm assays.
2.3.1 Soil DNA extraction
Soil DNA was extracted using the commercial Ultra Clean soil DNA isolation kit (MoBio). DNA quantity and quality were verified by spectrophotometry (Nanodrop, ThermoScientific).
2.3.2 High-throughput sequencing of 16S rRNA gene and data processing
The V3-V4 hypervariable region of 16S rRNA gene was amplified from microbial genomic DNA using universal primers 341F (5’-CCTACGGGNGGCWGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The following PCR conditions were used: 94 °C for 3 min followed by 35 cycles of 94 °C for 15 s, 55 °C for 45 s, and 72 °C for 1 min, followed by a final elongation step of 8 min at 72 °C. Library construction and high-throughput sequencing were performed at Macrogen Inc. (Seoul, South Korea) on an Illumina MiSeq platform and 2 bp/300 bp paired-end reads were generated. The datasets generated in this study are available under request.
The initial processing and quality control of the paired-end reads was performed using Trimmomatic v0.33 (Bolger et al. 2014), which remove all primers and adapters sequences and perform a quality filtering allowing a quality Phred cut-off score of at least Q20. FLASH was used to merge the pairs of reads from the original DNA fragments (Magoč and Salzberg 2011). Sequencing reads were assigned to each sample according to the unique barcode of each sample. Sequences were analyzed with the QIIME software package (Quantitative Insights Into Microbial Ecology) (Caporaso et al. 2010). Briefly, chimeric sequences were filtered out using USERCH algorithm and those remaining sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs). Singletons and OTUs with abundance below 0.005% were removed from the final OTU table, to avoid microbial diversity overestimation (Bokulich et al. 2013). A representative sequence for each OTU was picked and the SILVA v.128 database was used to obtain the taxonomic information.
Normalization of OTU counts was carried out by performing multiple rarefactions from 1000 to 47,300 sequences with steps of 1000 and 10 repetitions at each rarefaction depth. The distributions of the OTUs obtained were visualized using the average of the replicates in a hierarchically clustered heatmap implemented in R. Venn diagram was done to show the relationships between different communities associated to peanut and maize plants in all treatments analyzed. The observed species richness was defined as the number of OTUs present in each sample. Also, the Chao 1 (Chao 1984) and Shannon (Margelef 1958) indices, for analyzed richness and diversity of the communities respectively, were calculated using QIIME. The Good’s coverage index, which is considered a relative measure of how well the sequences obtained represent the entire populations was calculated using QIIME.
2.4 Abundance of culturable rhizobacterial populations
The abundances of culturable heterotrophic bacteria (CHB), diazotrophic bacteria (DB) and phosphate solubilizing bacteria (PSB) at beginning and the end of the assays were determined. For this, soils and rhizospheric samples from all treatments were collected. Three replicates per treatment were used to analyze the culturable diversity. Each sample (0.5 g) was placed into flasks containing 4.5 ml of 0.1% sterile sodium pyrophosphate (NaPP) and mixed for 30 min at 180 rpm. For CHB, DB and PSB count, 0.1 ml aliquots of serial dilutions (up to 10–8) were streaked onto Petri dishes containing tenfold diluted tryptic soy agar (110–1 TSA) (Smit et al. 2001), nitrogen-free bromothymol blue malate medium (NFb) (Döebereiner 1995) and National Botanical Research Institute’s phosphate grown medium with bromophenol blue (NBRIP-BPB) (Mehta and Nautiyal 2001), respectively. Media were supplemented with 2.6-dicloro-4-nitroanilina (dicloran50 µg ml−1) to prevent fungal growth. Plates were incubated at 28 °C during seven days and CFU ml−1 from rhizospheric soil were counted. Individual colonies grown on plates containing NFb solid medium were inoculated into assay tubes containing semisolid NFb medium. Pellicle formation under the medium surface was indicative of growth of free living nitrogen fixing bacteria. On the other hand, in NBRIP-BPB solid medium, the halo of clearance around the bacterial colony, evidenced by acidification of the medium (turn to yellow), indicated solubilizing ability.
2.5 Determination of growth parameters, chlorophyll, P and N content of peanut and maize plants
The growth, chlorophyll and P and N content of peanut and maize plants were analyzed at the end of the microcosm assays. The following growth parameters were determined: aerial length (cm), root length (cm), aerial dry weight (g plant−1) and root dry weight (g plant−1). Chlorophyll content was determined by following Cassol et al. (2008). P and N content of aerial tissues (mg g plant−1) were determined following the procedure of Jackson with modifications (1973) and Nelson and Sommers with modifications (1973), respectively. The presence and number of nodules and boxes were determined in peanut plants.
2.6 Data analysis
The statistical analyses were performed using Infostat software (Balzarini et al. 2008). Principal component analysis (PCA) was performed and the results of this analysis are presented as biplot graphs. Data obtained from the determination of abundance of culturable rhizobacterial populations and plants growth and content of chlorophyll, P and N were subjected to one-way analysis of variance (ANOVA) and differences among treatments were detected by LSD test (P < 0.05).
3 Results
3.1 Rhizospheric bacterial communities associated to peanut and maize show the most structural changes during plant growth and with fertilized treatment
The high-throughput sequencing analysis indicated a total of 6,803,162 reads obtained from a total of 22 samples, with an average of 309,235 ± 26,926.05 reads per sample, and a minimum and maximum range of 240,560 and 363,926 reads, respectively. After quality filtering, merge, and removal of chimeric reads, the number of bacterial OTUs detected ranged from 2723 to 2968 (Table 1). In all communities analyzed it was possible to observe a Good’s coverage index greater than 99%, indicating that the analyzed sequences covered almost the entire diversity of bacterial populations (Fig. S1-supplementary material). Alpha diversity indexes of soil samples are shown in Table 1. The highest richness value (Chao 1 index and observed OTUs) was detected in the ES sample and the lowest corresponded to M_Fert treatment. Shannon diversity index showed the highest value on the samples of bacterial rhizospheric community of treatment P_IJ49 and the lowest corresponded to that of treatment M_Fert.
The community structure showed the presence of 10 phyla with more than 1% of relative abundance in all the samples, comprising 96 classes (18 > 1%), 161 orders (33 > 1%), 248 families (41 > 1%) and 351 genera (38 > 1%) (data not shown). The most abundant bacterial phyla across all treatments were Proteobacteria, Acidobacteria and Actinobacteria (Fig. 1A, B). No statistically significant differences could be found in the relative abundance of phyla present between treatments. Notable trends were observed in the rhizobacterial communities of both plants with respect to ES (Fig. S2 and S3-supplementary material). In the rhizobacterial communities associated to peanut, all treatments and control samples showed an increase in the relative abundances of Actinobacteria and Chloroflexi, and a decrease in the relative abundances of Bacteroidetes, Gemmatimonadetes, Nitrospirae, β-, δ-, and γ-Proteobacteria (Fig. S2-supplementary material).
Rhizobacterial communities structure associated to peanut and maize plants from different treatments. Stacked bar graph represents the relative abundance of the most abundant phyla. ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB: seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize
On the other hand, the rhizobacterial communities of treatment M_Fert and M_IP.A showed variations in the relative abundances in several of the analyzed phyla with respect to ES (Fig. S3-supplementary material). M_Fert treatment produced an increase in the relative abundances of Planctomycetes and α-Proteobacteria, and a decrease in Acidobacteria, Gemmatimonadetes, Nitrospirae, β-, δ-, and γ-Proteobacteria, respect to ES. In rhizospheric samples of M_IP.A treatment, an increase in Bacteroidetes was observed with respect to ES, while a decrease in Gemmatimonadetes, Nitrospirae, Planctomycetes, and α-Proteobacteria was detected.
A cluster analysis, based on the family level, showed that the relative abundance of the families analyzed could be split in two clusters (Fig. 2). One cluster showed all treatments associated to maize plant and ES, and the other cluster contained all treatments associated to peanut plant. Both fertilized treatments, P_Fert and M_Fert, showed the greatest differences within each group. In addition, the most abundant families found in peanut and maize rhizosphere were Sphingomonadacea, Chitinophagaceae, Hyphomicrobiaceae, and one unclassified family belonging to the Acidimicrobiales order.
Hierarchically clustered heatmap of bacterial distribution of different communities associated to peanut and maize plants from different treatments at the family level. Row represents different samples and column indicates the abundance of each bacterial group. The relative abundance for each bacterial family was depicted by color intensity with the legend indicated at the top of the figure. ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB: seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize
The communities associated to peanut and the M_Fert treatment showed an increase in the relative abundance of the families Microccacea, Gaiellacea, Rhizobiaceae and Bacillaceae, with respect to ES. On the contrary, a decrease in the bacterial families Xanthomonadacea and Comamonadaceae was detected in these treatments respect to the initial sample ES. Furthermore, all peanut samples showed an increase in the relative abundance of Bradyrhizobiaceae and Streptomitaceae families. Moreover, these rhizobacterial communities presented a decrease in the relative abundance of the Chitinophagaceae and Sphingomonadacea families. Within maize samples, it was possible to observe that M_IJ260 treatment produced an increase in the relative abundance of the Xanthomonadacea family, with respect to ES and M_IJ49 treatment an increase on the relative abundance of members of the Pseudomonadacea and Sphingomonadacea families.
Venn diagram indicated that the rhizospheric bacterial communities of each treatment share 236 bacterial families in peanut rhizospheric samples (Fig. 3). In these communities, the P_C and P_IJ49 treatments presented one unshared OTUs for each treatment, been classified as a family of the Bacteroidetes phylum and a member of the Clostridiaceae family, respectively. In maize rhizosphere samples, Venn diagram showed that all samples shared 235 bacterial families. The M_Fert treatment community was the only one that presented bacteria belonging to the Brevibacterium family (Fig. 3).
Venn diagram showing relationships between different communities associated to peanut (A) and maize (B) plants from different treatments at family level. ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB:seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize
When the analysis of the rhizospheric bacterial communities of peanut and maize plants were analyzed at genus level, the results indicated that samples of all treatments of both plants presented many genera associated with beneficial mechanisms of plant growth promotion (Fig. 4). The most abundant genera detected in all rhizobacterial communities of both plants were Rhodoplanes and Kaistobacter. The results indicate that the P_IJ260 treatment showed a slight increase in the genera Bacillus, Rhizobium, and Bradyrhizobium, with respect to ES. On the other hand, the members of this genus presented an increase in all samples associated with peanuts samples. On the other hand, the results indicated an increase in the abundance of the Pseudomonas genus in maize rhizospheric samples, mainly in the M_IJ49 treatment, and a decrease in samples associated with the M_Fert treatment.
Hierarchically clustered heatmap of bacterial distribution of different communities associated to peanut and maize plants from different treatments at the genera level. Row represents different soil samples and column indicates the abundance of each bacterial group. The relative abundance for each bacterial genus was depicted by color intensity with the legend indicated at the top of the figure.ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB: seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize
3.2 Culturable rhizobacteria associated to peanut and maize plants are influenced by bacterial inoculation and fertilizer management
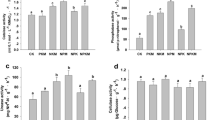
In general, the analysis of the abundance of culturable CHB, DB and PSB indicated an increase in the two former groups with the inoculation of native strain Enterobacter sp. J49 and the fertilization treatment in peanut plants. In particular, the results obtained from peanut microcosm assays indicated that the abundance of culturable heterotrophic bacteria (CHB) in rhizosphere presented a significant increase with the treatments P_IJ49 and P_Fert, with respect to the number detected in ES (Fig. S4A-supplementary material). These same treatments and P_C showed to produce a significant increase in the number of diazotrophic bacteria (DB) (Fig. S4B-supplementary material). All treatments significantly enhanced the abundance of phosphate solubilizing bacteria (PSB), respect to ES (Fig. S4C-supplementary material).
The analysis of the maize rhizospheric communities, indicated that the abundance of CHB and PSB showed a significant increase in all treatments, respect to ES (Fig. S4D and F-supplementary material). With exception of the control, M_C, the abundance of DB significantly enhanced in all treatments compared to ES (Fig. S4E-supplementary material).
3.3 Bacterial inoculation produced the most significative increases on growth, chlorophyll, P and N content of peanut plants
Growth parameters of peanut plants inoculated with either native strains or commercial strains increased at least one of the plant growth parameters analyzed with respect to uninoculated plants (Fig. S5-supplementary material). In general, the results indicated that plants inoculated with the native PSB Enterobacter sp. J49 showed the best grow parameters chlorophyll and N and P content. The aerial and root dry weight of plants inoculated with the native PSB Enterobacter sp. J49 showed a significant increase respect to control plants (Fig. S5C and D-supplementary material). Also, the inoculation with Enterobacter sp. J49 and Serratia sp. J260 and peanut reference strain SEMIA 6144 significantly increased chlorophyll content of peanut plants with respect to uninoculated plants, while fertilized treatment showed no differences with the control treatment (Fig. S5E-supplementary material). It was possible to observe that number of peanut boxes was enhanced in plants inoculated with native strains Enterobacter sp. J49 and Serratia sp. J260 and with the treatment P_Fert (Fig. 5F-supplementary material) respect to control plants. Aerial P content values of plants indicated that the treatments P_IJ49 and P_Fert significantly increased this nutrient respect to P_C (Table 2). Soil P content increased with the treatment P_Fert and N soil content was significantly enhanced in peanut plants inoculated with the native strain Serratia sp. J260 respect to the soil of uninoculated plants. The Principal Component Analysis (PCA) of the 7 variables evaluated on peanut plants indicated that PC1 explained 45.8% of total variance of the assay and PC2 explained 28.4% (Fig. 5A). PC1 showed that the bacterial inoculation with strains Enterobacter sp. J49 (treatment P_IJ49), Serratia sp. J260 (treatment P_IJ260) and Bradyrhizobium sp. SEMIA 6144 (treatment P_IB) clustered together and were separated from treatment P_FERT and control plants (P_C). The treatment P_IB was the most associated with the variables nodule number and root length. Also, the treatment P_IJ49 showed association with the variables pod number and root and aerial dry weight.
Biplotdisplay of PCA of the parameters analyzed with the different treatments in peanut plants (A) and in maize plants (B). Variables analyzed in peanut plants: aerial length, aerial dry weight, root length, root dry weight, chlorophyll content, number of nodules and number of pods. Variables analyzed in maize plants: aerial length, aerial dry weight, root length, root dry weight and chlorophyll content. The sense and size of the vectors indicate the way and weight of each variable respectively to separate the different treatments studied. ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB: seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize
3.4 Fertilizer management produced the most significative increases on growth, chlorophyll, P and N content on maize plants
In general, maize plant treated with chemical fertilizer (treatment M_Fert) showed to significantly increase growth and nutrient content. Results obtained from these maize assays indicated that treatments M_IJ49, M_IP.A and M_Fert significantly increased plant root length, with respect to uninoculated plants (Fig. S6B-supplementary material). Also, the results obtained indicated a significant increase in aerial and root dry weight and chlorophyll content at the end of microcosm assays in fertilized maize plants, respect to M_C (Fig. S6C, D, E-supplementary material). Aerial N content of maize plants indicated that the treatments M_IJ49, M_IJ260 and M_Fert significantly increased this nutrient respect to M_C (Table 2). An increase of P content was observed in pots of maize plants with the treatment M_Fert and N content was higher in maize plants inoculated with the native strain Serratia sp. J260 respect to uninoculated plants. PCA of the maize plant assays indicated that PC1 explained 74.3% and PC2 the 16.5% of the variance. In general, the treatment M_Fert was associated with the variables aerial and root dry weight and chlorophyll content (Fig. 5B).
4 Discussion
The application of PGPB as a bio-inoculant allows replacing the use of synthesis fertilizers, being this an environmentally sustainable alternative (Khan et al. 2021). Regardless of the agricultural practice employed, it is necessary to analyze the effect of the selected strategy on the structure and functionality of soil microbiota. In this study, we aimed to conduct a comparative analysis between the effect of bacterial inoculation and chemical fertilizer on the composition of the rhizospheric bacterial community associated to peanut and maize plants. We also aimed to compare the effect of the inoculation of native bacteria with respect to commercial bacterial strains, commonly used for these crops. Although there are many studies that analyze the effect of fertilizer application on bacterial community composition of several plants (Gu et al. 2017; Zhou et al. 2017; Ma et al. 2018; Wang et al. 2019), there are scarce reports regarding peanut and maize rhizospheric bacterial communities (Peiffer et al. 2013; Liu et al. 2015). The results obtained in this study show that the analysis of the structure of rhizobacterial communities associated to peanut and maize plants exhibits the characteristic distribution pattern described for agricultural soils (Gu et al. 2017; Anzuay et al. 2021), with the presence of Proteobacteria, Acidobacteria, Actinobacteria, Verrucomicrobia, Bacteroidetes, Chloroflexi, Planctomycetes, Gemmatimonadetes, and Firmicutes as the more abundant phyla (Schulz et al. 2013; Hong et al. 2015). The changes observed in the rhizobacterial community of the samples of peanut plants were similar to those observed in the control peanuts sample, indicating that they are more attributed to the phenological stage of plants rather that the different treatments. On the other hand, in the communities associated with maize, a differential effect associated with the different treatments was observed with respect to ES. Particularly, a notable change in the bacterial structure associated with the chemical fertilization treatment was observed, suggesting that this treatment causes more significant disturbances in the structure of the rhizosphere community associated with maize. Similarly, Li et al. (2017) found differential modulation of the soil microbial community under inorganic fertilization management or under combined organic–inorganic fertilization.
Regarding the application of fertilizers, in the present study it was possible to detect that this treatment produced notable effects on the bacterial communities associated to maize and peanut plants at the family level. In particular, it was interesting to note that this treatment increased the growth of the maize plants while showing lower values of richness and diversity compared to the rest of the treatments. In this sense, Celestina et al. (2019) reported that differences in microbial community structure, reduced microbial community richness, and soil diversity should be associated with the direct or indirect effect of fertilization. Additionally, in the rhizosphere of maize plants inoculated with commercial strains (M_IP.A), differential structural changes were found. These changes were also previously described by Saadouli et al. (2021), who reported that inoculation with P. agglomerans showed modifications in the overall edaphic bacterial community, significantly impacting its structure and composition. Other studies showed that these effects depend on the strain used, for example Trabelsi et al. (2011) analyzed the effect of field inoculation of rhizobia strains on the soil bacterial structure associated to common bean plants and found that this treatment improved the populations of alpha- and gamma-proteobacteria, along with Firmicutes and Actinobacteria. Conversely, Herschkovitz et al. (2005) observed that the inoculation of Azospirillum strains did not show outstanding effects on the population structure of rhizobacterial communities of maize.
The most abundant genera detected in all rhizospheric soil samples belong to the Proteobacteria phylum and within them, those associated with plant growth promoting mechanisms were detected. It was interesting to note that the abundance of bacterial species belonging to the genera Bacillus and Pseudomonas, which contain species with several and well characterized PGP properties (Gutierrez-Manero et al. 2001; Richardson et al. 2009; Abbasdokht and Gholami 2010; Zachow et al.2017), showed to be affected with some of the analyzed treatments. Thus, an increase in the abundance of Bacillus was observed in the rhizospheric samples of both plants when they were inoculated with the native strain Serratia sp. J260. On the other hand, a decrease in the abundance of Pseudomonas was observed in the communities of the M_Fert treatment.
The effect of these agricultural practices was also analyzed by culturable approaches from which it was possible to detect and increase of CHB and DB cells in peanut rhizospheric samples inoculated with the native strain Enterobacter sp. J49 and chemical fertilized. On the other hand, in the maize samples, the treatments did not show a significant difference in abundance compared to control samples in none of the populations analyzed. An increase in the abundance of PSB associated with the rhizosphere of both peanut and maize was detected in all treatments and in control samples. This could be attributed to a selection by the plant of that group of microorganisms associated with phosphorus found at low levels in the agricultural soils employed in this study. Alternatively, it could be suggested that PSB bacteria would be a must robust population or either a more diverse group which could change its structure with treatments applied but maintain the pool of bacterial cells.
Peanut plants inoculated with the native phosphate solubilizing Enterobacter sp. J49 strain presented the highest diversity values compared to the rest of the rhizospheric bacterial communities analyzed. This strain is also a free-living nitrogen fixer and consequently the inoculation of Enterobacter sp. J49 could influence the abundance of certain microbial populations; either because they increase the availability of nutrients or because they indirectly benefit their growth. The competitiveness of a phosphate-solubilizing microorganism in natural environments will depend on its ability to survive and multiply in the soil. However, understanding the dynamics of PSB in soils is the most limiting factor and it is difficult to predict the behavior and efficacy of this inoculation in a particular location (Gyaneshwar et al. 2002).
An increase in the abundances of CHB, DB, and PSB was detected in the rhizospheric communities of peanut and maize, with respect to ES, in the fertilized treatments. Similarly, Mandic et al. (2011) reported that the fertilizers had a significant effect on the soil microbial characteristics, noting that the treatments with the Klebsiella planticola SL09-based biofertilizer and nitrogen fertilizers induced the greatest increase in the total microbial count. These authors suggested that rhizospheric microorganisms could use the fertilizer components as a source of macro and micronutrients, thus increasing the growth of certain microbial populations.
The two native phosphate solubilizing bacteria employed in this study (Enterobacter sp. J49 and Serratia sp. J260) present N2 fixing ability and production of indole acetic acid (IAA). Also, their inoculation in peanut and maize plants have shown positive results in promoting the plant growth parameters and P content in previous microcosm assays (Taurian et al. 2010, 2013; Anzuay et al. 2015, 2017). Field assays in the peanut growing region of Córdoba Province (Argentina) of peanut and maize plants inoculated with Enterobacter sp. J49 showed a growth promoting effect on the yield of these crops (Anzuay et al. 2023). In this study, peanut plants inoculated with native strains of Enterobacter sp. J49 increased a large number of peanut plant growth parameters evaluated. The inoculation of peanut with BSP has shown encouraging results in several studies (Mudalagiriyappa et al. 1997; Dey et al. 2004; Taurian et al. 2010; Anzuay et al. 2015, 2017). Anzuay et al. (2023) reported a growth promoting effect on peanut crop yield, mainly under drought stress, in peanut plants inoculated with BSP Enterobacter sp. J49. In line with this, Jiang et al. (2018) analyzed inoculations with BSPs (Bacillus megaterium, Enterobacter sp., Providencia rettgeri and Ensifer adhaeren) under saline stress conditions and observed growth promotion in peanut plants.
In this study, plants inoculated with Enterobacter sp. J49 presented a better performance than the commercial nitrogen-fixing strain Bradyrhizobium sp. SEMIA 6144. The efficiency of phosphate solubilization can be expressed by the accumulation of P in plant tissues. In this sense, the increases in P in plant tissues could be due to the efficiency in the solubilization of phosphates or to an increase in the P content in the soil. In line with this, other authors reported growth promotion in maize and peanut plants inoculated with native BSP in field and microcosm assays (Viruel 2014; Pradhan et al. 2017). On the other hand, although peanut plants fertilized (P_Fert) also showed a significant increase in aerial P content and plant growth, these parameters were not statistically significant. This could be attributed to the fact that peanut plants have been described as not responding to the direct application of fertilizers (Pedelini and Monetti 2021).
Regarding maize plants, Anzuay et al. (2017) and Ludueña et al. (2017) reported that inoculation with BSP in microcosm assays produced increases in plant growth parameters. However, the results of this paper indicate that chemical fertilization presented the best results to promote maize growth.
The results in peanut plants indicate that inoculation with native bacteria significantly promotes plant growth, producing minimal disturbances in soil communities.These results encourage the use of this practice as an alternative to the application of chemical fertilizers. In addition to this, although chemical fertilizers increase the growth parameters of maize plants, they notably alter the structure of soil microbial communities, since diversity and specific richness decreased with this treatment.
5 Conclusion
From the results obtained it is possible to conclude that rhizobacterial community structure is highly dynamic and influenced by different factors such as type of plant, the fertilizer input and bio-inoculant applied. In particular, chemical fertilizer application is a practice that exert a more significative impact on bacterial community associated to peanut and maize plants and thus its replacement with biological inoculants based on PGPB would be a better ecological strategy.
Data availability
Data will be made available on request.
References
Abbasdokht H, Gholami A (2010) The Effect of Seed Inoculation (Pseudomonas putida+Bacilluslentus) and Different Levels of Fertilizers on Yield and Yield Components of Wheat (Triticumaestivum L.) Cultivars. World Acad Sci Eng Technol Int J Agric Biosyst Eng 4:8
Agrovoz (2015) Los suelos pampeanos pierden fertilidad. http://agrovoz.lavoz.com.ar/agricultura/los-suelos-pampeanos-pierden-fertilidad
Anzuay MS, Frola O, Angelini JG, Ludueña LM, Fabra A, Taurian T (2013) Genetic diversity of phosphate-solubilizing peanut (Arachis hypogaea L.) associated bacteria and mechanisms involved in this ability. Symbiosis 60:143–154. https://doi.org/10.1007/s13199-013-0250-2
Anzuay MS, Ludueña LM, Angelini JG, Fabra A, Taurian T (2015) Beneficial effects of native phosphate solubilizing bacteria on peanut (Arachis hypogaea L.) growth and phosphorus acquisition. Symbiosis 66:89–97. https://doi.org/10.1007/s13199-015-0337-z
Anzuay MS, Ruíz Ciancio MG, Ludueña LM, Angelini JG, Barros G, Pastor N, Taurian T (2017) Growth promotion of peanut (Arachis hypogaea L.) and maize (Zea mays L.) plants by single and mixed cultures of efficient phosphate solubilizing bacteria that are tolerant to abiotic stress and pesticides. Microbiol Res 199:98–109. https://doi.org/10.1016/j.micres.2017.03.006
Anzuay MS, Pin Viso N, Ludueña LM, Morla FD, Angelini JG, Taurian T (2021) Plant beneficial rhizobacteria community structure changes through developmental stages of peanut and maize. Rhizosphere 19:100407. https://doi.org/10.1016/j.rhisph.2021.100407
Anzuay MS, Prenollio A, Ludueña LM, Morla FD, Cerliani C, Lucero C, Angelini JG, Taurian T (2023) Enterobacter sp. J49: A Native Plant Growth‑Promoting Bacteria as Alternative to the Application of Chemical Fertilizers on Peanut and Maize Crops. Curr Microbiol. 80:85. https://doi.org/10.1007/s00284-023-03181-8
Balzarini MG, Gonzalez L, Tablada M, Casanoves F, Di Rienzo JA, Robledo CW (2008) Manual del Usuario, Editorial Brujas, Córdoba, Argentina.
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. Gen Microbiol 84:188–198
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. https://doi.org/10.1038/nmeth.2276
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2010) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108:4516–4522. https://doi.org/10.1073/pnas.1000080107
Cassol D, Silva FSP, Falqueto AR, Bacarin MA (2008) An evaluation of nondestructive methods to estimate total chlorophyll content. Photosynthetica 46:634–636. https://doi.org/10.1007/s11099-008-0109-6
Chao A (1984) Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Celestina C, Wood JL, Manson JB, Wang X, Sale PWG, Tang C, Franks AE (2019) Microbial communities in top- and subsoil of repacked soil columns respond differently to amendments but their diversity is negatively correlated with plant productivity. Sci Rep 9:8890. https://doi.org/10.1038/s41598-019-45368-9
Dey R, Pal KK, Bhatt DM, Chauhan SM (2004) Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth promoting rhizobacteria. Microbiol Res 159:371–394. https://doi.org/10.1016/j.micres.2004.08.004
Döebereiner J (1995) Isolation and identification of aerobic nitrogen fixing bacteria from soil and plants. In: Alef K (ed) Methods in applied soil microbiology and biochemistry. Nannipieri P AcademicPress, London, pp 134–141
Fertilizar (2020) Evolución del Consumo de Fertilizantes en Argentina. https://fertilizar.org.ar/wp-content/uploads/2020/10/Evolucio%CC%81n-del-consumo-de-FERTILIZANTES.pdf
Food and Agriculture Organization of the United Nations (2018) http://www.fao.org/faostat/en/#data/QC/visualize
Food and Agriculture Organization of the United Nations (2022) https://www.fao.org/statistics/en/
Gu Y, Wang Y, Lu S, Xiang Q, Yu X, Zhao K, Zou L, Chen Q, Tu S, Zhang X (2017) Long-term fertilization structures bacterial and archaeal communities along soil depth gradient in a Paddy soil. Front Microbiol 8:1516. https://doi.org/10.3389/fmicb.2017.01516
Gudelj V, Vallone P, Galarza C, Anselmi H, Donadío H, Conde B (2016) Fertilización en maíz. Resultados de experimentos de fertilización con fósforo y zinc en el ciclo 2015–16. Actualización. Información Para Extensión INTA Marcos Juárez. https://inta.gob.ar/sites/default/files/inta_mj_actualizacion_maiz2016.pdf
Gutierrez-Manero FJ, Ramos-Solano B, Probanza A, Mehouachi J, Tadeo FR, Talon M (2001) The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high aoumts of physiological active gibberellins. Physiol Plantarum 111:206–211. https://doi.org/10.1034/j.1399-3054.2001.1110211.x
Gyaneshwar P, Naresh Kumar G, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93. https://doi.org/10.1023/A:1020663916259
Herschkovitz, Y, Lerner A, Davidov Y, Okon Y, Jurkevitch E (2005)Azospirillum brasilense does not affect population structure of specific rhizobacterial communities of inoculated maize (Zea mays). Environ Microbiol 7:1847–52. https://doi.org/10.1111/j.1462-2920.2005.00926.x
Hong Y, Liao D, Hu A, Wang H, Chen J, Khan S, Su J, Li H (2015) Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartinaalterniflora and native mangrove using Illumina amplicon sequencing. Can J Microbiol 61:723–33. https://doi.org/10.1139/cjm-2015-0079
Jackson ML (1973) Soil Chemical Analysis. Prentic Hall (India) Pvt. Ltd., New Delhi.
Jiang H, Qi P, Wang T, Chi X, Wang M, Chen M, Chen N, Pan L (2018) Role of halotolerant phosphate-solubilising bacteria on growth promotion of peanut (Arachis hypogaea) under saline soil. Ann Appl Biol 174:20–30. https://doi.org/10.1111/aab.12473
Jones DL, Cross P, Withers PJA, DeLuca TH, Robinson DA, Quilliam RS, Harris IM, Chadwick DR, Edwards-Jones G (2013) Nutrient-stripping: The global disparity between food security and soil nutrient stocks. J Appl Ecol 50:851–862. https://doi.org/10.1111/1365-2664.12089
Khan MA, Sahile AA, Jan R, Asaf S, Hamayun M, Imran MLIJ (2021) Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Bio 21:1–15. https://doi.org/10.1186/s12870-021-02937-3
Li F, Chen L, Zhang J, Yin J, Huang S (2017) Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front Microbiol 8:187. https://www.frontiersin.org/article/https://doi.org/10.3389/fmicb.2017.00187
Liu W, Wang Q, Wang B, Wang X, Franks AE, Teng Y, Li Z, Luo Y (2015) Changes in the abundance and structure of bacterial communities under long-term fertilization treatments in a peanut monocropping system. Plant Soil 395:415–427. https://doi.org/10.1007/s11104-015-2569-3
Ludueña LM, Anzuay MS, Angelini JG, Barros G, Luna MF, Monge MP, Fabra A, Taurian T (2017) Role of bacterial pyrroloquinolinequinone in phosphate solubilizing ability and in plant growth promotion on strain Serratia sp. S119. Symbiosis 72:31–43. https://doi.org/10.1007/s13199-016-0434-7
Ma M, Zhou J, Ongena M, Liu W, Wei D, Zhao B, Guan D, Jiang X, Li J (2018) Effect of longterm fertilization strategies on bacterial community composition in a 35-year field experiment of Chinese Mollisols. AMB Express 8:20. https://doi.org/10.1186/s13568-018-0549-8
Magoč T, Salzberg SL (2011) FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Margelef D (1958) Information theory in ecology. Gen Syst 3:36–71
Mandic L, Djukić D, Beatovic I, Jovovic Z, Pesakovic M, Stevovic V (2011) Effect of different fertilizers on the microbial activity and productivity of soil under potato cultivation. Afr J Biotechnol 10:6954–6960. https://doi.org/10.5897/AJB11.947
McBratney A, Field DJ, Koch A (2014) The dimensions of soil security. Geoderma 213:203–213. https://doi.org/10.1016/j.geoderma.2013.08.013
Mehta S, Nautiyal ChSh (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56. https://doi.org/10.1007/s002840010259
Miller JH (1972) In: Experiments in molecular genetics. Cold Spring Harbor Laboratory. Cold Spring Harbory, NY, p. 433
Mudalagiriyappa S, Agasimani CS, Veeranna HK, Nanjappa HV (1997) Growth analysis and pattern of dry matter accumulation in groundnut (Arachis hypogaea) as influenced by phosphate solubilizers. Crop Res 13:541–546
National Institute of Agricultural Technology (2021) https://intainforma.inta.gob.ar/presentan-dos-nuevas-variedades-de-mani/
Nelson DW, Sommers LE (1973) Determination of total nitrogen in plant material. Agron J 65:109–112
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J MicrobiolBiotechnol 33:197. https://doi.org/10.1007/s11274-017-2364-9
Pedelini R, Monetti M (2021) Maní, guía práctica para su cultivo. INTA-FMA; Manfredi (Cba.) - Argentina. http://www.ciacabrera.com.ar/docs/Guia/20Mani/202018.pdf
Peiffer JA, Spor A, Koren O, Jinb Z, Tringed SG, Dangle JL, Bucklera ES, Ley RE (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. PNAS 110:16. https://doi.org/10.1073/pnas.1302837110
Pereira P, Ibáñez F, Rosenblueth M, Etcheverry M, Martínez-Romero E (2011) Analysis of the Bacterial Diversity Associated with the Roots of Maize (Zea mays L.) through Culture-Dependent and Culture-Independent Methods. ISRN Ecol https://doi.org/10.5402/2011/938546
Pradhan M, KumarSahoo R, Pradhan CH, Tuteja N, Mohanty S (2017) Contribution of native phosphorous-solubilizing bacteria of acid soils on phosphorous acquisition in peanut (Arachis hypogaea L.). Protoplasma 254:2225–2236. https://doi.org/10.1007/s00709-017-1112-1
Pretty J, Bharucha ZP (2014) Sustainable intensification in agricultural systems. Ann Bot 114:1571–1596. https://doi.org/10.1093/aob/mcu205
Richardson AE, Barca JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104-009-9895-2
Saadouli I, Mosbah A, Ferjani R, Stathopoulou P, Galiatsatos I, Asimakis E, Marasco R, Daffonchio D, Tsiamis G, Ouzari HI (2021) The impact of the inoculation of phosphate-solubilizing bacteria Pantoea agglomerans on phosphorus availability and bacterial community dynamics of a semi-arid soil. Microorganisms 9:1661. https://doi.org/10.3390/microorganisms9081661
Sainju UM, Ghimire R, Pradhan GP (2019) Nitrogen Fertilization I: Impact on crop, Soil, And Envoronment. Edited by Everlon Cid Rigobelo and Ademar Pereira Serra. IntechOpen. https://doi.org/10.5772/intechopen.86028
Sainz Rozas H, Echeverría HE, Angelini H (2012) Fósforo disponible en suelos agrícolas de la región Pampeana y Extra-pampeana Argentina. RIA 38:33-39. http://ria.inta.gob.ar/sites/default/files/numeros/ria-38-1-2012_0.pdf
Sainz Rozas H, Eyherabide M, Echeverría HE, Barbieri P, Angelini H, Larrea GE, Ferraris G, Barraco M (2013) ¿Cuál es el estado de la fertilidad de los suelos argentinos? Actas del Simposio Fertilidad 2013. IPNI-FERTILIZAR. https://inta.gob.ar/sites/default/files/inta.estado-fertilidad-suelos-argentinos.pdf
Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microb Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Schulz S, Brankatschk R, Dümig AD, Kőgel-Knabner IK, Schloter M, Zeyer J (2013) The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 10:3983–3996. https://doi.org/10.5194/bg-10-3983-2013
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M, Hurek T, Sarkar A, Bodrossy L, van Overbeek L, Brar D, van Elsas JD, Reinhold-Hurek B (2012) Functional characteristics of an endophyte community colonizing Rice roots as revealed by metagenomic analysis. MPMI 25:28–36. https://doi.org/10.1094/MPMI-08-11-0204
Singh DP, Singh HB, Prabha R (2016) Microbial inoculants in sustainable agricultural productivity: Vol. 1: Research Perspectives. Springer, XV, p 535. https://doi.org/10.1007/978-81-322-2647-5
Smit E, Leeflansg P, Gommans S, van den Broek J, van Mil S, Wernars K (2001) Diversity and seasonal fluctuations of the dominant members of the bacterial soil community in a wheat field as determined by cultivation and molecular methods. Appl Environ Microbiol 67:2284–2291. https://doi.org/10.1128/AEM.67.5.2284-2291.2001
Somasegaran P, Hoben H (1994) Quantifying the growth of rhizobia. In: Garber R (ed.) Handbook for Rhizobia: Methods in Legume Rhizobia Technology. Springer, New York, pp. 382–390, Section 3
Taurian T, Aguilar OM, Fabra A (2002) Characterization of nodulating peanut rhizobia isolated from a native soil population in Córdoba, Argentina. Symbiosis 33:59–72
Taurian T, Anzuay MS, Angelini JG, Tonelli MA, Ludueña L, Pena D, Ibañez F, Fabra A (2010) Phosphate-solubilizing peanut associated bacteria: Screening for plant growth-promoting activities. Plant Soil 329:421–431. https://doi.org/10.1007/s11104-009-0168-x
Taurian T, Anzuay MS, Ludueña LM, Angelini JG, Muñoz V, Valetti L, Fabra A (2013) Effects of single and co-inoculation with native phosphate solubilising strain Pantoea sp. J49 and the symbiotic nitrogen fixing bacterium Bradyrhizobium sp. SEMIA 6144 on peanut (Arachis hypogaea L.) growth. Symbiosis 59:77–85. https://doi.org/10.1007/s13199-012-0193-z
Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC (2017) Perspectives and challenges of microbial application for crop improvement. Front Plant Sci 8:49. https://doi.org/10.3389/fpls.2017.00049
Trabelsi D, Mengoni A, Ben Ammar H, Mhamdi R (2011) Effect of on-field inoculation of Phaseolus vulgaris with rhizobia on soil bacterial communities. FEMS Microbiol Ecol 77:211–222. https://doi.org/10.1111/j.1574-6941.2011.01102.x
Trabelsi D, Mhamdi R (2013) Microbial inoculants and their impact on soil microbial communities: A review. Biomed Res Int. https://doi.org/10.1155/2013/863240
Tringe SG, Hugenholtz P (2008) A renaissance for the pioneering 16S rRNA gene. Curr Opin Microbiol 11:442–446. https://doi.org/10.1016/j.mib.2008.09.011
Vicario JC, Primo ED, Dardanelli MS, Giordano W (2016) Promotion of Peanut growth by co-inoculation with selected strains of Bradyrhizobium and Azospirillum. J Plant Growth Regul 35:413–419. https://doi.org/10.1007/s00344-015-9547-0
Vincent JM (1970) A Manual for the Practical Study of Root Nodule Bacteria. Blackwell Scientific, Oxford
Viruel EM (2014) Inoculation of maize with phosphate solubilizing bacteria: Effect on plant growth and yield. J Soil Sci Plant Nutr 14:819–831. https://doi.org/10.4067/S0718-95162014005000065
Wang Z, Liu Y, Zhao L, Zhang W, Liu L (2019) Change of soil microbial community under long-term fertilization in a reclaimed sandy agricultural ecosystem. Peerj 6497. https://doi.org/10.7717/peerj.6497
Zachow C, Müller H, Monk J, Berg G (2017) Complete genomesequence of Pseudomonas brassicacearum strain L13–6–12, a biological control agent from the rhizosphere of potato. Stand Genomic Sci 12:6. https://doi.org/10.1186/s40793-016-0215-1
Zhou J, Jiang X, Wei D, Zhao B, Ma M, Chen S, Cao F, Shen D, Guan D, Li J (2017) Consistent effects of nitrogen fertilization on soil bacterial communities in black soils for two crop seasons in China. Sci Rep 7:3267. https://doi.org/10.1038/s41598-017-03539-6
Acknowledgements
This work used computational resources from the Bioinformatics Unit, Instituto de Biotecnología (CICVyA-INTA), part of the Consorcio Argentino de Tecnología Genómica (CATG) (PPL Genómica, MINCyT).N.P. is fellowships from CONICET, F.D.M. is professor of UNRC and M.S.A., L.M.L, J.G.A. and T.T. are members of research career of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Contributions
María Soledad Anzuay: Investigation, formal analysis, writing. Natalia Pin Viso: Investigation, formal analysis, writing. Liliana Mercedes Ludueña: Writing. Federico Daniel Morla: Formal analysis. Romina Yanet Dalmasso: Writing. Jorge Guillermo Angelini: Writing. Tania Taurian: Investigation, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conficts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13199_2023_959_MOESM1_ESM.doc
Fig. S1. Rarefaction curves of OTUs of the rhizobacterial communities associated with peanut (P) and maize (M) from different treatments clustered at 97% sequence identity. ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB : seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize (DOC 136 KB)
13199_2023_959_MOESM2_ESM.docx
Fig. S2. Phylum relative abundance (%) present in rhizobacterial communities associated with peanut (P). ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB: seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut (DOCX 165 KB)
13199_2023_959_MOESM3_ESM.docx
Fig. S3. Phylum relative abundance (%) present in rhizobacterial communities associated with maize (M). ES: experimental soil, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize (DOCX 166 KB)
13199_2023_959_MOESM4_ESM.doc
Fig. S4. Number of culturable heterotrophic bacteria (CHB), diazotrophic bacteria (DB) and phosphate solubilizing bacteria (PSB) grown in 10% TSA, NFb and NBRIP-BPB medium from rhizospheric soil samples associated to peanut (A, B and C) and maize (D, E and F) plants from different treatments. Data aremeans ± S.E.,of 8–10 replicates, according to LSD test (P <0.05). *: indicates statistically significant difference comparedwith time at the beginning of the assay.ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB : seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maízandRIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize (DOC 894 KB)
13199_2023_959_MOESM5_ESM.doc
Fig. S5. Aerial length (cm) (A), root length (cm) (B), aerial dry weight (g plant -1) (C), root dry weight (g plant -1) (D), chlorophyll content (units) (E), number of pods (F) and number of nodules (G) of peanut plants from different treatments. Data are means ± S.E. of 8-10 replicates, according to LSD-Fisher test (P< 0.05). Different letters indicate statistically significant difference between treatments.ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB : seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize (DOC 755 KB)
13199_2023_959_MOESM6_ESM.doc
Fig. S6. Aerial length (cm) (A), root length (cm) (B), aerial dry weight (g plant -1) (C), root dry weight (g plant -1) (D) and chlorophyll content (units) of maize plants from different treatments. Data are means ± S.E. of 8-10 replicates, according to LSD-Fisher test (P< 0.05). Different letters indicate statistically significant difference between treatments.ES: experimental soil, P_IJ49: seeds of peanut inoculated with Enterobacter sp. J49, P_IJ260: seeds of peanut inoculated with Serratia sp. J260, P_IB : seeds of peanut inoculated with Bradyrhizobium sp. SEMIA 6144, P_Fert: uninoculated seeds of peanut grown on fertilized soil, P_C: uninoculated and unfertilized seeds of peanut, M_IJ49: seeds of maize inoculated with Enterobacter sp. J49, M_IJ260: seeds of maize inoculated with Serratia sp. J260, M_IP.A: seeds of maize inoculated with Nodumax® Azo maíz and RIZOFOS®, M_Fert: uninoculated seeds of maize grown on fertilized soil, M_C: uninoculated and unfertilized seeds of maize (DOC 442 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anzuay, M.S., Viso, N.P., Ludueña, L.M. et al. Biological inoculants and chemical fertilizers application produce differential effects on rhizobacterial community structure associated to peanut (Arachis hypogaea L.) and maize (Zea mays L.) plants. Symbiosis 92, 75–90 (2024). https://doi.org/10.1007/s13199-023-00959-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00959-z