Abstract
In this study, the impact of formulated emulsion was studied on strawberries which were coated using dip and electrostatic spray coating methods. The coated strawberries were kept at room temperature for a period of 12 days. A significant level of chargeability of w/o emulsion was achieved in terms of charge-to-mass ratio of 2.81 mC/kg at an applied high voltage of 2.0 kV, applied air pressure of 0.3 MPa, and liquid flow rate of 33.6 ml/min. The distance of 170 mm from the nozzle tip to Faraday cage was maintained during the measurements. As compared to uncoated and dip coated strawberries, the water-in-oil based electrostatically charged sprays considerably (p < 0.05) reduced the weight loss, decay rate, pH, titrable acidity, TSS, and antioxidant activity. In both the cases, i.e. strawberries coated with dip and electrostatic spray coating methods, the same weight loss was observed, however, there was a considerably less weight loss as compared to uncoated samples. The textures of the uncoated (9.02 N) and dip coated (12.58 N) samples were significantly different from the electrostatic spray coated (15.85 N) samples. Since, the coating formulation had no impact on the sensory attributes, the samples were considered as acceptable at the end of the storage. Furthermore, compared to uncoated, water-in-oil based electrostatically charged spray coating was more effective at delaying the decay by 12 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strawberry (Fragaria ananassa) is a non-climacteric fruit having a high phytochemical content and strong antioxidant capacity associated with anti-cancer and anti-mutagenic properties (Liu et al. 2019; Meyers et al. 2003). Strawberries have many marketing challenges, like, being highly perishable, high metabolic activity, high water content and contamination by fungi, especially grey mold (Botrytis cinerea). The strawberries have acidic pH, which makes a healthy environment for fungus to grow. The shelf life of strawberries is around 4–5 days at ambient temperature (Han et al. 2004). Major strawberry varieties grown for commercial purposes are Confitura, Chandler, and Tyoga. To attain the highest quality in terms of visual appeal, texture, flavour, and nutritional content, strawberries are collected at full maturity stage. During storage, strawberries are highly perishable and prone to mechanical damage, physiological decline, water loss, and microbial degradation (Hussain et al. 2012). Fungal spoilage is the most challenging problem associated with strawberries, which affects shelf life, storage, and distribution (Tumbarski et al. 2018).
In recent years, more advanced techniques have been used in food bio preservation, such as the application of edible coatings to extend the shelf life of strawberries. Edible coatings are widely used in food products to enhance the shelf life, and their basic function is to protect from mechanical damage and physical, chemical, and microbiological activities (Falguera et al. 2011). The edible coatings reduce moisture loss, which is the main cause of fruit quality deterioration, by acting as protective barriers against dehydration (Mehta 2023). Further, edible coatings prevent respiration, enhance textural quality, aid in preserving the natural colour and volatile taste, ingredients, and slow down microbial, particularly fungal growth, in addition to reducing the loss of product mass (Corbo et al. 2015). Coating material plays a vital role in the effectiveness of edible coatings, which can be developed by using various combinations of materials. There are three types of edible coatings: hydrocolloid, lipid, and composite. Hydrocolloids include proteins, cellulose derivative, alginate, pectin, starch, and other polysaccharides. In general, lipids include fatty acids, waxes, and acyl glycerol. Composites can be a conglomerate, a film with lipid and hydrocolloid interstices, or a bilayer produced by both lipid and hydrocolloids. The most commonly used structural matrices are polysaccharides, proteins, lipids, or their derivatives. Emulsions are typically described as heterogeneous systems with two immiscible layers, each with a liquid spread within it in the form of droplets that are typically larger than 0.1 µm in diameter. The system is called water-in-oil (w/o) emulsion when water or aqueous solutions are disseminated in a continuous oil medium (Sarathchandraprakash et al. 2013).
The most popular coating methods are spraying, brushing, pouring, fluidized bed processing, and dip coating. The cost-effectiveness, minimal coating material waste, the avoidance of microbiological contamination etc. are considered while choosing a coating process (Jiang et al. 2019). Currently, electrostatic spraying is one of the most efficient and effective methods that is widely used in disinfection, food safety and nutrition, locust and pest control, and thin film deposition for bio-sensing (Chauhan et al. 2023; Khan et al. 2017; Patel et al. 2017; Ghanshyam et al. 2013). There are several advantages of electrostatic spray coating which makes it an attractive method for applying antimicrobials to perishable food commodities. More adhesion and less waste are produced as a result of the electrostatically charged particles being drawn to the target surface and minimizing overspray. In case of electrostatic spray coating, the charge present in the droplets which inhibits all kinds of microorganisms, hinders the further growth of pathogenic activity (Khan et al. 2014). Electrostatic spray coating produces a homogeneous sized charged droplets of coating material which provides a way to control the thickness and uniformity of coating. This method reduces the processing costs, reduces material waste, recycles & reuses the material, improves coating material application efficiency and quality (Patel, et al. 2022). In the present study, an edible coating was developed with tween-80, glycerol, rice bran oil and water with the addition of Eugenol oil. The edible coating developed with Eugenol oil has antifungal and antioxidant effects. Advanced electrostatic spray coating and traditional dip coating methods were used to coat the strawberries with innovative formulations, which were studied over a period of 12 days at room temperature. During the storage period, the physiochemical changes of coated strawberries have been studied in comparison to uncoated strawberries.
Material and methods
Materials
Rice bran oil was purchased from a local market in Chandigarh, India. Rice bran oil belongs to the Ricela brand and is manufactured at the A.P. Organics Ltd. in Dhuri, Punjab. Glycerol was purchased from S.D. Fine Chemical Ltd., Mumbai, India. Eugenol was purchased from Synthite Industries Pvt. Ltd., Kerala, India. Tween 80 was purchased from Sigma-Aldrich, Bangalore, India.
Preparation of w/o emulsion
The water-in-oil-based emulsions were prepared as shown in Fig. 1A by a mixer grinder with different concentrations. Emulsions were prepared using different concentrations of RBO and water (50–50, 60–40, 70–30, 80–20 and 90–10%). Plasticizer, stabilizer and Eugenol were added in the ratio (0.5:1:1) and the final volume was made up to 100 mL with the prepared water-in-oil based emulsion. Then, while continuously stirring the jar at a speed of 18,000 rpm for 2 min, rice bran oil was slowly poured into it with each emulsion. After that, all emulsions were kept at room temperature for 20 days before analysis.
Characterization of coating material
Stability of w/o emulsion
To check the stability, all the formulated emulsions were transferred into glass vials and kept for a period of 20 days at room temperature. The volume fraction of oil on the surface of the w/o emulsions was measured to determine their stability. Glass vials with a 1.5 cm diameter and a 10 cm height were used to keep 1 ml of emulsions at room temperature for 20 days. The heights of the oil layer (\({H}_{o}\)) and the overall emulsion (\({H}_{e}\)) were measured. The proportion of the oil phase’s volume from the emulsion is calculated from the Eq. (1):
Electrical conductivity of w/o emulsion
The electrical conductivity of the w/o emulsion was measured by a bench-top conductivity meter (inoLab Multi 9620IDS, WTW, Weilheim, Germany) working within the range of 10 µS/cm-2000 mS/cm and according to the ASTMD2624 standard test procedure. The analysis was carried out in triplicate.
pH of w/o emulsion
A digital pH meter (WTW inoLab Multi 9620IDS) was used to analyse the pH of the emulsions. Before analysis, pH 4, pH 7, and pH 9 buffer solutions were used to calibrate the pH meter. The average value of emulsion samples was evaluated in triplicate.
Viscosity measurement of w/o emulsion
This study evaluated the viscosity of emulsions at 25 °C using a stress-controlled Rheometer MCR302 (Anton-Paar) with a concentric cylinder (bob diameter: 16.664 mm, cup diameter: 18.080 mm). In order to eliminate any preceding stresses, 10 ml of each emulsion and rice bran oil were added separately into the cup at 25 °C for 1 min and then undergo a steady pre-shear for 2 min at a shear rate of 15 rad/s. The flow ramp test was conducted between 0.01 and 100 1/s shear rate after the emulsion was adjusted for additional one-minute homogenization at 25 °C before measurement. The analysis was carried out in parallel and in triplicates.
Surface tension and density of w/o emulsion
The surface tension of w/o emulsion was measured with an automated Surface Tensiometer (Kyowa Dy-300) at room temperature, equipped with a thin probe such as a platinum plate. The Wilhelmy method was used to measure the surface tension of w/o based emulsion using a platinum plate. This measurement was repeated thrice, and the final mean value was calculated. Additionally, the density was measured using DuNouy method with the same Surface Tensiometer using the Pycnometer.
Pre-processing of fruit
Fresh strawberries (Winter Dawn) were purchased from a local farm (Hisar, Haryana, India). Strawberries were selected based on their uniform size, colour, and absence of any physical or microbiological defects. Strawberries without defects or imperfections were picked and washed with tap water. After removing the remaining water, strawberries were immersed for 2 min in a solution of 30 ppm sodium hypochlorite and afterwards kept in a tray for drying at room temperature.
Method of coating of Strawberries
Dip coating
Approximately 5.0 kg of strawberries were selected and divided into three groups. Strawberries were immersed into the emulsion for at least 30 s. After dipping, the coated sample was kept at room temperature for 1 h to remove excess solution from the fruit surface as shown in Fig. 2A. After fully drying, strawberries were weighed, labelled, and then packed in a plastic basket and stored at room temperature for 10 days until tests were performed. The physio-chemical performance was evaluated every day for the entire coated and uncoated sample throughout the storage. 210 strawberries were divided into three groups (uncoated, dip and electrostatic) with three replicates of each treatment. Each tray with six fruits made up each replicate, which contained 18 fruits in total.
A Visual aspect of the dip and electrostatic spray coated strawberries during the drying process, and B Visual appearances of strawberries a Uncoated strawberries on 4th day, b Dip coated strawberries on 4th day, c Electrostatic spray coated strawberries on 4th day, d Uncoated strawberries on 10th day, e Dip coated strawberries on 10th day, and f Electrostatic spray coated strawberries on 12th day
Electrostatic spray coating
Emulsion coating materials were sprayed onto the surface of strawberries using an electrostatic spray coating technique as shown in Fig. 2B. The electrostatically charged edible coating material was sprayed on the strawberries for around 40 s after they were placed on a conveyer belt. The strawberry samples were dried for 1 h at room temperature. After being sprayed electrostatically, strawberries were weighed, labelled, and then placed in plastic baskets. For each treatment, 78 strawberries were divided into groups, with three replicates of each treatment. Three trays with six fruits each made up each replicate, which contained 18 fruits in total.
Experimental set-up
Emulsions of different concentrations were prepared and various characteristic parameters were measured to identify the best concentration on the basis of stability. The prepared emulsion was coated on strawberries using different coating methods. The coated strawberry samples were stored at room temperature. The coating has been performed on strawberries using an advanced electrostatic spray coating system. The coating material is passed through the high electric field, where the droplets attain the negative charge. The charged droplets of coating material travel downward and reach to the target surfaces of food commodities. The Ultravolt one-channel power supply (Brand: Ultravolt, Model: HV-RACK-1-250-00287, 20 kV, 1.5 mA, 30 W) was used for the experiments to supply the high voltage to charging electrode.
Shelf life studies
Physiological weight loss (PWL)
Using a digital balance, the weight of strawberries was measured every day until the completion of shelf-life studies. The weight loss was calculated as a gram loss in weight compared with the fruit initial weight. This measurement was performed in triplicate.
Decay percentage
Strawberries were examined every day for visual decomposition and considered as spoiled due to the presence of visual fungal growth or significant softness. The percentage of decay for each treatment was calculated by dividing the number of decaying fruits by the total number of fresh fruits and multiplying the result by 100 using an Eq. (2):
Firmness analysis of strawberry
The TA.HD plus C texture analyser (Stable Micro System, UK) was used to measure the firmness of coated and uncoated strawberries. Using a P/75 compression plate with a load cell of 50.0 kg and test speed of 1 mm/sec at the distance of 10 mm, the force was delivered to equatorial locations on the surface of strawberries. Five fruits from each treatment were used in the measurement of force (N), to find out the firmness of uncoated and coated strawberry samples.
pH
A WTW inoLab Multi 9620IDS digital pH meter was used to analyse the pH of the strawberry samples. Before analysis, buffer solutions with pH 4, 7, and 9 were used to calibrate the pH meter. Before proceeding with strawberry juice for pH analysis, it was homogenized in a blender before filtering it out through the muslin cloth. The average values were noted for coated and uncoated samples.
Total soluble sugars (TSS)
The TSS of coated and uncoated strawberries was analysed at room temperature by an Atego refractometer (Tokyo, Japan). Prior to analysing the TSS of the strawberry sample, the measuring device was calibrated with distilled water. The average values were measured in degree Brix units.
Titrable acidity (TA)
The titration method was used to determine the titrable acidity of strawberry samples. A 0.1 N NaOH solution containing phenolphthalein as an endpoint indicator was used to titrate 10 ml of strawberry juice. Citric acid (%) was calculated as TA according to the method developed as shown in Eq. (3) (Kumar et al. 2020):
Antioxidant activity
The 1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay was used to investigate antioxidant activity of coated and uncoated strawberry samples towards scavenging free radicals. A 0.09 ml of deionized water, 3.9 ml of methanolic DPPH solution (0.25 g/L), and 0.01 ml of strawberry juice were mixed in glass vials and kept at dark place for 30 min. The absorbance of the sample at 515 nm was measured using a spectrophotometer compared to a control using the initial DPPH absorbance value. The percentage of radical DPPH inhibition due to a decrease in the absorption with respect to the control value was used as estimation for antioxidant activity by using an Eq. (4):
Sensory analysis
Using a 9-point hedonic scale, the sensory characteristics of the samples were evaluated as part of a pilot-scale consumer. The samples were evaluated for colour, firmness, aroma, flavour, and overall acceptability on every day of storage. The panellists were chosen from the institute staff and students (aged 25 to 34). The hedonic scale for each fruit was rated from 0 to 9, with 0 being extremely dislike to 9 being very likeable.
Statistical analysis
The experimental data was evaluated using ANOVA (Analysis of Variance), and the mean and standard deviation were reported. Subsequently, the Tukey (Origin Pro® 2020) test was used to determine statistical significance of results.
Result and discussion
Rheological and material properties of w/o emulsion
Stability of w/o emulsion
The stability of w/o emulsions was studied at different rice bran oil concentrations. In can be seen from the Fig. 1B; that except 70% rice bran oil, all emulsions containing 90, 80, 60, and 50% v/v rice bran oil were unstable to phase separation and formed a layer of oil on top just after 1 day of storage. During the storage period of 20 days, the volume percentage of the separated oil phase was increased. On the other hand, sample 3 (S3) emulsion containing 70% of rice bran oil was more stable at room temperature compared to other samples. It is concluded that sample S3 was more stable than other samples for 20 days and led the study further as an emulsion based coating material.
Electrical conductivity of w/o emulsion
According to the study, the critical voltage is needed for stable flow manufacturing. Although, it was considered that liquids with electrical conductivities outside of 10−8–10−4 s/m could not be atomized as stable sprays (Abu-Ali and Barringer 2005). The conductivity of the material to be sprayed determines the amount of charge a liquid can acquire while passing through the high electric field created by the charging electrode of spray nozzle. The present study showed that the electrical conductivity range can be expanded because of w/o emulsion, which included 70% rice bran oil and 30% water for successful optimization with a conductivity of 14.66 µs/m. This is perhaps because water is a good conductor of electricity, but oil is not and another aspect could be that the slightly acidic solutions are suitable for good electrical conductivity.
pH of w/o emulsion
It is important to monitor the pH to determine the stability of the emulsions, and pH change indicates the onset of chemical reactions which can affect the quality of the final product. It also ensures other covalent bonding, to increase compression of edible coating layers (Kocira et al. 2021). The pH of a w/o based emulsion was observed to be 5.83, which is slightly acidic and inhibits fungal growth.
Viscosity measurement of w/o emulsion
Viscosities of rice bran oil and w/o emulsion were tested using a cylinder system at a temperature of 25 °C. The ideal viscosity behaviour of rice bran oil has been shown in Fig. 3a, the estimated viscosity was 82.41 mPa.s. The viscosity curve showed that the viscosity remains constant across the entire measuring range, even at very low shear stress. The viscosity of the w/o based emulsion was estimated to be 113.98 mPa.s, which is a desirable value of viscosity to be suitable for electrostatic spray coating. In general, stored emulsion had a shear-thinning behaviour at the checked shear rates of 0.01 to 100 1/s, i.e. the viscosity decreased gradually with increasing shear rate as shown in Fig. 3b. This process has been connected to the hydrodynamic forces produced by the shear rates, which lead to the deformation and eventual disruption of aggregates and a decrease in viscosity (Liu et al. 2007; McClements et al. 2017). The result can be attributed to the large contact surface area between the oil droplets and the phase that inhibits the emulsion free movement. The system viscosity increases when there is a high oil concentration because the oil droplets can interact with one another.
Surface tension and density of w/o emulsion
The surface tensions of w/o based emulsion and rice bran oil were estimated at 36.98 and 33.63 mN/m, respectively, as shown in Table 1. The surface tension of w/o based emulsion was higher than the rice bran oil. The result showed that the w/o emulsion was more likely to spread over the skin of the strawberries due to its cohesive force. It was stated that the liquid surface tension is less than 100 mN/m (low-energy surfaces), the contact angle from a drop of liquid to a solid surface will be a linear function of the surface tension of the liquid (Zisman 1969).
The densities of w/o based emulsion and rice bran oil were determined to be 0.9477 and 0.9070 g/cm3, respectively as shown in Table 1. The surface tension and density were found suitable for electrostatic spray coating which will contribute to higher electrical flux and chargeability of emulsion (Cakmak et al. 2018).
Shelf life studies of strawberry
Physiological weight loss reduction
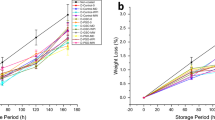
As strawberry physiological weight loss reduction was measured, both coated and uncoated strawberries exhibited an increase in storage time as shown in Fig. 4a. It can be noticed that dip-coated strawberries (21.36%) were less effective at preventing weight loss than an electrostatic spray coating (17.72%). Electrostatic spray-coated and dip-coated strawberries were significant throughout the entire experiment (p < 0.05) when compared to control strawberries (31.36%). This could be the result of the uniform coating generated by electrostatic spraying, which developed a barrier to the exchange of water vapour and controlled enzymatic activity. Furthermore, it can be observed that electrostatic spraying of w/o based emulsion coating was significantly more effective at decreasing strawberry weight loss. An electrostatic spray coating had a higher transfer efficiency and uniformity than a conventional spray coating, resulting in a decrease in water loss in electrostatically coated strawberries. In this review showed that higher weight loss for coated strawberries, up to 45% (Peretto et al. 2017). All treatments improved weight loss over the controls and varied significantly, except for emulsion-based electrostatic sprays and dip coated strawberries.
Decay percentage
The short shelf life of strawberries is caused by their significant physiological activities and hence called perishable fruit in nature. The results obtained from the shelf life study showed an increase in fruit decay in all treatments. However, the coating of strawberries significantly decreased the amount of visible mold and fungi compared to control samples (p < 0.05). Dip coated and uncoated strawberries showed spoilage (visible fungus) on the 4th day as shown in Fig. 4b. Out of the total amount of strawberries, uncoated strawberries showed 7.14% deterioration, whereas emulsion-based dip-coated strawberries showed 1.78% spoilage on the 4th day. On the 10th day, 33.25% spoilage was observed in uncoated strawberries; while emulsion based dip coated strawberries had 9.25% spoilage. Emulsion-based electrostatic treatment provided superior control over spoilage and did not show any fungal growth until the 12th day. In the case of emulsion based electrostatic coatings on strawberries, spoilage initiated on the 5th day with 1.26% spoilage and showed 8.12% spoilage till the 12th day as shown in Fig. 3B. This might be due to electrostatic-based anti-microbial spraying, which inhibits fungal growth. Furthermore, electrostatic spraying created a uniform coating on the surface of strawberries, which might have preserved tissue integrity and delayed ripening. By preventing the strawberries from being exposed to microbes and delaying fruit ripening, edible coatings lowered the rate of degradation. The deterioration of strawberries with chitosan coating (1% w/v) after 9 days was 72% lower than the control sample. The strawberries coated with CMC (1% w/v) had less degradation after 12 days of shelf life than untreated fruit. By reducing the decay of strawberries (up to 22%) when compared to untreated samples, the CMC edible coating extended the shelf life of strawberries (Dong and Wang 2017).
Firmness of strawberry
The firmness of strawberries was evaluated from 0 to 12 days, and the findings are shown in Fig. 4(c). Strawberries were chosen for their good, firm cell walls, and due to an increased ripening process, fruits lose firmness while being stored. When strawberries are stored, their firmness decreases, whether they are coated or uncoated. The difference between coating treatments was statistically significant (p < 0.05), despite the electrostatic (19.94%) and dip coated (33.43%) treatments having better firmness than the control (53.16%). The fruit softens as a result of senescence, cellular breakdown, and pectin degradation or de-polymerization. Tanada-Palmu & Grosso (2005) reported that the firmness reduction in the strawberries coated with gluten was limited to 40%, whereas it was approximately 80% in the control group (Tanada-Palmu and Grosso 2005). Edible coatings stop moisture loss and delay fruit ripening in producing a barrier layer around the fruit.
pH
In this investigation, the pH level has been increased in both the coated and uncoated strawberry samples during the storage as shown in Fig. 4d. The shelf life study of strawberries showed that the pH increased, which could have affected the fruits enzymatic activities and senescence, leading to a decrease in acid content. However, coated strawberries control the enzymatic activity compared with uncoated strawberries. Electrostatic and dipping methods are very efficient for controlling the pH. Zheng et al. (2007) reported that pH may rise while it is being stored due to the fruit’s increased respiration rate caused by the increased oxygen content (Zheng et al. 2007). Fresh strawberry pH ranges from 2.5 to 3.9 and is affected by the fruit’s variety, ripening stage, storage conditions, and microbial contamination (Gross et al. 2016). Moreover Jiang et al. (2019) showed that the control sample’s pH value increased to 4.623, however the sample treated by chitosan with 81.0% only had a value of 4.13 (Jiang et al. 2019).
Total soluble solid (TSS)
The alterations in the TSS of uncoated and coated strawberry samples throughout storage are shown in Fig. 4e. The TSS pattern is higher for preserved strawberries, regardless of the coating material or method used, as compared to the control samples (p < 0.05). The increase in TSS levels in strawberries may be caused by the breakdown and conversion of complex sugars to simple sugars during storage. The TSS parameter in strawberries is related to fruit maturity and fundamental metabolic processes that raise the fruits sugar content and sweetness over the course of storage. Moreover, w/o emulsion based electrostatic coatings have a significantly controlled increment of TSS as compared to dip coating. This may be due to the uniform coating, which has restricted the metabolic change in strawberries.
Titrable acidity (TA)
TA has been measured in milligrams of citric acid per gram of fresh weight, and it is directly correlated with the amount of organic acids present in the fruit. According to the Fig. 4f, TA decreases while strawberries are stored, independent of the coating material applied. Furthermore, after 10 to 12 days of storage period, emulsion-based dipping and electrostatic coatings revealed less reduced titrable acidity than uncoated strawberry samples. It’s possible that an electrostatically sprayed layer significantly lowered the respiration rate and delayed the use of organic acids during storage. A decrease in acidity may be expected when fruit undergoes metabolic changes or when organic acids are used in the respiratory process during storage (Gol et al. 2013). Increased respiration rate and phenolic component oxidation have been found to be the reasons for decreased acidity during storage.
Antioxidant activity
Antioxidants are free radical scavengers that can be used for an assortment of disorders. The Fig. 4g showed that how the antioxidant activity of coated and uncoated strawberries changed throughout storage. In the current study, antioxidant activity was reduced throughout the period of storage. Emulsion-based dip and electrostatic coating considerably reduced the loss of antioxidant activity in strawberries as compared to untreated strawberries throughout the storage period (p < 0.05). This shows that the metabolic changes in strawberries during storage are successfully inhibited by electrostatic and dip coating. The total antioxidant capacity of the samples was slightly lower than that of the alginate-based coated strawberries by electro spraying; however, this variation in total antioxidant capacity could be due to cultivar type, harvesting season, or maturity. According to Martinez et al. (2018), the edible coating of chitosan with thymus capitatus essential oil provided protection to strawberries by delaying the loss of their antioxidative properties and increasing their shelf life. This study found that the electrostatic coating based on strawberries had very little influence on the coating formed from alginate (Martínez et al. 2018).
Sensory evaluation
The sensory attributes of strawberries, such as colour, aroma, flavour and texture, are important indicators of quality and have a direct impact on consumers’ purchasing decisions. Therefore, strawberry sensory characteristics were evaluated over the duration of a 12 days storage period as shown in Fig. 5. The colour, aroma, texture, flavour, and overall acceptability scores of the control, dip, and electrostatic spray-coated strawberries were significantly different at intervals, despite the coating composition (p < 0.05). The standard deviation for each treatment is shown by the error bar. Overall, these sensory parameters were comparable/similar to those in the control sample. Compared to the uncoated sample, each sample obtained scores above the acceptance threshold. This may be due to the fact that the coating substance is used in such small quantities that it is unclear whether something has been coated or not. Conversely, there were significant differences obtained from the instrumental texture analysis (p < 0.05). Regardless of the dip and electrostatic spray, flavour and odour ratings eventually declined in all samples. The prior art reported the strawberries coated with alginate and chitosan-based coatings had poorer odour and taste scores after 7 days of storage, some of the samples were even worse than the control. The result findings of the present study found that strawberry coated with electrostatic spray coating was still acceptable after 12 days storage period, despite a slight decrease in sensory scores. By focusing on analysing how the coating material changes over time, as is done in the literature, the sensory study of coated strawberries depends on the first perception (Dong and Wang 2017).
Conclusion
Fresh strawberries were coated with w/o based emulsions by dip and electrostatic spray coating, and the effects of both the application methods on strawberry quality evaluation were critically examined. Fresh strawberry quality parameters were used to evaluate the effectiveness of a novel electrostatic spray coating and dip coating methods. The results were compared to uncoated strawberries to determine the effectiveness of the coating techniques. The w/o emulsion based coating material was found to have high electrical conductivity and hence suitable for the electrostatic spray coated to strawberries. The electrostatic spray coating extended the shelf life of perishable food products while minimizing losses of coating materials. Also, electrostatic spray coating material on strawberries had better surface tension, viscosity, and homogeneity than dip coating, it had no effect on the quality characteristics of the product, such as weight reduction, acidity, colour, firmness, sensory indices, and total antioxidants, during the period of storage. The electrostatic spray coating method had the same or even greater protective effect with lesser coating material, while the dip coating method was efficient in preventing strawberry weight loss. The electrostatically sprayed edible coatings have potential to increase the shelf life of perishable food products.
Data availability
The manuscript has no associated data.
Code availability
Not applicable.
References
Abu-Ali J, Barringer S (2005) Method for electrostatic atomization of emulsions in an EHD system. J Electrostat 63(5):361–369
Cakmak H, Kumcuoglu S, Tavman S (2018) Production of edible coatings with twin-nozzle electrospraying equipment and the effects on shelf-life stability of fresh-cut apple slices. J Food Process Eng 41(1):e12627
Chauhan A, Patel MK, Nayak MK, Saini SS (2023) Chargeability study of disinfectants and the optimization of design parameters of a handheld electrostatic disinfection device for small scale applications. PLoS ONE 18(6):e0286740
Corbo MR, Campaniello D, Speranza B, Bevilacqua A, Sinigaglia M (2015) Non-conventional tools to preserve and prolong the quality of minimally-processed fruits and vegetables. Coatings 5(4):931–961
Dong F, Wang X (2017) Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int J Biol Macromol 104:821–826
Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A (2011) Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol 22(6):292–303
Ghanshyam C, Bagchi S, Kapur P (2013) Optimization of spray parameters in the fabrication of SnO2 layers using electrostatic assisted deposition technique. J Electrostat 71(1):68–76. https://doi.org/10.1016/j.elstat.2012.10.001
Gol NB, Patel PR, Rao TR (2013) Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol 85:185–195
Gross KC, Wang CY, Saltveit ME (2016) The commercial storage of fruits, vegetables, and florist and nursery stocks. United States Department of Agriculture, Agricultural Research Service
Han C, Zhao Y, Leonard S, Traber M (2004) Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria× ananassa) and raspberries (Rubus ideaus). Postharvest Biol Technol 33(1):67–78
Hussain PR, Dar MA, Wani AM (2012) Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int J Food Sci Technol 47(11):2318–2324
Jiang Y et al (2019) Electrostatic spraying of chitosan coating with different deacetylation degree for strawberry preservation. Int J Biol Macromol 139:1232–1238
Khan MKI, Cakmak H, Tavman Ş, Schutyser M, Schroёn K (2014) Anti-browning and barrier properties of edible coatings prepared with electrospraying. Innov Food Sci Emerg Technol 25:9–13
Khan MKI, Nazir A, Maan AA (2017) Electrospraying: a novel technique for efficient coating of foods. Food Eng Rev 9:112–119
Kocira A, Kozłowicz K, Panasiewicz K, Staniak M, Szpunar-Krok E, Hortyńska P (2021) Polysaccharides as edible films and coatings: characteristics and influence on fruit and vegetable quality—A review. Agronomy 11(5):813
Kumar N, Neeraj P, Singla M (2020) Enhancement of storage life and quality maintenance of Litchi (Litchi Chinensis Sonn.) fruit using Chitosan:pullulan blend antimicrobial edible coating. Int J Fruit Sci 20(sup3):S1662–S1680. https://doi.org/10.1080/15538362.2020.1828224
Liu H, Xu X, Guo SD (2007) Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT-Food Sci Technol 40(6):946–954
Liu Y, Wang S, Lan W, Qin W (2019) Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int J Biol Macromol 121:1329–1336
Martínez K, Ortiz M, Albis A, Castañeda CGG, Valencia M, Tovar CG (2018) The effect of edible chitosan coatings incorporated with Thymus capitatus essential oil on the shelf-life of strawberry (Fragaria x ananassa) during cold storage. Biomolecules 8(4):155. https://doi.org/10.3390/biom8040155
McClements DJ, Bai L, Chung C (2017) Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu Rev Food Sci Technol 8:205–236
Mehta D et al (2023) Quality evaluation of tomatoes coated by an advanced electrostatic spray coating system. Int J Food Sci Technol 585(12):6351–6361
Meyers KJ, Watkins CB, Pritts MP, Liu RH (2003) Antioxidant and antiproliferative activities of strawberries. J Agric Food Chem 51(23):6887–6892
Patel MK et al (2017) An advance air-induced air-assisted electrostatic nozzle with enhanced performance. Comput Electron Agric 135:280–288. https://doi.org/10.1016/j.compag.2017.02.010
Patel MK et al (2022) Real-time measurement of droplet size and its distribution of an air-induced air-assisted electrostatic nozzle. J Electrostat 115:103665. https://doi.org/10.1016/j.elstat.2021.103665
Peretto G, Wen-Xian D, Avena-Bustillos RJ, Jose DJ, Berrios PS, McHugh TH (2017) Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol 10(1):165–174. https://doi.org/10.1007/s11947-016-1808-9
Sarathchandraprakash N, Mahendra C, Prashanth S, Manral K, Babu U, Gowda D (2013) Emulsions and emulsifiers. Asian J Exp Chem 8:30–45
Tanada-Palmu PS, Grosso CR (2005) Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharvest Biol Technol 36(2):199–208
Tumbarski Y, Lante A, Krastanov A (2018) Immobilization of bacteriocins from lactic acid bacteria and possibilities for application in food biopreservation. Open Biotechnol J 12(1):25–32
Zheng Y, Wang SY, Wang CY, Zheng W (2007) Changes in strawberry phenolics, anthocyanins, and antioxidant capacity in response to high oxygen treatments. LWT-Food Sci Technol 40(1):49–57
Zisman W (1969) Surface chemistry of plastics reinforced by strong fibers. Ind Eng Chem Prod Res Dev 8(2):98–111
Acknowledgements
The authors are thankful to Council of Scientific and Industrial Research (CSIR), Government of India and Sree Padmavathi Venkateswara Foundation (Sree PVF), Vijayawada, Andhra Pradesh, India for the financial support under project grants HCP0031 and GAP0467 respectively. The authors are also thankful to the CSIR-CSIO, Chandigarh for providing the analytical facilities and assistance to conduct the study.
Funding
The study was supported under the project grants HCP0031 and GAP0467 by the Council of Scientific and Industrial Research (CSIR), Government of India and Sree Padmavathi Venkateswara Foundation (Sree PVF), Vijayawada, Andhra Pradesh, India respectively.
Author information
Authors and Affiliations
Contributions
AS: Conceptualization, Methodology, Data curation, Formal analysis, Validation and Writing—original draft. Raj Rani: Data curation, Formal analysis, VW—original draft. DM: Methodology, Formal analysis. MKN: Resources, Investigation and Writing—review & editing. MKP: Conceptualization, Methodology, Formal analysis, Validation, Resources, Funding acquisition, Investigation and Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethical approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sohel, A., Rani, R., Mehta, D. et al. Quality evaluation of strawberries coated with water-in-oil based emulsion using an advanced electrostatic spray coating system. J Food Sci Technol 61, 1492–1502 (2024). https://doi.org/10.1007/s13197-023-05915-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05915-9