Abstract
Acute lung injury (ALI), is a severe inflammatory lung disease. We tested the prophylactic effect of a functional food mix comprising three anti-inflammatory plant products: turmeric, amla, and black pepper (TAB) against lipopolysaccharide (LPS)-induced ALI in rats. Two-month-old male Wistar rats were randomly divided into three groups: control (C), LPS (5 mg/kg), and LPS with TAB (TAB). After 6 h of LPS injection, the rats were sacrificed by cervical decapitation to collect the lung tissue. Results showed that TAB partially ameliorated LPS-induced increase in circulating inflammatory cytokines (TNFα and IL6) and significantly prevented lung histopathological changes. TAB also suppressed LPS-activated ER stress markers (GRP78, pIRE1, and CHOP) and apoptotic markers (caspase-3 and − 12) in the lung. The anti-inflammatory effects of the TAB support its potential use as an adjuvant to mitigate ALI. Importantly, TAB’s ingredients have been used for centuries as part of the diet with limited or no toxic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lung injury (ALI), and its severe condition, acute respiratory distress syndrome (ARDS) are serious inflammatory lung diseases affecting around 1 million people annually resulting in high morbidity and mortality (Rubenfeld and Herridge 2007; Yuan et al. 2018). The most common cause of ALI is sepsis, due to microbial infection including current SARS-CoV-2 (Leist et al. 2020; Habashi et al. 2021). Increased vascular permeability, leukocyte recruitment, and overproduction of cytokines causing alveolar and interstitial pulmonary edema, alveolar collapse, and hypoxemia are the characteristics of ALI (Tomashefski 2000). Despite much research, no effective therapies are available for ALI / ARDS in clinical practice. As of now, corticosteroid therapy in ALI is beneficial only in the early phase of lung inflammation but with several adverse effects like hyperglycaemia, hypokalaemia, dyslipidaemia, hypertension, peptic ulcers, immunosuppression, neuropsychiatric disturbances, osteoporosis, myopathy, etc. (Schacke et al. 2002). Scientific evidence indicates the role of an overactive inflammatory response in the pathogenesis of early steps of ALI / ARDS. The host’s uncontrolled inflammatory response, including leukocyte recruitment and cytokine storm, could impair the host pulmonary epithelial or endothelial layer (Karbian et al. 2020; Liu et al. 2020). Hence, a strong anti-inflammatory agent or formulation could alleviate this pathology.
Turmeric (Curcuma longa), Amla (Emblica Officinalis Gaertn.), and Black pepper (Piper nigrum L.) are popular culinary plants and are also used for several centuries in the Indian Ayurvedic medicine system for curing numerous inflammatory disorders. Turmeric is a food component used as a spice in many parts of the world. Curcumin, responsible for the vibrant yellow color is the major active principle of turmeric has been attributed to numerous pharmacological activities of which the best-explored is its anti-inflammatory effect (Jurenka 2009). Curcumin was shown to modulate the inflammatory response by suppressing the production of tumor necrosis factor-alpha (TNF-α), IL-1, − 2, − 6, − 8, and − 12 (Abe et al. 1999). Black pepper has been reported to have anti-inflammatory potential is commonly used in food preparation and traditional medicine in several countries (Tasleem et al. 2014; Pei et al. 2020). Further, black pepper was shown to increase curcumin’s bioavailability by 20 folds (Shoba et al. 1998; Patil et al. 2016). Studies have also shown that amla extract has strong anti-inflammatory effects and inhibits microbial infection-induced expression of the neutrophil chemokines interleukin (IL)-8, GRO-alpha, GRO-gamma, and pro-inflammatory cytokine, IL-6 in the bronchial epithelial cells (Nicolis et al. 2008). In addition to the immunomodulatory effect of amla (Singh et al. 2013), the synergistic effects of turmeric, amla, and black pepper are well reported in the scientific literature (Rawal et al. 2014; Pitchaiah et al. 2017; Abdul Manap et al. 2019; Chaitanya et al. 2020). Furthermore, our earlier studies suggested the prophylactic efficacy of these three components individually against diabetic complications (Suryanarayana et al. 2004; Muthenna et al. 2009; Puppala et al. 2012; Rao et al. 2012) which motivated us to investigate the combined effect of turmeric, amla, and black pepper (TAB) against sepsis-induced ALI using a rat model.
Materials and methods
Chemicals and reagents
Lipopolysaccharides from Escherichia coli O55:B5 (LPS), horseradish peroxidase (HRP) conjugated anti-rabbit, and anti-mouse antibodies, immunoblot chemicals, and protease inhibitor cocktail were purchased from Sigma Chemicals (MO, USA). Primary antibodies for NF-kB, pNF-kB, PI3K, AKT, pAKT, ATG5, LC3, BAX, caspase 3, and caspase 12 were purchased from Cell Signaling Technology, (Massachusetts, USA). TNFa, pERK1, pJNK1, pPI3K, GRP78, pIRE1, CHOP, Beclin1, P62, Bcl2, and β-actin antibodies were purchased from Thermo Scientific (Massachusetts, USA).
Animal experiment
Two-month-old Wistar rats were received from the animal facility of ICMR- National Institute of Nutrition and adapted for a week. Rats were fed with an AIN93 pellet diet and drinking water ad libitum. The animal room was maintained at standard conditions of 22 ± 2 °C temperature, 50–60% of humidity, and 12:12 h dark: light cycle. All the procedures of animal work were approved and accorded by the Institutional Animal Ethical Committee. Rats were randomly divided into the following three groups: control (C), LPS, and LPS with a functional food mixture of turmeric, amla, and black pepper (TAB). The Group-TAB was pre-treated with the TAB in the diet for a week before LPS administration, while Groups -C and -LPS received a regular chow diet.
Functional food mixture of turmeric, amla and black pepper (TAB)
Fine quality dried turmeric root/rhizome and black pepper (dried fruits) were purchased from a local market and grounded to a fine powder. Good quality fresh amla was collected in the winter season from a local market and washed under tap water, air-dried to remove water, seeds were removed, pericarp was cut into small pieces and air-dried at room temperature under shade. The dried amla pieces were grounded into a fine powder and stored in an airtight glass container. One gram of amla powder, 0.25 gram of turmeric powder, and 0.5 gram of black pepper were added to 100 grams of the chow diet. These specified individual doses were decided based on the active principle content (curcumin in turmeric, β-glucogallin in amla, and piplartine in black pepper) required for optimal efficacy, back-calculated based on our earlier studies on diabetic complications (Suryanarayana, Kumar et al. 2004, Suryanarayana, Saraswat et al. 2005, Mrudula, Suryanarayana et al. 2007, Suryanarayana, Satyanarayana et al. 2007, Saraswat, Muthenna et al. 2008, Muthenna, Suryanarayana et al. 2009, Puppala, Ponder et al. 2012, Rao, Muthenna et al. 2012).
LPS was administered on the eighth day to all rats except Group-C at a dose of 5 mg/kg body weight, dissolved in saline via intraperitoneal route. The LPS dose used in the study was based on our pilot study conducted according to the earlier reported studies (Ayaz et al. 2017; Deng et al. 2017). After 30, 60, and 120 min of LPS injection blood sample was collected from the retro-orbital plexus for analysis of inflammatory cytokines. Rats were sacrificed by cervical decapitation after 6 h of LPS injection and a part of lung tissue was fixed in formalin solution and the remaining was snap-frozen for further analysis.
Analysis of circulatory cytokines by ELISA
Plasma concentrations of cytokines IL-6, IL-10 and TNF-α were measured by the Solid Phase Sandwich ELISA method (R&D Systems, MN, USA) by following the manufacturer’s instructions. Briefly, 100 µL of plasma sample was mixed with 100 µL of the Detection Antibody, diluted in 1% BSA in PBS, pH 7.2 and incubated for 2 h in dark at room temperature. After washing with 0.05% Tween-20 in PBS, pH 7.2, added 100 µL of the working dilution of Streptavidin-HRP conjugate, and incubated for 20 min at room temperature. Following a wash, added 100 µL of Substrate Solution (1:1 mixture of H2O2 and Tetramethylbenzidine) and incubated at room temperature for 20 min. 50 µL of 2 N H2SO4 was added to each well to stop the reaction and gently tapped the plate to ensure proper mixing. Absorbance at 450 nm was measured immediately, using a microplate reader. A standard curve in the range of 62.5 to 4000 pg/mL was constructed by reducing the data generating a four-parameter logistic (4-PL) curve-fit.
Immunoblotting
Lung tissue was homogenised in 20 mM Tris lysis buffer (100 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1 µg/mL each of aprotinin, leupeptin, and pepstatin; pH7.5) and protein concentration was measured by the Lowry method. An equal amount of protein from each of the three experimental groups was subjected to SDS PAGE and the resolved bands were transferred to nitrocellulose membrane to probe with primary antibodies each at a specific dilution. Then incubated with respective secondary antibody conjugated with HRP enzyme. Finally, the bands are visualised after developing with enhanced chemiluminescence detection reagent (Bio-Rad Laboratories, California, USA) and images were captured by an Image Analyzer (G-Box iChemi XR; Syngene, Maryland, USA). Images were quantitated and analyzed using ImageJ software (NIH, Bethesda, USA).
Immunohistochemistry
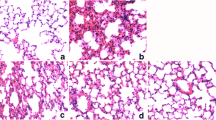
The lung tissue was perforated with and fixed in formalin solution and embedded in paraffin. The lung Sect. (4 μm thickness) were stained with hematoxylin and eosin (H&E) and were visualized in a microscope (Leica Microsystems, Germany) at 10X and 40X magnifications.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using the one-way ANOVA by GraphPad Prism 8 scientific software (GraphPad Software, California, USA). Tukey’s multiple comparisons test was used for post hoc analysis. p < 0.05 was considered as statistically significant.
Results and discussion
TAB ameliorated LPS-induced inflammatory markers
We observed no changes in the food intake and body weights among the experimental groups (Fig. 1A, B).
Pre-treatment with TAB prevented LPS-induced inflammation. A Average food intake/day. B Bodyweight of rats. C Plasma TNFα levels. D Plasma IL6 levels. E Plasma IL10 levels. F Representative immunoblots of lung tissue inflammatory markers. G Quantitative data of immunoblots. Data are presented as the mean ± SEM. *p < 0.05 when compared with the control group, #p < 0.05 when compared with the LPS group. C = control group, LPS = lipopolysaccharide injected group, TAB = functional food mixture (turmeric + amla + black pepper) pre-treated group
A profound increase in cytokines (cytokine storm) causing severe inflammation and lung damage is manifested in sepsis-induced ALI and the same is displayed in LPS injected rats. The LPS after binding to TLR4 induces the events, which converge at the activation of nuclear factor kappa light-chain-enhancer of activated B cell (NF-κB) inducing the gene expression of pro-inflammatory mediators (Fig. 7) (Akira and Takeda 2004). We observed increased phospho-NF-κB expression in the lungs of LPS injected rats while TAB pre-treatment significantly prevented LPS induced NF-κB activation (Fig. 1F, G). TNFα, an inflammatory cytokine secreted by macrophages during acute inflammation, triggers several cell-signalling events. We have observed higher circulating TNFα by 30 min of LPS injection and its levels peaked by 60 min and later diminished by 120 min but still higher than control. Pre-treatment with TAB could partially ameliorate the rise in TNFα levels as seen in Fig. 1C. Immunoblotting of TNFα at the tissue level showed higher protein levels in the lungs of LPS injected rats while TAB intervention significantly prevented this rise of TNFα levels (Fig. 1F, G). Another important inflammatory cytokine, interleukin 6 (IL6) responsible for stimulating acute phase inflammatory response was increased in circulation by 60 min and a further increase till 120 min of LPS injection. However, TAB could completely ameliorate the rise of IL6 levels at 60 min and partially at 120 min of LPS injection (Fig. 1D). Interleukin 10 (IL-10), a potent anti-inflammatory cytokine that plays a vital role in avoiding damage to the host and preserving tissue homeostasis was found increased by 30 min of LPS injection and gradually decreased but greater than control even at 120 min. TAB intervened rats showed moderate levels of circulatory IL10 throughout the observed period (Fig. 1E).
TAB prevented LPS-induced lung histopathological changes
Inflammatory infiltrates, alveolar membrane thickening and severe alveolar space destruction were observed in LPS injected group compared to the control group (Fig. 2). However, pre-treatment with the TAB significantly prevented the severity of ALI compared with the rats that received LPS alone (Fig. 2).
Representative histology of LPS-induced lung injury in rats. (Hematoxylin and eosin staining; magnification, x10 and ×40; scale bar = 100 μm for x40 and 200 μm for x10). LPS = lipopolysaccharide group; TAB = functional food mixture (turmeric + amla + black pepper) pre-treated group. Black filled arrows are indicating decreased alveolar spaces, red arrows are indicating thickened alveolar membrane, black open arrows are indicating macrophage infiltration near alveolar space
TAB ameliorated LPS-induced MAPK (ERK/JNK) and PI3K/Akt pathway activation
There are several signalling pathways associated with the inflammation, however, MAPKs and PI3K/Akt are considered as two key signalling cascades affecting the translocation of NF-κB during inflammation (Andy SN 2017) (Fig. 7).
MAPKs are well-known signalling adaptors involved in inflammatory responses. To determine the outcome of TAB pre-treatment on LPS-induced MAPKs, the levels of extracellular signal-regulated protein kinase (ERK1/2), and c-Jun N-terminal kinase (JNK) were assessed by immunoblot. We observed increased levels of pERK1/2 and JNK1 protein expression in the lungs of the LPS group that were prevented by TAB (Fig. 3).
TAB prevented LPS-induced MAPK and PI3K/Akt excessive activation. A Representative immunoblot images of MAPK and PI3K/Akt pathway proteins. B Quantification data of immunoblots. Data are presented as the mean ± SEM. *p < 0.05 when compared with the control group, #p < 0.05 when compared with the LPS group. C = control group, LPS = lipopolysaccharide injected group, TAB = functional food mixture (turmeric + amla + black pepper) pre-treated group
Phosphoinositide 3-kinases (PI3Ks) regulate inflammatory response by modulating the activation and spreading of neutrophils and macrophages (Hawkins and Stephens 2015). Hence, we next investigated the status of PI3K and Akt protein expression. We observed increased phosphorylation of PI3K and Akt proteins in the LPS group that were prevented by TAB (Fig. 3).
.
TAB ameliorated LPS-induced ER stress
ER stress response plays a vital role in many infectious conditions including LPS induced sepsis (Zeng et al. 2017) and hence, we next investigated the status of ER stress markers in the rat lung. As shown in Fig. 4, we observed increased protein expression of ER stress markers like GRP78, pIRE1 and CHOP in the LPS group. However, TAB pre-treatment prevented LPS-induced ER stress.
TAB prevented LPS-induced ER stress. A Representative immunoblot images of ER stress markers. B Quantification data of immunoblots. Data are presented as the mean ± SEM. *p < 0.05 when compared with the control group, #p < 0.05 when compared with the LPS group. C = control group, LPS = lipopolysaccharide injected group, TAB = functional food mixture (turmeric + amla + black pepper) pre-treated group
ER stress is a protective response not only to restore protein homeostasis but also to restore cellular homeostasis by modulating several cellular signalling pathways by activating the unfolded protein response. ER stress recruits TRAF2 to the ER membrane to initiate inflammatory responses via the NF-κB pathway and is a major contributor to several inflammatory diseases (Keestra-Gounder et al. 2016; Li et al. 2020a). The ER stress inhibitor 4-PBA was shown to prevent LPS induced inflammation through modulating ER stress and autophagy in ALI models (Zeng et al. 2017; Wang et al. 2020). In the current study, the TAB could alleviate ER stress in LPS injected rats (Fig. 4). The tannoids of amla, curcumin and piperine present in the TAB were earlier shown to combat ER stress individually by various mechanisms in varied disease models (Guo et al. 2018; Dhivya Bharathi et al. 2019; Shakeri et al. 2019).
TAB ameliorated LPS-induced changes in autophagy- lysosomal system
To further understand the possible mechanisms of TAB interference in reducing inflammation, we analysed the level of Beclin1, ATG5, LC3-II and p62 proteins to inspect the possible alterations in the autophagy-lysosome system (Fig. 5). LPS-group showed increased protein expression of Beclin1, ATG5, LC3II and P62 while TAB- group showed a beneficial effect in preventing these alterations (Fig. 5).
TAB prevented LPS-induced alterations in autophagy- lysosomal system. A Representative immunoblot images of autophagy proteins. B Quantification data of immunoblots. Data are presented as the mean ± SEM. *p < 0.05 when compared with the control group, #p < 0.05 when compared with the LPS group. C = control group, LPS = lipopolysaccharide injected group, TAB = functional food mixture (turmeric + amla + black pepper) pre-treated group
TAB ameliorated LPS-induced apoptotic markers
Along with the inflammation lung-cell apoptosis is another key pathologic feature of ALI and modulation of apoptosis was shown to prevent LPS induced ALI (Ju et al. 2018; Xie et al. 2018). Hence, we next investigated the status of apoptotic protein expression in the lungs of experimental rats. As shown in Fig. 6, the LPS- group showed increased protein levels of Bax that plays a crucial role in the mitochondrial apoptotic process. Bax/Bcl2 ratio estimated by quantitative immunoblotting was greater in LPS-group when compared with the control group but with no statistical significance. Caspase-3 protein another marker of apoptosis was elevated in LPS-group but prevented in TAB-group. Cleaved caspase 12, a marker of ER stress-induced apoptosis was elevated in LPS-group which is partly prevented in TAB-group.
TAB prevented LPS-induced alterations in apoptotic markers. A Representative immunoblot images of apoptotic and anti-apoptotic proteins. B Quantification data of immunoblots. Data are presented as the mean ± SEM. *p < 0.05 when compared with the control group, #p < 0.05 when compared with the LPS group. C = control group, LPS = lipopolysaccharide injected group, TAB = functional food mixture (turmeric + amla + black pepper) pre-treated group
Turmeric offers several biological activities most of which are attributed to the presence of curcumin. Curcumin exerts strong anti-oxidant and anti-inflammatory activities and more than 100 clinical trials were conducted on curcumin in various diseases including autoimmune disorders (Naksuriya et al. 2014). Curcumin was shown to regulate the inflammatory response by down-regulating the activity of cyclooxygenase-2 (COX-2), lipoxygenase, and inducible nitric oxide synthase (iNOS) enzymes. It also inhibits the production of the inflammatory cytokines TNF-α, IL-1, -2, -6, -8, and − 12, and down-regulates mitogen-activated JNK (Abe et al. 1999; Jagetia and Aggarwal 2007; Goel et al. 2008). Curcumin dose-dependently inhibited LPS-induced NF-κB with an IC50 of 18 µM, interestingly turmeric extract showed an IC50 of 15 µM (Edwards et al. 2020). In another study, curcumin-free turmeric was also shown to have anti-inflammatory activity (Aggarwal et al. 2013).
Amla has been used in treating several disorders especially inflammatory diseases such as pneumonia, hepatitis, and even cancer, and is well-known for a wide range of biological activities (Wang et al. 2017). Phytochemical studies on amla showed that it is rich in tannins, polyphenols, flavonoids, gallic acid, vitamin C, and emblicol (Sarin et al. 2014). The immunomodulatory effects of Amla were mainly due to the down-regulation of pro-inflammatory genes, COX-2, iNOS, IL-16, IL-6, and TNF-α (Chatterjee et al. 2011; Sripanidkulchai and Junlatat 2014). Amla also alleviated LPS induced inflammation in macrophages by decreasing the release of pro-inflammatory mediators (Li et al. 2020). Chang et al. reported that beta-glucogallin isolated from amla could inhibit LPS-induced oxidative stress by preventing activation of JNK and p38 in murine macrophages (Chang et al. 2013).
Black pepper is used in traditional food formulations, perfumery, alternative medicine, and cosmetics in many Asian countries. The active phenolic component of pepper, piperine, is shown to have beneficial health effects. Piperine was shown to inhibit the translocation of activator protein 1 (AP-1) in IL1β-treated cells thereby modulating inflammation (Bang et al. 2009). Piperine was reported to exert anti-inflammatory activity by decreasing the expression of ICAM-1 on the macrophage surface thereby inhibiting macrophage polarization (Gholijani et al. 2021). Piperine could suppress the pro-inflammatory factors IL-1β and TNF-α, and enhance the anti-inflammatory effects of IL-10 and TGF-β1 (Yu et al. 2021). Though curcumin has several health benefits, one of the drawbacks is its poor bioavailability. Interestingly, piperine was found to increase the bioavailability of curcumin by 2000% in both humans and animals with no adverse effects (Shoba et al. 1998). Hence, the issue of curcumin’s poor bioavailability could be resolved by the addition of pepper, consequently enhancing the efficacy of the formulation by synergism.
Conclusion
As summarised in Fig. 7, sepsis induces severe inflammation in which NF-kB activation is crucial and multifactorial. Further, the uncontrolled inflammation finally damages the lung tissue causing ALI. The results showed that pre-treatment with TAB constituting turmeric, amla, and black pepper powder at specified doses prevented LPS-induced NF-kB activation and its downstream pathologies in rat lungs. TAB has ameliorated LPS-induced ER stress response, MAPK signalling, autophagy, and cell death processes. The anti-inflammatory effect of the TAB is well supported by these results and hence, can be used at least as an adjuvant for ALI and other inflammatory conditions. The formulation can be easily prepared as the ingredients are economical and readily available. Further, people regularly consume these constituents for centuries as part of the diet without any signs of toxicity.
Schematic diagram showing the key mediators of LPS induced acute lung injury. LPS activates TLR4 on the cell membrane that in turn triggers several cell-signalling molecules. TLR4 initiates translocation of the pro-inflammatory transcription factor NF-κB into the nucleus from the cytoplasm to induce gene transcription. TLR4 also activates PI3K/Akt signalling to stabilise NF-kB. MAPKs activated by TLR4 also induces pro-inflammatory cytokine synthesis via AP1. TLR4 induces apoptosis in multiple ways including ROS mediated, ER stress mediated and cytokine-mediated apoptosis. ER stress further increases the translocation of NF-kB for the induction of pro-inflammatory cytokine gene expression. Severe inflammation in the lung caused by excessive proinflammatory cytokines, ER stress, oxidative stress, and apoptosis collectively disturbs organ homeostasis and finally leads to acute lung injury. Pre-treatment with functional food mixture TAB could modulate the activities of several key mediators of LPS-induced ALI in rats. LPS- lipopolysaccharide, TLR4- Toll-like receptor 4, NF-kB- nuclear factor kappa light-chain-enhancer of activated B cell, AP1- Activator protein 1
Data availability
Authors confirm that all relevant data are included in the article. Raw data are available with the corresponding author on request.
Abbreviations
- TAB:
-
Functional food consisting of turmeric, amla, and black pepper
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- TNF-α:
-
Tumor necrosis factor-alpha
- IL:
-
Interleukin
- MCP:
-
Monocyte chemoattractant protein
- NF-κB:
-
Nuclear factor kappa B
- LPS:
-
Gram-negative bacterial cell wall lipopolysaccharide
- ER:
-
Endoplasmic reticulum
References
Abdul Manap AS, Wei Tan AC, Leong WH, Yin Chia AY, Vijayabalan S, Arya A, Wong EH, Rizwan F, Bindal U, Koshy S, Madhavan P (2019) Synergistic Effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in SH-SY5Y cells via computational molecular modeling and in vitro assay. Front Aging Neurosci 11:206
Abe Y, Hashimoto S, Horie T (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res 39(1):41–47
Aggarwal BB, Yuan W, Li S, Gupta SC (2013) Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res 57(9):1529–1542
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4(7):499–511
Andy SN, Kadir CC HA (2017) Deoxyelephantopin from Elephantopus scaber modulates neuroinflammatory response through MAPKs and PI3K/Akt-dependent NF-κB signaling pathways in LPS-stimulated BV-2 microglial cells. J Funct Foods 38:221–231
Ayaz G, Halici Z, Albayrak A, Karakus E, Cadirci E (2017) Evaluation of 5-HT7 receptor trafficking on in vivo and in vitro model of lipopolysaccharide (LPS)-induced inflammatory cell injury in rats and LPS-treated A549 cells. Biochem Genet 55(1):34–47
Bang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS (2009) Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther 11(2):R49
Chaitanya N, Badam R, Aryasri AS, Pallarla S, Garlpati K, Akhila M, Soni P, Gali S, Inamdar P, Parinita B, Zaheer K, Prabhath T, Swetha A (2020) Efficacy of improvised topical zinc (1%) ora-base on oral mucositis during cancer chemo-radiation-a randomized study. J Nutr Sci Vitaminol 66(2):93–97 (Tokyo)
Chang KC, Laffin B, Ponder J, Enzsoly A, Nemeth J, LaBarbera DV, Petrash JM (2013) Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem Biol Interact 202(1–3):283–287
Chatterjee A, Chattopadhyay S, Bandyopadhyay SK (2011) Biphasic effect of Phyllanthus emblica L. extract on NSAID-induced ulcer: an antioxidative trail weaved with immunomodulatory effect. Evid Based Complement Alternat Med 2011:146808
Deng G, He H, Chen Z, OuYang L, Xiao X, Ge J, Xiang B, Jiang S, Cheng S (2017) Lianqinjiedu decoction attenuates LPS-induced inflammation and acute lung injury in rats via TLR4/NF-kappaB pathway. Biomed Pharmacother 96:148–152
Dhivya Bharathi M, Justin-Thenmozhi A, Manivasagam T, Ahmad Rather M, Saravana Babu C, Mohamed Essa M, Guillemin GJ (2019) Amelioration of aluminum maltolate-induced inflammation and endoplasmic reticulum stress-mediated apoptosis by tannoid principles of Emblica officinalis in neuronal cellular model. Neurotox Res 35(2):318–330
Edwards RL, Luis PB, Nakashima F, Kunihiro AG, Presley SH, Funk JL, Schneider C (2020) Mechanistic differences in the inhibition of NF-kappaB by turmeric and its curcuminoid constituents. J Agric Food Chem 68(22):6154–6160
Gholijani N, Hashemi E, Amirghofran Z (2021) Piperine from black pepper decreased the expression of intercellular adhesion molecule-1 in macrophages. Antiinflamm Antiallergy Agents Med Chem 20(2):201–205
Goel A, Kunnumakkara AB, Aggarwal BB (2008) Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol 75(4):787–809
Guo J, Cui Y, Liu Q, Yang Y, Li Y, Weng L, Tang B, Jin P, Li XJ, Yang S, Li S (2018) Piperine ameliorates SCA17 neuropathology by reducing ER stress. Mol Neurodegener 13(1):4
Habashi NM, Camporota L, Gatto LA, Nieman G (2021) Functional pathophysiology of SARS-CoV-2-induced acute lung injury and clinical implications. J Appl Physiol (1985) 130(3):877–891
Hawkins PT, Stephens LR (2015) PI3K signalling in inflammation. Biochim Biophys Acta 1851(6):882–897
Jagetia GC, Aggarwal BB (2007) "Spicing up” of the immune system by curcumin. J Clin Immunol 27(1):19–35
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu D, Cang J, Luo Z (2018) MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting in flammation and apoptosis through modulating TLR4/MyD88/NF-kappaB pathway. Cell Cycle 17(16):2001–2018
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14(2):141–153
Karbian N, Abutbul A, El-Amore R, Eliaz R, Beeri R, Reicher B, Mevorach D (2020) Apoptotic cell therapy for cytokine storm associated with acute severe sepsis. Cell Death Dis 11(7):535
Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chavez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR, de Jong MF, Kerrinnes T, Ravindran R, Luciw PA, McSorley SJ, Baumler AJ, Tsolis RM (2016) NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532(7599):394–397
Leist SR, Dinnon KH III, Schafer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, Gully KL, Scobey T, Brown AJ, Sheahan TP, Moorman NJ, Boucher RC, Gralinski LE, Montgomery SA, Baric RS (2020) A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183(4):1070–1085
Li W, Cao T, Luo C, Cai J, Zhou X, Xiao X, Liu S (2020) Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol 104(14):6129–6140
Li W, Zhang X, Chen R, Li Y, Miao J, Liu G, Lan Y, Chen Y, Cao Y (2020) HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J Ethnopharmacol 254:112740
Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X (2020) Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55:102763
Mrudula T, Suryanarayana P, Srinivas PN, Reddy GB (2007) Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem Biophys Res Commun 361(2):528–532
Muthenna P, Suryanarayana P, Gunda SK, Petrash JM, Reddy GB (2009) Inhibition of aldose reductase by dietary antioxidant curcumin: mechanism of inhibition, specificity and significance. FEBS Lett 583(22):3637–3642
Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE (2014) Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 35(10):3365–3383
Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bianchi N, Bezzerri V, Mancini I, Giri MG, Rizzotti P, Gambari R, Cabrini G (2008) Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. Int Immunopharmacol 8(12):1672–1680
Patil VM, Das S, Balasubramanian K (2016) Quantum chemical and docking insights into bioavailability enhancement of curcumin by piperine in pepper. J Phys Chem A 120(20):3643–3653
Pei H, Xue L, Tang M, Tang H, Kuang S, Wang L, Ma X, Cai X, Li Y, Zhao M, Peng A, Ye H, Chen L (2020) Alkaloids from black pepper (Piper nigrum L.) exhibit anti-inflammatory activity in murine macrophages by inhibiting activation of NF-kappaB pathway. J Agric Food Chem 68(8):2406–2417
Pitchaiah G, Akula A, Chandi V (2017) Anticancer potential of nutraceutical formulations in MNU-induced mammary cancer in Sprague Dawley rats. Pharmacogn Mag 13(49):46–50
Puppala M, Ponder J, Suryanarayana P, Reddy GB, Petrash JM, LaBarbera DV (2012) The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 7(4):e31399
Rao VR, Muthenna P, Shankaraiah G, Akileshwari C, Babu KH, Suresh G, Babu KS, Chandra Kumar RS, Prasad KR, Yadav PA, Petrash JM, Reddy GB, Rao JM (2012) Synthesis and biological evaluation of new piplartine analogues as potent aldose reductase inhibitors (ARIs). Eur J Med Chem 57:344–361
Rawal S, Singh P, Gupta A, Mohanty S (2014) Dietary intake of Curcuma longa and Emblica officinalis increases life span in Drosophila melanogaster. Biomed Res Int 2014:910290
Rubenfeld GD, Herridge MS (2007) Epidemiology and outcomes of acute lung injury. Chest 131(2):554–562
Saraswat M, Muthenna P, Suryanarayana P, Petrash JM, Reddy GB (2008) Dietary sources of aldose reductase inhibitors: prospects for alleviating diabetic complications. Asia Pac J Clin Nutr 17(4):558–565
Sarin B, Verma N, Martin JP, Mohanty A (2014) An overview of important ethnomedicinal herbs of Phyllanthus species: present status and future prospects. Sci World J 2014:839172
Schacke H, Docke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96(1):23–43
Shakeri A, Zirak MR, Wallace Hayes A, Reiter R, Karimi G (2019) Curcumin and its analogues protect from endoplasmic reticulum stress: mechanisms and pathways. Pharmacol Res 146:104335
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353–356
Singh MK, Yadav SS, Gupta V, Khattri S (2013) Immunomodulatory role of Emblica officinalis in arsenic induced oxidative damage and apoptosis in thymocytes of mice. BMC Complement Altern Med 13:193
Sripanidkulchai B, Junlatat J (2014) Bioactivities of alcohol based extracts of Phyllanthus emblica branches: antioxidation, antimelanogenesis and anti-inflammation. J Nat Med 68(3):615–622
Suryanarayana P, Kumar PA, Saraswat M, Petrash JM, Reddy GB (2004) Inhibition of aldose reductase by tannoid principles of Emblica officinalis: implications for the prevention of sugar cataract. Mol Vis 10:148–154
Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB (2005) Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci 46(6):2092–2099
Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB (2007) Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit 13(12):BR286-292
Tasleem F, Azhar I, Ali SN, Perveen S, Mahmood ZA (2014) Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac J Trop Med 7S1:S461-468
Tomashefski JF Jr (2000) Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med 21(3):435–466
Wang CC, Yuan JR, Wang CF, Yang N, Chen J, Liu D, Song J, Feng L, Tan XB, Jia XB (2017) Anti-inflammatory effects of Phyllanthus emblica L. on benzopyrene-induced precancerous lung lesion by regulating the IL-1beta/miR-101/Lin28B signaling pathway. Integr Cancer Ther 16(4):505–515
Wang Y, Zhou X, Zhao D, Wang X, Gurley EC, Liu R, Li X, Hylemon PB, Chen W, Zhou H (2020) Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS ONE 15(5):e0232630
Xie W, Lu Q, Wang K, Lu J, Gu X, Zhu D, Liu F, Guo Z (2018) miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J Cell Physiol 233(9):6615–6631
Yu JW, Li S, Bao LD, Wang L (2021) Piperine treating sciatica through regulating inflammation and MiR-520a/P65 pathway. Chin J Nat Med 19(6):412–421
Zeng M, Sang W, Chen S, Chen R, Zhang H, Xue F, Li Z, Liu Y, Gong Y, Zhang H, Kong X (2017) 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett 271:26–37
Yuan Z, Bedi B, Sadikot RT (2018) Bronchoalveolar Lavage Exosomes in Lipopolysaccharide-induced Septic Lung Injury. J Vis Exp(135).
Author information
Authors and Affiliations
Contributions
MN: Performed the experiments, data collection, and analysis. KKK, KPR, MS, and URA: data collection and analysis. GBR: Project administration, manuscript review, and funding acquisition. SSR: Study conception and design, supervision and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Funding
Author G.B.R has received grants (Q-11/16/2019-R&D) from the Ministry of Food Processing Industries, Government of India. M.N was supported by a postdoctoral fellowship from the Science and Engineering Research Board (SERB, PDF/2020/001907), Government of India.
Ethical approval
Approved by Institutional Animal Ethics Committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagaraju, M., Kalahasti, K.K., Reddy, K.P. et al. Anti-inflammatory potential of turmeric, amla, and black pepper mixture against sepsis-induced acute lung injury in rats. J Food Sci Technol 60, 252–261 (2023). https://doi.org/10.1007/s13197-022-05610-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-022-05610-1