Abstract
Shrimp lipid (SL) from Pacific white shrimp (Litopenaeus vannamei) cephalothorax was subjected to ethanol separation with subsequent cholesterol removal. Around 98.4% of cholesterol was removed from cholesterol rich polar lipid fraction (PLF), in which PLF/β cyclodextrin (β-CD)/mixed solvents (ethyl acetate/water,1:1) at the ratio of 1:10:20 (w/w/v) were used. Thereafter, PLF with lowered cholesterol was combined with non-polar fraction rich in triglycerides to obtain lowered cholesterol shrimp lipid (LC-SL). Astaxanthin content in LC-SL was augmented by three-fold, compared to that found in SL. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) contents of LC-SL were also significantly increased, contrasted with SL. Peroxide value and phospholipids were decreased in LC-SL (4.56 ± 0.15 meq/kg and 9.94 ± 1.9%) compared to those of SL (4.80 ± 0.25 meq/kg and 49.11 ± 2.1%), while TBARS and p-Anisidine values remained unchanged. However, conjugated dienes and free fatty acids were augmented, plausibly due to hydrolysis. FTIR spectra confirmed the increased degree of unsaturation of lipids. Thus, the lowered cholesterol shrimp lipid could be used as functional foods or nutraceutical for health promotion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrimp is one of the highly demanding aquatic foods widely consumed around the world in the form of fresh as well as processed products. Around 3.05 million tonnes of shrimp were documented for the global trade in 2019, in which the top three importers included European Union, China, and United States of America (FAO 2020). The enormous growth of shrimp export leads to the generation of substantial amount of shrimp waste by the processing industries (FAO 2020). Cephalothorax is one of the shrimp wastes, that can be utilized for the production of shrimp oil. The presence of highly valuable eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and astaxanthin makes shrimp oil an excellent source of nutraceuticals (Gulzar et al. 2020). Nevertheless, shrimp oil contains cholesterol, which is not desirable, especially in terms of health risks. Cholesterol consumed through diet could lead to heart disorders (Zhong et al. 2019). In order to increase shrimp oil benefits, the cholesterol level should either be reduced or depleted. Commonly used cholesterol reducing agents in various foods were β cyclodextrin (β-CD) and saponin (Sieber 1993).
β-CD, a non-toxic oligosaccharide, has been used widely as a food additive, pharmaceutical, cosmetic, and also utilized in the agricultural industries (Brewster and Loftsson 2007). Due to the complex structure of inner hydrophobic and outer hydrophilic nature, β-CD showed its ability to trap or remove cholesterol from numerous food products such as milk, pork lard, and butter (Astray et al. 2009). The cholesterol removal process with the aid of β-CD was applied without major changes in other lipid constituents (Raju and Benjakul 2019). The cholesterol removal from shrimp lipid using β-CD led to the augmented proportion of astaxanthin, in which its concentration was increased by 89%, compared to that of untreated shrimp lipid. However, the content of some fatty acids, especially EPA, was slightly decreased during the process due to the oxidation (Raju and Benjakul 2019). Raju and Benjakul (2020) used saponin for cholesterol removal from shrimp lipid by firstly separating the polar lipids by ethanol separation, in order to protect the deterioration of triglycerides (non-polar fraction) during the process associated with the loss of fatty acids. Due to the amphiphilic nature, cholesterol and astaxanthin were separated by ethanol. However, cholesterol removal with saponin resulted in decrease in the amount of astaxanthin (Raju and Benjakul 2020). The lack of specific binding site of β-CD and hydrophobic binding nature of saponin more likely decreased the level of astaxanthin in treated shrimp lipid (Raju and Benjakul 2020). This study was aimed at using β-CD in combination with ethanol separation process to remove cholesterol and to maintain or improve the levels of astaxanthin and polyunsaturated fatty acids in shrimp lipids.
Materials and methods
Chemicals
β-CD was obtained from Wacker chemicals (Singapore). HPLC grade solvents (methanol and acetonitrile), ethyl acetate, hexane and propanol were obtained from Lab-Scan (Bangkok, Thailand). All other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA). Commercial astaxanthin and fatty acid methyl ester standard were procured from Dr. Ehrenstorfer GmbH (Augsburg, Germany) and Supelco (Bellefonte, PA, USA), respectively.
Shrimp lipid extraction
Shrimp (Litopenaeus vannamei) cephalothorax was acquired under frozen condition (− 18 °C) from Sea Wealth Frozen Food Co., Ltd., Songkhla province, Thailand. Frozen samples were transported to the laboratory, thawed, and ground to a homogenous paste with a blender (National Model MK-K77, Tokyo, Japan). Shrimp lipid extraction was carried out following the method of Gulzar and Benjakul (2018). Cephalothorax (200 g) paste was mixed with 1 L of hexane/isopropanol mixture (1:1) and homogenized at 9500 rpm by an IKA Labortechnik homogenizer (Selangor, Malaysia) for 2 min and the homogenate was filtered using a Whatman filter paper No.4. The filtrate was washed thrice with an equal volume of distilled water.
Ten grams (10 g) of anhydrous sodium sulphate were added to the collected solvent fraction and filtered. The solvent was evaporated by EYELA rotary evaporator (N-1000, Tokyo Rikakikai, Co., Ltd., Tokyo Japan) at 40 °C and shrimp lipid retained was collected, flushed with nitrogen and capped tightly. Shrimp lipid was stored at −20 °C for further process. The extracted lipid was termed shrimp lipid (SL).
Lipid fractionation using ethanol
Ethanol soluble lipid fractionation was done as per the procedure of Raju and Benjakul (2020). Ten (10) g of SL was added to 50 mL of ethanol and the extraction was performed twice. The bottom fraction was further dissolved in 5 mL of hexane, stored at − 20 °C and referred to as ‘non-polar lipid fraction (NPLF). Thereafter ethanol soluble lipid fraction was evaporated using a rotary evaporator and taken for the cholesterol removal process. The polar lipid fraction was termed as ‘PLF’.
Impact of β-CD at varying concentrations and ethyl acetate volumes on cholesterol removal in shrimp lipid
PLF (5 g) was added to β-CD to obtain different PLF/β-CD ratios (1:2, 1:5 and 1:10 W/W). The mixture was further dissolved in various volumes of ethyl acetate (25, 50, and 100 mL). The same volume of water (25, 50, and 100 mL) was subsequently added, in which PLF/mixed solvent (MS) (ethyl acetate + water) ratios of 1:10, 1:20 and 1:40 (W/V) were obtained. All the mixtures were homogenized using a homogenizer for 10 min and centrifuged at 8000 × g for 20 min at 15 °C. The ethyl acetate phase was collected and combined with the stored NPLF rich in triglycerides. The mixture was further subjected to solvent evaporation at 40 °C using a rotary evaporator. All the combined lipid samples were analyzed for cholesterol and astaxanthin contents.
Astaxanthin and cholesterol contents were analyzed using the method of Raju and Benjakul (2019). High performance liquid chromatography system (Waters 2695 series, Milford, MA, USA) equipped with reverse-phase Thermo scientific BDS-C18 column (5 µm; 150 × 4 mm) was used. The peaks were detected by a photo diode-array detector using the methanol–acetonitrile (50:50) mobile phase. Astaxanthin was recorded at 480 nm and cholesterol peak was identified at 201 nm. Identification of peaks was confirmed with the retention time of standards and astaxanthin content was expressed as mg/g lipid.
Characterization of cholesterol lowered shrimp lipid
Combined lipids obtained under the optimum condition yielding the lowest cholesterol and highest astaxanthin termed as ‘LC-SL’ was selected. Characterization was done in comparison with ‘SL’.
Fatty acid profile
Fatty acid profile was determined using the method of Olatunde et al. (2019). Ten (10) mg of lipid sample was transmethylated using 2 M methanolic sodium hydroxide and further neutralized by 2 M methanolic hydrochloric acid. Peaks were detected using Agilent 7890B (Santa Clara, CA, USA) gas chromatography system with flame ionization detector (FID). Supelco FAME mix (Bellefonte, PA, USA) standards were used for the identification of peaks and the fatty acid content was expressed as g/100 g lipid.
Conjugated diene
Conjugated dienes (CD) were analyzed using the method of Pudtikajorn and Benjakul (2020) with the aid of UV/vis spectrophotometer (Shimadzu UV-1800, Kyoto, Japan) at 234 nm.
Peroxide value
Peroxide value (PV) was measured using the method of Gulzar and Benjakul (2019). Samples were firstly dissolved in ten-fold of 75% ethanol (v/v). The prepared sample mixture was added to 2.35 mL of 75% ethanol (v/v), 50 µL of 30% ammonium thiocyanate (w/v) and 50 µL of 20 mM ferrous chloride solution in 3.5% HCl (w/v), mixed well and measured at 500 nm using a spectrophotometer. Hydroperoxide concentrations were measured from a standard curve of cumene hydroperoxide (0.5–2 ppm) and expressed as meq/kg lipid.
Thiobarbituric acid reactive substances (TBARS)
The method of Olatunde et al. (2019) was adopted for the determination of TBARS value. The standard curve of 1,1,3,3- tetramethoxypropane (0–6 ppm) was prepared. Quantification of the sample was done and the value was expressed as mg malonaldehyde/kg lipid.
p-Anisidine value (AV)
AV was determined following the method of Pudtikajorn and Benjakul (2020). Approximately 0.1 g of sample was mixed with 25 mL of p-anisidine reagent and the absorbance of the mixture was read at 350 nm. The calculation of AV was done as follows.
where W denotes the sample weight (g) and A1 and A2 stand for the absorbance recorded before and after the addition of anisidine reagent.
Free fatty acid content
Free fatty acid (FFA) content was measured according to the method of Lowry and Tinsley (1976). Sample (100 mg) was mixed with 5 mL of isooctane and 5% (W/V) cupric acetate-pyridine reagent (1 mL). The reaction mixture was measured at 715 nm after 90 s. The FFA content was expressed as g FFA/100 g lipid, in which the standard curve of palmitic acid (0–10 µmol/mL) was prepared.
Phospholipid content
The procedure of Miwa and Low (1992) was adopted for the determination of phospholipid contents. Lipid sample (500 mg) was dissolved in 1 mL of chloroform/hexane and passed through the glass column containing 5 g of silica (100 mesh). The neutral and non-polar components were eluted using 125 mL of chloroform and the remaining phospholipid was eluted using 100 mL of methanol. The solvent in both fractions were evaporated by a rotary evaporator followed by measurement of weight. The total phospholipid content was reported as a percentage of mass of the sample used.
Fourier Transform Infrared (FTIR) Spectra
FTIR spectra were recorded using Bruker Model Vector 33 FTIR spectrometer (Bruker Co., Ettlingen, Germany) (Gulzar and Benjakul 2018). Wavenumber range was selected between 4000 and 500 cm− 1 with 16 scans. Normalization was done with a clean empty cell at 25 °C and considered as the reference background. The data was collected using OPUS 3.0 data collection software (Bruker Co. Billerica, MA, USA).
Statistical analysis
Complete randomized design (CRD) was applied for the initial data analysis. All the samples were analyzed in triplicates using three different sample lots. Mean comparison was done using the Duncan’s multiple range and t-test (Steel and Torrie 1980) with the aid of the Statistical Package for Social Science (SPSS software (IBM software, New York, NY, USA).
Results and discussion
Cholesterol removal using different β-CD concentrations and mixed solvent (MS) ratios
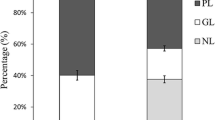
Around 34.5 ± 1.1% of non-polar lipids was obtained on extraction. Figure 1a shows the cholesterol removal with different PLF/β-CD and PLF/MS ratios. Initially, β-CD was used at the lowest concentration (PLF/β-CD ratio of 1:2), while varying PLF/MS ratios of 1:10, 1:20 and 1:40 were used (Fig. 1a). The cholesterol removal was highest (P < 0.05) (23.20 ± 1.2%) when the lowest PLF/MS (1:10) ratio was used. However, cholesterol removal efficacy was reduced, when higher PLF/MS ratios were applied. The result revealed that β-CD bound with cholesterol to a lower extent. In the presence of higher PLF/MS ratio, cholesterol could migrate or was distributed in ethyl acetate phase with ease. As a result, the interaction between β-CD and cholesterol was reduced due to dilution effect. Cholesterol removal with β-CD was more efficient when saturated β-CD with lesser solvent was utilized (Astray et al. 2009). In order to obtain the highest cholesterol removal efficiency, β-CD amount was further increased to mix with PLF up to 1:10. Approximately 98.4 ± 0.02% cholesterol was removed when PLF/β-CD ratio of 1:10 and PLF/MS ratio of 1:20 were used (Fig. 1a), in which this optimum condition was further used. β-CD is an amphiphilic compound containing an inner hydrophobic and an outer hydrophilic region (Sharma and Baldi 2016). The inner hydrophobic cavity structure of β-CD is more appropriate to trap the steroid compounds such as cholesterol selectively without affecting the astaxanthin content (Del Valle 2004). Moreover, a slightly lower decrease in cholesterol removal was found as PLF/MS ratio of 1:10 was used, compared to 1:20 and 1:40 (P < 0.05). Physical force is one of the prime factors for forming the inclusion complex with β-CD (Astray et al. 2009). When PLF/β-CD ratio of 1:10 and PLF/MS ratio of 1:10 was used, the mixture was highly saturated due to the low volume of solvent in the system. As a result, the interaction between cholesterol and β-CD was reduced.
Cholesterol removal (a) and astaxanthin content (b) of shrimp lipid treated using different PLF/β- CD ratios and PLF/MS ratios of fractionated polar fraction, prior to combination with non-polar fraction. Note X2, X5 and X10 denotes PLF/β- CD ratio of 1:2, 1:5 and 1:10, respectively and a,b and c denote PLF/MS ratios 1:10, 1:20 and:40, respectively. Different lowercase letters on the bar within the same PLF/β- CD ratios including SL denote significant difference (p ≤ 0.05)
Previously, cholesterol removal was done by direct addition of β-CD in SL, which led to the loss of some fatty acids, especially EPA (Raju and Benjakul 2019). To mitigate this drawback, ethanol fractionation was firstly applied to separate the triglycerides from SL. Cholesterol was also removed from SL using saponin along with ethanol separation and the resulting lipid had lower loss in fatty acids (Raju and Benjakul 2020). Ethanol fraction was mainly composed of polar lipids, pigments, cholesterol and other sterols. Apart from cholesterol, β-CD has been reported to have the tendency to bind with other polar lipids such as phospholipids (Crini et al. 2018). In our previous study, β-CD/SL of 1:4 was used to achieve the highest cholesterol removal in shrimp lipid (Raju and Benjakul 2019). Gulzar and Benjakul (2018) reported that phospholipid content was around 46% in shrimp lipid. During the ethanol fractionation, those phospholipid content was partitioned to PLF along with cholesterol. To achieve the optimal cholesterol removal in phospholipid rich fraction (PLF), PLF/β-CD ratio was increased to 1:10. However, increasing β-CD with PLF/MS ratio of 1: 20 reduced the yield of resulting lipid (42.1 ± 0.56%) (P < 0.05), while astaxanthin content was increased (Fig. 1b).
Astaxanthin content was augmented in all samples after cholesterol removal was implemented, regardless of treatments (Fig. 1b). Thus astaxanthin was not negatively affected when cholesterol was bound with β-CD. Astaxanthin content was increased significantly (P < 0.05) compared to that found in SL, when PLF/β-CD of 1:10 was used (Fig. 1b). The increased astaxanthin favored more reddish color in LC-SL, compared to SL (Fig. 2). The augmentation of astaxanthin might be due to the removal of other lipid components. Also, astaxanthin is linear in structure and could not fit well in the cup of β-CD (Del Valle 2004). As a consequence, astaxanthin was still abundant in the treated lipid. The result was concomitant with the previous study, in which the increased astaxanthin (8.99%) during cholesterol removal with β-CD was achieved (Raju and Benjakul 2019). At PLF/β-CD ratio of 1:10, astaxanthin content was declined when PLF/MS ratio was decreased. It could be due to the insufficient volume of solvent, which is required for astaxanthin to migrate from β-CD to solvent phase.
Fatty acid profile
Fatty acid compositions of SL and LC-SL were different for each fatty acids before and after cholesterol removal (Table 1). Major fatty acids were increased in LC-SL. The predominant fatty acid in both lipid samples was palmitic acid. After the cholesterol removal, palmitic acid was 50% higher in LC-SL (15.86 ± 0.18 g/100 g) than in SL (10.17 ± 0.11 g/100 g). Polyunsaturated fatty acids, especially EPA (7.98 ± 0.07 g/100 g) and DHA (8.63 ± 0.03 g/100 g) were augmented in LC-SL, compared to those of SL (4.88 ± 0.08 and 6.01 ± 0.02 g /100 g, respectively). Cholesterol removal by direct addition of β-CD in shrimp lipid resulted in the loss in EPA and other fatty acids due to hydrolysis (Raju and Benjakul 2019). Nevertheless, via ethanol fractionation, triglycerides were separated from polar compounds, in which they could be preserved during the cholesterol removal process (Raju and Benjakul 2020). Cholesterol removal in shrimp lipid using saponin was performed under ethanol fractionation process and aided to maintain the fatty acids by preventing hydrolysis of triglycerides (Raju and Benjakul 2020). Moreover, β-CD has been documented to induce hydrolysis of triglycerides (Kwak et al. 2002; Raju and Benjakul 2019). It can be confirmed that ethanol fractionation process provided promising and efficient method for reducing the breakdown of triglycerides and retaining fatty acids. Overall the cholesterol removal using β-CD with prior ethanol fractionation was able to reduce the loss in fatty acids of LC-SL.
Oxidation and hydrolysis of shrimp lipid
Conjugated dienes (CD) are the initial products formed during lipid oxidation (Saleh et al. 2013). CD was significantly higher in LC-SL (P < 0.05) than SL (Table 2). Ethanolic fraction rich in polar lipids was mainly composed of phospholipids, astaxanthin or astaxanthin esters, sterols, vitamins and other pigments. Most of astaxanthin were conjugated with polyunsaturated fatty acids (Gómez-Estaca et al. 2017). Double bonds found in PUFA are highly reactive to the free radicals, leading to the formation of highly thermodynamically stable dienes (Shima and Sakashita 2016). In general, PV was decreased (P < 0.05) in LC-SL and the secondary oxidation products such as TBARS and AV were found to be unaltered (Table 2). These results were in tandem with the findings of Raju and Benjakul (2019). The loss of lipid via degradation when β-CD was used for cholesterol removal in milk fat and cream have been reported (Kumar et al. 2010; Bhatia et al. 2019). The stability of lipid might also be due to the increased astaxanthin content in LC-SL, which could provide the increased antioxidant activity for lipids. This led to the prevention of lipid deterioration, particularly via oxidation.
Hydrolytic rancidity as measured by free fatty acids (FFA) was found to increase in LC-SL. β-CD has the tendency to induce the formation of FFA (Table 2). Endogenous lipase or phospholipase in shrimp cephalothorax also played a role in hydrolysis (Gulzar and Benjakul 2019). When β-CD was present, its hydrophobic inner cavity can promote the transformation of bound hydrophobic substrates to the reaction region of lipase (Wang et al. 2019). Additionally, when the hydrophobic domain of lipid, mainly fatty acid domain was inserted in the cup of β-CD, the glycerol on polar head of phospholipid which is hydrophilic in nature was more likely protruded to the aqueous phase and bound to outer region of adjacent β-CD. This could favour hydrolysis induced by mechanical shearing mediated by high speed homogenization. As a consequence, FFA were produced to a higher extent in LC-SL. Cholesterol removal in shrimp lipid using β-CD also resulted in the augmentation of FFA (Raju and Benjakul 2019). FFA production was more pronounced when β-CD was used for cholesterol removal in cheddar cheese (Kwak et al. 2003). Majority of PLF was composed of phospholipids, but they were decreased in LC-SL (P < 0.05). The amphiphilic nature of β-CD tends to bind the phospholipids (Crini et al. 2018) and contributed to the reduction of phospholipids in LC-SL.
FTIR spectra
The infrared spectra of SL and LC-SL depicted the changes in their functional groups and
also oxidation status of lipids (Fig. 3). Hydrogen peroxide was formed ascertained by OH stretching peak in the range of 3400–3100 cm−1 (Gulzar and Benjakul 2020), which was lower in LC-SL than SL. However, there was a notable increase in the amplitude of LC-SL in the range of 1790-1590 cm−1, which represent CH stretch, C=O ester, especially C=C representing double bond (Guillén and Cabo 1997; Laurens and Wolfrum 2011). The wavenumber 1711 cm−1, which represents the presence of free fatty acids C=O ester bonds, was increased slightly in LC-SL. This corresponded with the increase in content of free fatty acids (Table 2) (Laurens and Wolfrum 2011). Unsaturation was noted at wavenumber 1635 cm−1 and 3025 cm−1, indicating C=C and =CH, respectively confirming the increased unsaturated fatty acids in LC-SL (Table 1). Moreover, the peak found at 1765–1720 cm−1 was attributed to the presence of triglycerides at a higher level in LC-SL (Nzai and Proctor 1998). These augmented triglycerides were owing to the separation of non-polar lipid fraction via ethanol fractionation. Phospholipids prominent at 1145–970 cm−1 and 830–740 cm−1 (Nzai and Proctor 1998) were lowered after cholesterol removal process. During the separation of polar and non-polar lipids, polar lipids were mainly composed of phospholipids (Raju and Benjakul 2020). These phospholipids interacted with β-CD, resulting in their reduction after cholesterol removal. The cholesterol removal conducted by Raju and Benjakul (2019) using β-CD also showed the lowering of phospholipids. Overall, FTIR spectra demonstrated that LC-SL had lower oxidation and lower content of phospholipids, while unsaturation content of lipids increased.
Conclusion
Cholesterol was removed up to 98.4% in shrimp lipid when β-CD at PLF/β-CD ratio of 1:10 and PLF/MS (ethyl acetate/water, 1:1) ratio of 1:20 (V/V) were applied to treat ethanolic fraction rich in cholesterol. Astaxanthin and fatty acids were augmented after cholesterol removal process. For cholesterol lowered shrimp lipid, the content of astaxanthin increased by three-fold and EPA and DHA contents were augmented by 56% and 43%, respectively. Nevertheless, oxidation and hydrolysis occurred to some extent. Conversely, degree of unsaturation was increased after cholesterol removal, reflecting the advantage of the developed process. Overall, cholesterol could be removed from shrimp lipid under appropriate condition, leading to augmented astaxanthin and PUFA.
References
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gándara J (2009) A review on the use of cyclodextrins in foods. Food Hydrocoll 23:1631–1640
Bhatia P, Sharma V, Arora S, Rao PS (2019) Effect of cholesterol removal on compositional and the physicochemical characteristics of anhydrous cow milk fat (cow ghee). Int J Food Prop 22:1–8. https://doi.org/10.1080/10942912.2018.1564762
Brewster ME, Loftsson T (2007) Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev 59:645–666. https://doi.org/10.1016/j.addr.2007.05.012
Crini G, Fourmentinn S, Lichtfouse E (2018) Cyclodextrin fundamentals reactivity and analysis. Springer, Cham
Del Valle EMM (2004) Cyclodextrins and their uses: a review. Process Biochem 39:1033–1046. https://doi.org/10.1016/S0032-9592(03)00258-9
FAO (2020) GLOBEFISH Highlights January 2020 ISSUE, with Jan. – Sep. 2019 Statistics- A quarterly update on world seafood markets. Globefish Highlights no. 1–2020. FAO, Rome
Gómez-Estaca J, Calvo MM, Álvarez-Acero I, Montero P, Gómez-Guillén MC (2017) Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem 216:37–44
Guillén MD, Cabo N (1997) Characterization of edible oils and lard by fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. J Am Oil Chem Soc 74:1281–1286. https://doi.org/10.1007/s11746-997-0058-4
Gulzar S, Benjakul S (2018) Ultrasound waves increase the yield and carotenoid content of lipid extracted from cephalothorax of Pacific white shrimp (L. vannamei). Eur J Lipid Sci Technol 120:1700495. https://doi.org/10.1002/ejlt.201700495
Gulzar S, Benjakul S (2019) Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp (L. vannamei) by ultrasonic assisted process. LWT 100:106–113. https://doi.org/10.1016/j.lwt.2018.10.051
Gulzar S, Benjakul S (2020) Impact of pulsed electric field pretreatment on yield and quality of lipid extracted from cephalothorax of Pacific white shrimp (L. vannamei) by ultrasound-assisted process. Int J Food Sci Technol 55:619–630
Gulzar S, Raju N, Chandragiri Nagarajarao R, Benjakul S (2020) Oil and pigments from shrimp processing by-products: extraction, composition, bioactivities and its application—A review. Trends Food Sci Technol 100:307–319. https://doi.org/10.1016/j.tifs.2020.04.005
Kumar M, Sharma V, Lal D, Kumar A, Seth R (2010) A comparison of the physico-chemical properties of low-cholesterol ghee with standard ghee from cow and buffalo creams. Int J Dairy Technol 63:252–255. https://doi.org/10.1111/j.1471-0307.2010.00572.x
Kwak HS, Jung CS, Shim SY, Ahn J (2002) Removal of cholesterol from cheddar cheese by beta-cyclodextrin. J Agric Food Chem 50:7293–7298
Kwak HS, Jung CS, Seok JS, Ahn J (2003) Cholesterol removal and flavor development in cheddar cheese. Asian-Australasian J Anim Sci 16:409–416
Laurens LML, Wolfrum EJ (2011) Feasibility of spectroscopic characterization of algal lipids : chemometric feasibility of spectroscopic characterization of algal lipids : chemometric correlation of NIR and FTIR spectra with exogenous lipids in algal biomass. BioEnergy Res 4:22–35. https://doi.org/10.1007/s12155-010-9098-y
Lowry RR, Tinsley IJ (1976) Rapid colorimetric determination of free fatty acids. J Am Oil Chem Soc 53:470–472. https://doi.org/10.1007/BF02636814
Miwa K, Low SJ (1992) Laboratory manual on analytical methods and procedures for fish and fish products, 2nd edn. Marine Fisheries Research Department, Southeast Asian Fisheries Development Center All, Singapore
Nzai JM, Proctor A (1998) Determination of phospholipids in vegetable oil by fourier transform infrared spectroscopy. J Am Oil Chem Soc 75:1281–1289. https://doi.org/10.1007/s11746-998-0173-x
Olatunde OO, Benjakul S, Vongkamjan K (2019) Comparative study on nitrogen and argon-based modified atmosphere packaging on microbiological, chemical, and sensory attributes as well as on microbial diversity of Asian sea bass. Food Packag Shelf Life 22:100404
Pudtikajorn K, Benjakul S (2020) Simple wet rendering method for extraction of prime quality oil from skipjack tuna eyeballs. Eur J Lipid Sci Technol 122:2000077
Raju N, Benjakul S (2019) Use of beta cyclodextrin to remove cholesterol and increase astaxanthin content in shrimp oil. Eur J Lipid Sci Technol 122:1900242
Raju N, Benjakul S (2020) Application of saponin for cholesterol removal from Pacific white shrimp (L. vannamei) lipid. Eur J Lipid Sci Technol 122:2000078
Saleh Z, Ezzatpanah H, Aminafshar M, Safafar H (2013) The effect of refining process on the conjugated dienes in soybean oil. J Agric Sci Technol 15:1185–1193
Sharma N, Baldi A (2016) Exploring versatile applications of cyclodextrins: an overview. Drug Deliv 23:739–757. https://doi.org/10.3109/10717544.2014.938839
Shima M, Sakashita H (2016) Kinetic analysis of the concentration of conjugated diene structures in glyceryl trilinoleate during oxidation. Food Sci Technol Res 22:733–738
Sieber R (1993) Cholesterol removal from animal food-can it be justified? LWT Food Sci Technol 26:375–387. https://doi.org/10.1006/fstl.1993.1076
Steel RGD, Torrie JH (1980) Principles and procedures of statistics, a biometrical approach, 2nd edn. McGraw-Hill Kogakusha Ltd, Newyork
Wang P, Ke Z, Yi J, Liu X, Hao L, Kang Q, Lu J (2019) Effects of β-cyclodextrin on the enzymatic hydrolysis of hemp seed oil by lipase Candida sp.99–125. Ind Crops Prod 129:688–693. https://doi.org/10.1016/j.indcrop.2018.11.046
Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM, Allen NB (2019) Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. J Am Med Assoc 321:1081–1095. https://doi.org/10.1001/jama.2019.1572
Acknowledgements
This research was supported by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No. AGR6505012S). Thailand’s Education Hub for Southern Region of ASEAN Countries (TEH-AC, 2018) scholarship was acknowledged. Prachayacharn grant from Prince of Songkla University (Grant No. AGR6402088N) and Chair professor grant (P-20-52297) from NSTDA were also acknowledged.
Funding
This work was supported by National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No. AGR6505012S). Prince of Songkla University (Grant No. AGR6402088N) and Chair professor grant (P-20-52297) were also acknowledged.
Author information
Authors and Affiliations
Contributions
NR: Investigation, data analysis, Writing-original draft. TS: Methodology. KO: Data analysis. SB: Conceptualization, funding acquisition and editing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raju, N., Sae-leaw, T., Osako, K. et al. Improved cholesterol depletion with enhanced astaxanthin and polyunsaturated fatty acids of lipid from Pacific white shrimp cephalothorax using prior ethanolic separation of polar lipid and β-Cyclodextrin. J Food Sci Technol 59, 2255–2262 (2022). https://doi.org/10.1007/s13197-021-05238-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05238-7