Abstract
Xanthomonas citri (X. citri) is a quarentenary plant pathogen and the causal agent of the citrus canker. X. citri forms biofilms and remains fixed on the surface of plant tissues, especially on leaves and fruits. Considering this, all the citrus fruits have to be sanitized before they can be commercialized. NaOCl is the main sanitizer used to decontaminate fruits in the world. Due to its toxicity, treatment with NaOCl is no longer accepted by some Europe Union countries. Therefore, the aim of this work was to evaluate potassium bicarbonate (KHCO3), calcium hydroxide (Ca(OH)2), calcium hypochlorite (Ca(OCl)2) and peracetic acid (CH3CO3H) as alternatives to NaOCl for the sanitization of citrus fruit. By monitoring cell respiration and bacterial growth, we determined that peracetic acid and calcium hypochlorite exhibit bactericidal action against X. citri. Time-response growth curves and membrane integrity analyses showed that peracetic acid and calcium hypochlorite target the bacterial cytoplasmatic membrane, which is probably responsible for cell death in the first minutes of contact. The simulation of the sanitization process of citrus fruit in packinghouses showed that only peracetic acid exhibited a performance comparable to NaOCl. Among the tested compounds, peracetic acid constitutes an efficient and safer alternative to NaOCl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sanitization of equipment and food is a relevant subject to be considered in order to avoid microorganism contamination, cross-contamination and pathogen dissemination (Beltrame et al. 2012). Food sanitization also prevents the transit of etiological agents of quarentenary diseases to areas where they are not present yet. Considering this, the postharvest sanitization of fruit in packinghouses is an extremely important step in food processing, especially for those destined to be exported to other countries. In addition, since fruits will be ingested, less toxic but yet efficient sanitizers are sought (Jo et al. 2019).
Citrus production is one of the most profitable markets in the world, in which Brazil and the USA are responsible for almost half of all the global orange production (Neves et al. 2020). The Brazilian production reached 294.17 millions of orange boxes (40.8 kg) in the 2021/2022 season, according with the crop estimation survey from the Fund for Citrus Protection (Fundecitrus). Thus, citriculture has an enormous economic impact in Brazil, which represents nearly 35% of orange fruit production and 56% of the orange juice consumed in the world (Curtolo et al. 2017).
Citrus canker is a disease caused by the quarentenary pathogen Xanthomonas citri subsp. citri, a Gram-negative bacterium that can infect all the commercially important citrus cultivars around the world (Ge et al. 2015). Furthermore, there is no treatment available for the affected trees. The control of citrus canker is exerted by the application of a set of agricultural measures (risk mitigation system) with the intent to avoid the spread of X. citri to disease-free areas (Ference et al. 2018). Citrus canker has a great dispersion potential, being able to spread to new areas by the combined action of wind and rain (Bock et al. 2010). Another factor accounting for the great infection success of X. citri is the ability to form biofilms on the surface of the host plant tissues as stalks, branches, leaves, and fruits (Cubero et al. 2011; Sena-Vélez et al. 2016). Therefore, plants and fruits contaminated with the bacterium are prohibited to be commercialized, and to avoid the dissemination of the pathogen to disease free areas/countries, the post-harvest sanitization of citrus fruit are mandatory before commercialization in order to ensure the complete elimination of X. citri (Behlau et al. 2016; Zamuner et al. 2020). The postharvest sanitization of citrus fruits is mandatory by the Brazilian legislation (Brazil 2018). This legislation states that, before leaving the field, citrus fruits have to be sanitized for 2 min in a 200 ppm (0.2% v/v) sodium hypochlorite (NaOCl) solution (Brazil 2018). However, the public health concerns on the use of chlorine and chlorine-associated compounds as a sanitizer of fresh food are increasing, with some European countries not accepting its use anymore (Meireles et al. 2016). The risks for the human health caused by the presence of chlorate in food have been monitored by the European Union (EFSA 2015), leading their Council to issue the regulation EU 2018/605—European Union Delegated Commission Regulation–including now the category “Endocrine Disrupters” (EDs) in sanitizing products for use in foods intended for human consumption (European Commission 2018). This category lists a ban on the use of harmful substances, and establishes a maximum residue level that is allowed for chlorate on citrus fruits. Their risk is in part due to the possible accumulation of negative downstream byproducts, such as trihalomethanes, chloroform, and many other carcinogenic and toxic compounds, which can be generated by the reaction of NaOCl with the organic matter (São José and Vanetti 2012; Wang et al. 2013; EFSA 2015). Moreover, NaOCl is a corrosive agent that may inflict injuries to people involved in the sanitization processes (São José and Vanetti 2012).
A variety of alternative chemical sanitizers is now available, many of which have the potential to remove bacterial biofilms and/or inhibit microorganisms commonly associated with food (Beltrame et al. 2012; Jahid and Ha 2012). Among them, we mention peracetic acid, for which effectiveness against fungi and bacteria has been demonstrated (Jo et al. 2019). Neo et al. (2013) also obtained positive results when using peracetic acid for the inhibition of Escherichia coli O157:H7, Listeria monocytogenes, Salmonella spp. in mung bean sprouts. Other sanitizers that are described as antimicrobials are potassium bicarbonate (Fallir et al. 1997), calcium hydroxide (Kayali et al. 2020) and calcium hypochlorite (Wang and Kniel 2014). However, to date, none of these sanitizers have been tested in citrus sanitization. Their antibacterial action against X. citri, as well as their potential as a substitute for NaOCl are unknown matters.
The objectives of the present study were to evaluate peracetic acid, potassium bicarbonate, calcium hydroxide, and calcium hypochlorite as alternative sanitizers of citrus fruits allowed by the European Union countries. We tested their efficacy to inhibit growth and kill X. citri in vitro. We also investigated the main mechanisms of action of the compounds against this plant pathogen. Finally, by simulating the sanitization process used in commercial packinghouses, we show the potential of the suggested sanitizers as a substitute for NaOCl.
Materials and methods
Sanitizing agents
The sanitizing agents potassium bicarbonate (KHCO3), calcium hydroxide (Ca(OH)2), calcium hypochlorite (Ca(OCl)2), peracetic acid (CH3CO3H), and sodium hypoclorite (NaOCl) were all purchased from Sigma-Aldrich (Taufkirchen, Germany).
Bacterial strain and growth conditions
The bacterium used was Xanthomonas citri subsp. citri, isolate 306 (IBSBF 1594) (Schaad et al. 2006). Cells were cultivated in NYG/NYG-agar medium (Nitrogen-Yeast-Glycerol: 5 g L−1 of peptone, 3 g L−1 of yeast extract, 2% glycerol; for solid medium bacterial agar was added to 15 g L−1) at 29 ± 1 ºC, under agitation at 200 rpm for liquid cultures (Zamuner et al. 2020). Cell numbers per volume were determined by colony counting on plate, always relating the CFU mL−1 values to the absorbance of the liquid by using a spectrophotometer Beckman Coulter DU 730 (Brea, USA). For all the assays described in this work, cells were collected in the exponential growth phase (OD600nm ~ 1.0), and if necessary diluted before using.
Inhibitory concentration (IC) and minimum bactericidal concentration (MBC)
Inhibitory concentration was used to express the potency of the sanitizers to preclude X. citri growth, based on the Resazurin Microtiter Assay (REMA) described by Silva et al. (2013). Briefly, test sanitizers were added to the wells of a 96-multiwell plate to give the final concentrations of 100, 50, 25, 12.5, 6.25, 3.15, 1.6, or 0.8 ppm. The volume of each well was adjusted to 100 µL using NYG medium. The negative control was NYG medium without any sanitizer, the positive control was NYG medium containing 20 µg mL−1 kanamycin, and the vehicle control was 50% (v/v) NYG medium in deionized water. X. citri was then inoculated to the final concentration of 105 CFU/well (Optical Density—OD600nm of ~ 0.10). Plates were incubated for 16 h at 29 ± 1 ºC. Next, 15 µL of 0.1 mg mL−1 resazurin Sigma-Aldrich (Taufkirchen, Germany) was added to each well, followed by further two hours incubation at 29 ± 1 ºC. The percentage of viable cells was determined based on their ability to reduce the blue dye resazurin to the pink fluorescent compound resorufin, which was monitored using the plate reader Synergy H1N1 BioTek (Winooski, USA) with excitation and emission wavelengths set to 530 and 590 nm, respectively. Data was used to generate dose–response curves, and polynomial regression was employed to calculate the Inhibitory Concentrations (IC) of the compounds (Morão et al. 2019). MBC was used to verify if the sanitizers show bactericidal or bacteriostatic action. MBC test was accomplished by transferring samples from the REMA test to NYG-agar plates (before the development with resazurin), using a 96-replicator (8 × 12 Sigma-Aldrich, Germany). Plates were then incubated at 29 °C for two days to verify the ability of the cells to resume growth. Three independent experiments of REMA and MBC were performed in triplicates. Statistical analyses and the preparation of graphs were done using the software Origin 8.0.
Time-response growth curve
The bactericidal effect of the sanitizers was further evaluated through growth curve experiments. Cultures of X. citri using NYG liquid medium were prepared in 24-wells microtiter plates containing the sanitizers at their bactericidal concentrations. The final volume was 1500 µL, and the starting bacterial concentration was 105 CFU mL−1 (OD600nm of ~ 0.10) in each well. The negative control did not have any sanitizer, the positive control had kanamycin at 20 µg mL−1, and the vehicle control had deionized water and NYG medium 50% (v/v). The plates were incubated in a Synergy H1N1 plate-reader BioTek (Winooski, USA) set to 29 ± 1 °C, and the constant agitation of 120 rpm. Absorbance readings at 600 nm were taken every 30 min for a time-period of 24 h. Experiment was conducted three times, and in triplicates. Data analyses and graphs were prepared using the software Origin 8.0.
Fluorescence microscopy
Fluorescence microscopy was used to investigate some of the possible mechanisms of action of the sanitizers, such as their effects on the integrity of the cell cytoplasmic membrane. Cells of X. citri were exposed to the sanitizers at their bactericidal concentrations essentially as in the REMA test: reaction volume was 100 μL, and 105 CFU/test (OD600nm of ~ 0.10) were used per treatment. However, 1.5 mL microcentrifuge tubes were used in place of the 96-multiwell plates. After 15 min of exposure, 900 μL of saline solution (0.8% NaCl) was added to each tube in order to dilute the compound and stop the contact reaction between cell/sanitizer. Next, for the membrane integrity analyses, cells were stained using the Live/Dead BacLight kit (Thermo-Scientific L7012; Waltham, USA), where SYTO9 stains all the cells, and propidium iodide (PI) stains only the cells that have damaged cytoplasmatic membranes. Untreated cells were used as negative control (cells with intact cytoplasmatic membrane), while the positive control (cells with permeabilized membranes) was generated by thermal-stress (Savietto et al. 2018). Cells were then immobilized on agarose-covered slides prior to the microscope observations. All the visualizations were done using an Olympus BX-61 microscope (Tokyo, Japan), equipped with a monochromatic OrcaFlash-2.8 camera. Image acquisition and processing was conducted using the software CellSens version 11—Olympus (Tokyo, Japan).
Fruit sanitization
The potential of the compounds to be used as sanitizers of citrus fruit was evaluated by the method described by Zamuner et al. (2020). Tahiti limes (Citrus latifolia) of approximately 5 cm in diameter were used in the tests. Cells of X. citri were cultivated in 250 mL of liquid NYG medium until the OD600nm of ~ 0.50 (108 CFU mL−1). Cells were collected by centrifugation at 6000 xg for 7 min at room temperature. The supernatant was discarded and cells were dissolved in 250 mL of 1 × phosphate buffer (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) in order to keep the bacterial concentration at 108 CFU mL−1 (OD600nm of ~ 0.50). The limes were prepared by washing with autoclaved deionized water to remove debris and dust from the field, and then allowed to dry at room temperature (~ 23 °C) for 16 h. The bacterial suspension was sprayed on the limes until the run-off point by using a manual hand-sprayer, and then left to dry at room temperature for 3 h. After, the sanitizers or 200 ppm sodium hypochlorite (positive control; NaOCl, Chemical Abstract Service number 7681–52–9 from Sigma-Aldrich Company Inc—Taufkirchen, Germany) were sprayed on the limes until the run-off point. All the sanitizers were used at their bactericidal concentration, determined in the MBC and Time-response growth curve tests. As negative control, limes were sprayed with deionized water. Limes were exposed to the test solutions for 2 min at room temperature, and after this time-period, the excess of liquid was removed with the help of an air dried (without heat) for 30 s. In order to rescue the viable bacterial cells contaminating the fruits, limes were gently hand-washed inside sterile plastic bags containing 100 mL of 1 × PBS for 5 min. The washing liquid was centrifuged at 6000 xg for 7 min, the supernatant was discarded, and the pellet dissolved in 3 mL of 1 × PBS. One hundred µL were spread onto the semi-selective medium for X. citri MGY-KCC (16 μg mL−1 Kasugamycin, 16 μg mL−1 Cephalexin, and 50 μg mL−1 Cycloheximide) (Behlau et al. 2012). Plates were incubated for 72 h at 30 ± 1 ºC to allow bacterial growth. The identity of X. citri colonies was verified by diagnostic PCR (Coletta-Filho et al. 2006), and the total number of X. citri cells was determined by cell counting (expressed as CFU mL−1) after the sanitization tests. For each treatment, five test groups were used, containing 15 limes in each group. All the data obtained were submitted to non-parametrical statistical analysis of Kruskal–Wallis (Dunn). Three independent experiments were done in triplicates and subsequently submitted to standard deviation analyses to verify experimental errors. The statistical analyses and preparation of graphs were done using the software Origin 8.0.
Results and discussion
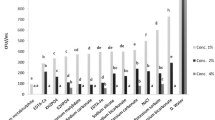
The evaluation of the bacterial growth inhibitory potential of the sanitizers by REMA showed that potassium bicarbonate was the only compound that did not exhibit any activity against X. citri cells. All the remainder, Ca(OH)2, Ca(OCl)2 and CH3CO3H, showed a dose–response pattern of inhibition, with some variations in their efficacy against X. citri (Fig. 1). Polynomial regression applied to the data enabled us to calculate the minimal concentrations of the compounds able to inhibit X. citri growth. Peracetic acid showed IC50 and IC100 of 3.10 ppm and 12.5 ppm, respectively, being the most potent growth inhibitor of X. citri. Showing an intermediate performance, calcium hypochlorite had IC50 and IC100 of 30.5 ppm and 44 ppm, respectively. Finally, calcium hydroxide was the least effective, with IC50 of 57.8 ppm and IC100 of 98.9 ppm.
Despite their good performance as growth inhibitors, only peracetic acid and calcium hypochlorite displayed bactericidal action against X. citri when used at ≥ 25 ppm and ≥ 100 ppm, respectively (Fig. 2, lane D). Beltrame et al. (2012) also found that peracetic acid at 20 ppm was bactericidal against bacteria of clinical interest, which is a result very close to ours. Calcium hydroxide was not bactericidal at the maximum concentration tested (100 ppm).
Minimum Bactericidal Concentration (MBC) test. Numbers on the right hand-side of the picture represent the concentrations of the sanitizers: 1, 100 ppm; 2, 50 ppm; 3, 25 ppm; 4, 12.5 ppm; 5, 6.25 ppm; 6, 3.15 ppm; 7, 1.6 ppm; 8, 0.8 ppm. Letters underneath: A, calcium hydroxide; B, calcium hypochlorite; C, potassium bicarbonate; D, peracetic acid; E, positive control, 20 µg mL−1 kanamycin, lines 1 to 4//Vehicle control, 50% deionized water in NYG medium, lines 5 to 8; F, negative control, untreated bacterial cells
The time-response growth curve showed that peracetic acid and calcium hypochlorite at 25 and 100 ppm, respectively, kill X. citri right at the first minutes of contact with the cells (Fig. 3). Note that the cell density, which can be inferred indirectly from the OD600nm measurements, remained unchanged throughout the whole test, and even after 24 h of exposure, no viable cell could be detected upon plating samples of the cultures on NYG-agar. Such results are promising, considering that compounds intended to be used as sanitizers are expected to act on the first few minutes of contact. Besides, food cannot be exposed for long periods to sanitizers due to their potential destructive action on fruit skin and vegetable quality (Hilgren and Salverda 2000).
Considering calcium hydroxide at 100 ppm, inhibition of X. citri cells was observed during the first 6 h of contact (Fig. 3). However, this effect ended after 6 h of exposure, and bacteria started exponential growth at approximately 8 h. This result argues against the stability and possible application of calcium hydroxide as an inhibitor of X. citri. A bacteriostatic action, such as the one shown by calcium hydroxide at 100 ppm, delays bacterial development and can potentially be applied in conjunction with other compounds that have bactericidal effect. Such practice can perhaps diminish the necessary dose of a more toxic and/or less potent bactericidal agent. However, bacteriostatic agents have to be used with extreme cautious in the food industry, since the remaining bacteria can still grow upon pressure lift and spoil the food very rapidly (Aziz and Karboune 2018).
Fluorescence microscopy was used to investigate one of the potential mechanisms of action of the sanitizers. Considering that the cytoplasmic membrane is one of the first structures contacted by the compounds, it is conceivable that attacks on it can potentially generate detectable ruptures and/or membrane permeabilization. Here, all the three active compounds (calcium hydroxide at 100 ppm, calcium hypochlorite at 100 ppm, and peracetic acid at 25 ppm) permeabilized the membrane of X. citri to Propidium Iodide (PI) (Fig. 4; compare the number of cells colored in red in panels C, D, and E with the negative control in A). PI is a nucleic acid dye that only penetrates cells with damaged cytoplasmic membranes. A quantitative analysis showed that calcium hydroxide permeabilized ~ 40% of the cells, while both calcium hypochlorite and peracetic acid induced the permeabilization of ~ 70% of the cells (Fig. 5). Despite the differences in efficacy against certain species of microorganisms, antimicrobials such as peracetic acid share a basic mechanism of action, which is the chemical oxidation of cellular components leading to similar effects of cell perturbation (Finnegan et al. 2010). In fact, H2O2, ClO−, HO− act mainly on the bacterial surface, causing rupture of the cytoplasmatic membrane and leading to cell death (Dilarri et al. 2021). Disinfectants generally have an initial action on the bacterial surface especially when they have ClO− in their chemical structure (Denyer and Stewart 1998; Dilarri et al. 2021), even though they might exert other effects internally.
Membrane integrity analysis of X. citri cells exposed to the sanitizers. Cells with intact membranes are colored in blue (A), while the cells with permeabilized membranes are colored in red (B). Panels: A negative control, untreated cells; B positive control generated by thermal stress; C Calcium hydroxide at 100 ppm; D Calcium hypochlorite at 100 ppm, and E Peracetic acid at 25 ppm. The pictures shown are an overlay of Tx Red and phase contrast images. Scale bar, 5 μm; magnification 100 ×
Percentage of X. citri cells with permeabilized membrane after 15 min of contact with the sanitizers. Negative control, untreated cells; positive control, cells with damaged membranes; 25 ppm peracetic acid; 100 ppm calcium hypochlorite; 100 ppm calcium hydroxide. The experiment was performed twice and at least 250 cells were evaluated per experiment (n > 500). Vertical bars correspond to the average percentage of permeabilized cells, and lines above the bars correspond to the standard deviation of the means
Fluorescence microscopy showed that bactericidal concentrations of peracetic acid or calcium hypochlorite permeabilized the cytoplasmatic membrane of more than 70% of the cells in up to 15 min exposure (Fig. 5). The membrane integrity analyses corroborated our MBC results for calcium hydroxide, where 60% of cells remained apparently intact when exposed to 100 ppm of the compound. Overall, our data is in line with the characteristics of the bactericidal sanitizers, which can probably alter pH and/or react with cellular chemical structures leading to a rapid destruction of cellular components following the first moments of contact (Guilhelmelli et al. 2013). Finally, we did not detect any morphological change of the cells, upon contact with the sanitizers. These changes (cell filamentation, formation of chains or the appearance of minicells) are usually associated with interferences in the cell division machinery and/or chromosome segregation processes. Taken together, our results indicate that the bactericidal effects of peracetic acid and calcium hypochlorite derive from interactions of these compounds with chemical structures of the X. citri cell surface, causing rupture on the cytoplasmic membrane and consequently the death of cells.
Treatment of Tahiti limes artificially contaminated with X. citri was used to evaluate the sanitization potential of the compounds. Treatment with peracetic acid at 25 ppm was able to disinfect the limes and showed a significant difference from the negative control in our fruit washing assays (Fig. 6). It was observed a reduction of up to 1.79 log CFU mL−1 compared to the negative control. This reduction was very close to that in the positive control, which promoted a reduction of 2.16 log CFU mL−1. Neo et al. (2013) also tested peracetic acid as a potential sanitizer for mung bean sprouts, and reported 2.3, 1.8, 2.1 and 1.1 log CFU mL−1 of reductions for Escherichia coli O157:H7, Listeria monocytogenes, Salmonella spp., and natural microflora when using the peracetic acid at 70 ppm. Purnell et al. (2014) also obtained good results in the decontamination of chicken carcasses using peracetic acid. In our tests, we did not detect significant differences in the disinfection efficacy of peracetic acid and NaOCl, which is the agent recommended by the Brazilian legislation to sanitize citrus fruit (Brazil 2018). However, by comparing between both sanitizers, NaOCl was slightly more effective (Fig. 6). Noteworthy, Neo et al. (2013) showed that peracetic acid was more efficient than sodium hypochlorite. In the studies conducted by Rybka et al. (2021) they also compare several sanitizers, including sodium hypochlorite solution, and they conclude that peracetic acid is the most effective sanitizer for biological decontamination, being better than sodium hypochlorite. Although this has not been observed in the present study, it is possible to consider that both can be efficient for citrus fruits and mung bean sprouts sanitization. Beltrame et al. (2012) also reported a rapid action of peracetic acid against the cells of Salmonella choleraesuis, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes, requiring only 2 min of contact with the acid at 20 ppm for an efficient fresh food sanitization, which was very similar to the results obtained in the present work. These results showed the efficacy of peracetic acid against X. citri cells, being this a novel and important result for citriculture. Peracetic acid can substitute NaOCl in packinghouse sanitation of citrus, and its use in food is allowed by the European Union.
Colony forming units of X. citri rescued from the surface of citrus fruits after the sanitization assays. Bars represent the averages of rescued cells; whiskers indicate the standard deviation of the means. Three independent experiments were performed. Data showing the same letters are not significantly different from each other based on the non-parametrical statistical analysis of Kruskal–Wallis (Dunn). Negative control, fruit sanitized (washed) using autoclaved deionized water; positive control, fruit sanitized with 200 ppm NaClO; peracetic acid at 25 ppm; calcium hypochlorite at 100 ppm; calcium hydroxide at 100 ppm
Although calcium hypochlorite at 100 ppm was bactericidal in our in vitro tests, this compound did not perform efficiently as a fruit sanitizer in our packinghouse simulation assay (Fig. 6). The number of cells rescued after the sanitization process using calcium hypochlorite was not significantly different from the negative control (~ 2.75 and ~ 3.25 log CFU mL−1, respectively). Perhaps if the concentration of calcium hypochlorite were higher, the results in the packinghouse simulation tests would be better. Sometimes the concentration to be applied needs to be higher than that determined as the IC derived from the in vitro assays. This higher concentration can be considered as a safe dose, because the effect of the sanitizer changes depending on the characteristics of the surface where it is supposed to act on (Beltrame et al. 2012). Finally, calcium hydroxide at 100 ppm did not prove to be a good sanitizer either. The number of cells rescued after the sanitization process with calcium hydroxide did not differ significantly from the negative control. This is in line with the fact that this compound has no bactericidal action.
Noteworthy, we did not observe any changes in color, texture, or smell of the limes after contact with any of the sanitizers evaluated. Treated limes did not show detectable alterations on their peel when compared to the untreated control. Hilgren and Salverda (2000), Neo et al. (2013), and Lepaus et al. (2020) tested peracetic acid in vegetables, mung bean sprouts, and strawberries, and in all the cases, they did not detect any alteration in the food material under study, which corroborates our results.
Conclusions
Peracetic acid and calcium hypochlorite show bactericidal action against X. citri cells. The time-response growth curves and fluorescence microscopy analyses showed that the effects of peracetic acid and calcium hypochlorite take place in the first minutes of contact. Membrane integrity analysis evidenced that the main target for these compounds is the cytoplasmatic membrane. Peracetic acid at 25 ppm was efficient as a sanitizer of citrus fruit, performing as well as NaOCl at 200 ppm. Therefore, peracetic acid can be considered as a substitute for NaOCl in the sanitization of citrus fruit in packinghouses. The use of peracetic acid in food processing is allowed by the European Union.
Availability of data and material
The data that support the findings of this study are available from the corresponding authors upon request.
References
Aziz M, Karboune S (2018) Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: a review. Crit Rev Food Sci Nutr 58:486–511
Behlau F, Canteros BI, Jones JB, Graham JH (2012) Copper resistance genes from different xanthomonads and citrus epiphytic bacteria confer resistance to Xanthomonas citri subsp. citri. Eur J Plant Pathol 133:949–963
Behlau F, Fonseca AE, Belasque J Jr (2016) A comprehensive analysis of the Asiatic citrus canker eradication programme in Sao Paulo state, Brazil, from 1999 to 2009. Plant Pathol 65:1390–1399
Beltrame CA, Kubiak GB, Lerin LA, Rottava I, Mossi AJ, Oliveira D, Cansian RL, Treichel H, Toniazzo G (2012) Influence of different sanitizers on food contaminant bacteria: effect of exposure temperature, contact time, and product concentration. Food Sci Technol 32:228–232. https://doi.org/10.1590/S0101-20612012005000046
Bock CH, Graham JH, Gottwald TR, Cook AZ, Parker PE (2010) Wind Speed Effects on the Quantity of Xanthomonas citri subsp. citri Dispersed Downwind from Canopies of Grapefruit Trees Infected with Citrus Canker. Plant Dis 94:725–736. https://doi.org/10.1094/PDIS-94-6-0725
Brazil (2018) Coordenadoria de Defesa Agropecuária do Estado de São Paulo – MAPA, Brasil. INSTRUÇÃO NORMATIVA MAPA Nº 21, DE 25 DE ABRIL DE 2018. https://www.defesa.agricultura.sp.gov.br/legislacoes/instrucao-normativa-mapa-n-21-de-25-de-abril-de-2018,1152.html
Coletta-Filho HD, Takita MA, Souza AA, Neto JR, Destefano SA, Hartung JS, Machado MA (2006) Primers based on the rpf gene region provide improved detection of Xanthomonas axonopodis pv. citri in naturally and artificially infected citrus plants. J Appl Microbiol 100:279–285. https://doi.org/10.1111/j.1365-2672.2005.02787.x
Cubero J, Gell I, Johnson EG, Redondo A, Graham JH (2011) Unstable green fluorescent protein for study of Xanthomonas citri subsp. citri survival on citrus. Plant Pathol 60:977–985
Curtolo M, Cristofani-Yaly M, Gazaffi R, Takita MA, Figueira A, Machado MA (2017) QTL mapping for fruit quality in Citrus using DArTseq markers. BMC Genomics 18:289. https://doi.org/10.1186/s12864-017-3629-2
Denyer SP, Stewart GSAB (1998) Mechanisms of action of disinfectants. Int Biodeterior Biodegradation 41:261–268. https://doi.org/10.1016/S0964-8305(98)00023-7
Dilarri G, Zamuner CFC, Mendes CR, Junior JRM, Morão LG, Montagnolli RN, Bidoia ED, Ferreira H (2021) Evaluating the potential of electrolysed water for the disinfection of citrus fruit in packinghouses. J Sci Food Agric 101:2584–2591. https://doi.org/10.1002/jsfa.10888
EFSA-Panel on Contaminants in the Food Chain (CONTAM) (2015) Risks for public health related to the presence of chlorate in food. EFSA J 13:4135. https://doi.org/10.2903/j.efsa.2015.4135
European Commission (2018) European Union Delegated Commission Regulation - (EU) 2018/605 (Endocrine Disrupters): https://eur-lex.europa.eu/eli/reg/2018/605/oj; https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02018R0605-20180420; https://ec.europa.eu/food/plant/pesticides/eu-pesticides-db_en; https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0749&rid=1
Fallir E, Grinberg S, Ziv O (1997) Potassium bicarbonate reduces postharvest decay development on bell pepper fruits. J Hortic Sci 72:35–41. https://doi.org/10.1080/14620316.1997.11515489
Ference CM, Gochez AM, Behlau F, Wang N, Graham JH, Jones JB (2018) Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol Plant Pathol 19:1302–1318. https://doi.org/10.1111/mpp.12638
Finnegan M, Linley E, Denyer SP, McDonnell G, Simons C, Maillard J (2010) Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother 65:2108–2115. https://doi.org/10.1093/jac/dkq308
Ge H, Li Y, Fu H, Long G, Luo L, Li R, Deng Z (2015) Production of sweet orange somaclones tolerant to citrus canker disease by in vitro mutagenesis with EMS. Plant Cell Tissue Org Cult 123:29–38. https://doi.org/10.1007/s11240-015-0810-7
Guilhelmelli F, Vilela N, Albuquerque P, Derengowski LS, Silva-Pereira I, Kyaw CM (2013) Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front Microbiol 4:353
Hilgren JD, Salverda JA (2000) Antimicrobial efficacy of a peroxyacetic/octanoic acid mixture in fresh-cut-vegetable process waters. J Food Sci 65:1376–1379
Jahid IK, Ha S (2012) A review of microbial biofilms of produce: Future challenge to food safety. Food Sci Biotechnol 21:299–316
Jo H, Moon H, Kim HJ, Hong JK, Park C (2019) Effect of a peroxyacetic acid mixture as green chemical on rice bacterial and fungal pathogens. J Plant Pathol 101:661–669
Kayali AY, Yamashita Y, Kawakami H, Kiyota H, Ozawa J, Nishibuchi M (2020) Development and Improvement of Methods to Disinfect Raw Beef Using Calcium Hydroxide–Ethanol–Lactate-Based Food Disinfectant for Safe Consumption. Front Microbiol 11:537889
Lepaus BM, Rocha JS, São José JFB (2020) Organic acids and hydrogen peroxide can replace chlorinated compounds as sanitizers on strawberries, cucumbers and rocket leaves. Food Sci Technol 40:242–249. https://doi.org/10.1590/fst.09519
Meireles A, Simões GE (2016) Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res Int 82:71–85. https://doi.org/10.1016/j.foodres.2016.01.021
Morão LG, Polaquini CR, Kopacz M, Torrezan GS, Ayusso GM, Dilarri G, Cavalca LB, Zielinska A, Scheffers D, Regasini LO, Ferreira H (2019) A simplified curcumin targets the membrane of Bacillus subtilis. Microbiologyopen. https://doi.org/10.1002/mbo3.683
Neo SY, Lim PY, Phua LK, Khoo GH, Kim S, Lee S, Yuk H (2013) Efficacy of chlorine and peroxyacetic acid on reduction of natural microflora, Escherichia coli O157:H7, Listeria monocyotgenes and Salmonella spp. on mung bean sprouts. Food Microbiol 36:475–480
Neves MF, Trombin VG, Marques VN, Martinez LF (2020) Global orange juice market: a 16-year summary and opportunities for creating value. Trop Plant Pathol 45:166–174. https://doi.org/10.1007/s40858-020-00378-1
Purnell G, James C, James SJ, Howell M, Corry JEL (2014) Comparison of Acidified Sodium Chlorite, Chlorine Dioxide, Peroxyacetic acid and Tri-Sodium phosphate spray washes for decontamination of chicken carcasses. Food Bioproc Tech 7:2093–2101
Rybka A, Gavel A, Kroupa T, Meloun J, Prazak P, Draessler J, Pavlis O, Kubickova P, Kratzerova L, Pejchal J (2021) Peracetic acid-based disinfectant is the most appropriate solution for a biological decontamination procedure of responders and healthcare workers in the field environment. J Appl Microbiol. https://doi.org/10.1111/jam.15041
São José JFB, Vanetti MCD (2012) Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enterica Typhimurium on cherry tomatoes. Food Control 24:95–99. https://doi.org/10.1016/j.foodcont.2011.09.008
Savietto A, Polaquino CR, Kopacz M, Scheffers D, Marques BC, Regasini LO, Ferreira H (2018) Antibacterial activity of monoacetylated alkyl gallates against Xanthomonas citri subsp. citri. Arch Microbiol 200:929–937. https://doi.org/10.1007/s00203-018-1502-6
Schaad NW, Postnikova E, Lacy G, Sechler A, Agarkova I, Stromberg PE, Stromberg VK, Vidaver AK (2006) Emended 422 classification of xanthomonad pathogens on citrus. Syst Appl Microbiol 29:690–695. https://doi.org/10.1016/j.syapm.2006.08.001
Sena-Vélez M, Redondo C, Graham JH, Cubero J (2016) Presence of extracellular DNA during biofilm formation by Xanthomonas citri subsp. citri strains with different host range. PLoS ONE 11:0156695. https://doi.org/10.1371/journal.pone.0156695
Silva IC, Regasini LO, Petronio MS, Silva DH, Bolzani VS, Belasque J, Sacramento LV, Ferreira H (2013) Antibacterial activity of alkyl gallates against Xanthomonas citri subsp. citri. J Bacteriol 195:85–94
Wang C, Chen Y, Xu Y, Wu J, Xiao G, Zhang Y, Liu Z (2013) Effect of dimethyl dicarbonate as disinfectant on the quality of fresh-cut carrot (Daucus carota L.). J Food Process Preserv 37:751–758
Wang Q, Kniel KE (2014) Effectiveness of calcium hypochlorite on viral and bacterial contamination of alfalfa seeds. Foodborne Pathog Dis 11:759–768
Zamuner CFC, Dilarri G, Cavalca LB, Behlau F, Silva TG, Bacci M Jr, Ferreira H (2020) A cinnamaldehyde-based formulation as an alternative to sodium hypochlorite for post-harvest decontamination of citrus fruit. Trop Plant Pathol 45:701–709. https://doi.org/10.1007/s40858-020-00338-9
Acknowledgements
G. Dilarri received a PhD scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/07306-9). This work was funded by FAPESP (Grant 2015/50162-2) and INCT Citros (FAPESP 2014/50880-0 and CNPq 465440/2014-2).
Funding
G. Dilarri received a PhD scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/07306–9). This work was funded by FAPESP (Grant 2015/50162–2) and INCT Citros (FAPESP 2014/50880–0 and CNPq 465440/2014–2).
Author information
Authors and Affiliations
Contributions
G.D.: conceptualization, methodology, validation, formal analysis, writing original draft; C.F.C.Z.: conceptualization, methodology, formal analysis, validation; M.B.Jr.: conceptualization, methodology, resources; H.F.: conceptualization, visualization, writing/review and editing, supervision, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dilarri, G., Zamuner, C.F.C., Bacci, M. et al. Evaluation of calcium hydroxide, calcium hypochlorite, peracetic acid, and potassium bicarbonate as citrus fruit sanitizers. J Food Sci Technol 59, 1739–1747 (2022). https://doi.org/10.1007/s13197-021-05185-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-021-05185-3