Abstract
A peroxyacetic acid mixture (CH3CO3H) has been used as a disinfectant for a low environmental impact. In this study, we investigated whether a commercial peroxyacetic acid mixture, Perosan, can be applied as a green chemical to control bacterial and fungal pathogens, Xanthomonas oryzae pv. oryzae (Xoo) and Rhizoctonia solani in rice plants. Paper discs carrying 25% Perosan caused obvious chlorosis on rice leaves. Ion leakage caused by the phytotoxicity was obviously observed at 5% and higher concentrations of Perosan. Perosan treatment resulted in a slightly decreased pH in rice growing water. In a bacterial growth inhibition assay using optical density measurements, 0.007% of Perosan almost completely inhibited the Xoo growth for 36 h. In an agar dilution assay for antifungal activity, mycelial growth inhibition of R. solani was observed with 0.05% addition of Perosan. In planta antimicrobial activity assay with 5% of Perosan inhibited the lesion lengths in Xoo-inoculated rice plants. A spray of 0.5% Perosan significantly inhibited the lesion developments on the leaves inoculated with an agar disc of R. solani. Our results suggest that Perosan can be applied to rice plants to control the pathogens, Xoo and R. solani, as a green chemical.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green chemistry, also called sustainable chemistry, aims to minimize the flow of hazardous chemicals into the environment. To this end, the chemical industry certainly ensures that chemical processes are not toxic and do not generate the residues that contain contaminants. Also, the industry should use degradable raw materials (Mestres 2005). The incorporation of green chemistry strategies in sustainable agriculture could lead to the release of non-toxic and degradable substances that do not contaminate the environment.
A peroxyacetic acid mixture (CH3CO3H), which is the reaction product from acetic acid (CH3CO2H) and hydrogen peroxide (H2O2), is very reactive and quickly decomposes to acetic acid, oxygen, and water (Beuchat et al. 2004; Carrasco et al. 2011). A peroxyacetic acid mixture has been used for disinfecting the wastewater from a sewage treatment plant (Arturo-Schaan et al. 1996; Bonadonna et al. 1999; Meyer 1976). Afterwards, because none of the products formed during its degradation process are harmful, possible application of the peroxyacetic acid was examined for sustainable aquaculture. For example, peroxyacetic acid mixture application inhibited the growth of the bacterial fish pathogens, Aeromonas salmonicida and Yersinia ruckeri (Meinelt et al. 2015) and it successfully managed the parasite, Ichthyophthirius multifiliis (Picon-Camacho et al. 2012). It has also been applied as a disinfectant for agricultural purposes (Beuchat et al. 2004; Carrasco et al. 2011; Carrasco and Urrestarazu 2010). For instance, its treatment significantly reduced the population of the Escherichia coli O157:H7 and the Listeria monocytogenes inoculated on romaine lettuce leaves and shredded the iceberg lettuce, respectively (Beuchat et al. 2004; Keskinen and Annous 2011). It was also successfully applied to control seed transmission of bacterial fruit blotch caused by Acidovorax avenae on watermelon (Hopkins et al. 2003) and bacterial leaf spot and pith necrosis caused by Xanthomonas perforans on tomato (Cinquerrui et al. 2014).
Recently, the excessive and widespread use of agricultural chemicals to control plant diseases has caused significant public concerns because of the risk of poisoning humans and domestic animals, its harmful effects on beneficial insects, the ecological disturbance at the micro-organism level, and being a cause of water pollution (Liu et al. 2015; Moss 2008). Therefore, researchers have tried to develop less toxic and less harmful green chemicals for sustainable agriculture. Presently, these efforts are found in the cultivation of rice, one of the most important food crops. Rice production in the world comes at a significant environmental expense. Rice production in irrigated fields uses almost a third of the Earth’s available fresh water (Bouman 2009; Norton et al. 2017), which is being significantly polluted by agricultural chemicals. Therefore, it is urgent to develop green chemicals that do not pollute the irrigation water utilized for rice production.
To our knowledge, the available studies on the peroxyacetic acid mixture as a chemical to control plant pathogens are still limited. Hence, the purpose of this study was to determine whether a peroxyacetic acid mixture, which has been successfully used for aquaculture, can be also applied to sustainable agriculture for rice production. We examined if a commercial peroxyacetic acid mixture, Perosan, exhibits enough antimicrobial activity to control the bacterial pathogen, Xanthomonas oryzae pv. oryzae PXO99 and the fungal pathogen, Rhizoctonia solani AG1-1A, in rice. These strains were selected because they are representative strains for Xoo and R. solani, respectively (Nadarajah et al. 2017; Salzberg et al. 2008; Taheri et al. 2007).
Materials and methods
Peroxyacetic acid mixture
The commercially available peroxyacetic acid mixture Perosan (peroxyacetic acid 4%, hydrogen peroxide 27%) (Daesung C&S Ltd) was used throughout the experiments.
Plant and pathogen materials
The rice cultivar Kitaake (Oryza sativa L. ssp. japonica) was generously provided by Prof. Pamela Ronald (University of California Davis, USA). Six to eight pots with three rice plants per pot were cultivated in a plastic tub under irrigated paddy field-mimicking conditions with standing water on the soil surface. Rice plants were maintained in the greenhouse facility at Sejong University in Korea. The bacterial and the fungal strains used in this study were Xanthomonas oryzae pv. oryzae PXO99 (Xoo) (Salzberg et al. 2008) and Rhizoctonia solani AG1-1A (R. solani) (Nadarajah et al. 2017). Xoo was cultured on peptone sucrose agar media (PSA: peptone 10.0 g/L, sucrose 1.0 g/L, L-glutamic acid 1.0 g/L, and agar 16.0 g/L) (MB cell) containing 7.5 mg/L cephalexin at 28 °C for 2 days (Bai et al. 2000). R. solani was cultured on potato dextrose agar media (PDA: potato infusion 4.0 g/L, dextrose 20.0 g/L, and agar 15.0 g/L) (MB cell) at 28 °C for 2 days.
Phytotoxicity test
For the phytotoxicity test, sterilized paper discs, carrying 20 μl of different concentrations of Perosan (0, 0.2, 5, 10, and 25%), were placed on the fully-expanded leaves harvested from eight-week-old rice plants. The paper discs were removed after 1 h, and the leaves were maintained in a humidity container at room temperature. Chlorotic spots were photographed 18 h after the Perosan disc treatments. Ion conductivity test was conducted as described previously (Kenyon et al. 1985) with minor modifications. Detached rice leaves were cut into approximately one cm pieces and then floated on the different concentrations of Perosan (0, 1, 5, 10, and 15%). Electrolyte leakage was measured at certain time intervals (0, 1, 20, 40, and 60 min) using an Orion 3 star benchtop conductivity meter (Thermo Fisher Scientific). The pH was measured using rice plants growing in a greenhouse. Rice plants were cultivated in a plastic tub containing 10 L of tap-water in six pots (three plants per pot) with standing water on the soil surface. Perosan was added into the plastic tub, which resulted in final concentrations of 0, 0.005, 0.03, 0.05, 0.07, and 0.1%. Five minutes after the treatments, the pH values were checked using the Starter3100 pH meter (Ohaus Corporation). All phytotoxicity tests were repeated more than three times with similar results and the statistical analysis was performed using the Tukey’s HSD test.
Seedling growth analysis
Seedling growth analysis was performed as described previously (Su et al. 2016) with minor modifications. Six to seven rice seeds were germinated in a glass bottle with 20 ml of distilled water. The seedlings were incubated in a growth chamber, set on a 16 h light and 8 h dark photoperiod at 23 °C. After 2 weeks, the seedlings were fixed using a mesh sponge in the glass bottle, and the distilled water was substituted with 20 ml of different concentrations of Perosan (0, 0.001, 0.005, 0.01, 0.03, and 0.05%). The weight of the seedlings was measured once every 2 days for 6 days using a PAG213 analytical balance (Ohaus Corporation). The statistical analysis was performed using the Tukey’s HSD test. The experiments were repeated two times with similar results.
Antibacterial disc diffusion
The disc dilution method was performed as previously described (Balouiri et al. 2016). Xoo harvested from PSA media containing 7.5 mg/L cephalexin was suspended in distilled water. One hundred microliters of the Xoo suspensions (OD600 of 0.8) were spread on the PSA media containing 7.5 mg/L cephalexin using a sterile spreader. Then, 8 mm paper discs impregnated with 20 μl of different concentrations of Perosan (0, 1, 2, 4, 6, 8, and 10%) were placed in the middle of the PSA media. The inhibition zones were measured 4 days after incubation at 28 °C. The statistical analysis was performed using the Tukey’s HSD test. The experiments were repeated more than three times with similar results.
Antibacterial broth dilution
Broth dilution method was conducted as described previously (Stalons and Thornsberry 1975; Wiegand et al. 2008). One milliliter of Xoo suspension (OD600 of 1.0) was inoculated into 50 ml peptone sucrose broth media (PSB) (7.5 mg/L cephalexin) containing different concentrations of Perosan (0, 0.0005, 0.001, 0.003, 0.005, and 0.007%), and it was cultured at 28 °C in a shaking incubator at 150 rpm. The Xoo growth in the PSB media was measured after 0, 12, 18, 24, 30, and 36 h with a CO8000 Cell Density Meter (OD600) (Biochrom). This experiment was repeated three times with similar results.
Colony counting
Colony counting assay was performed as previously described (Kim et al. 2016; Sha and Zhang 2016). Xoo suspension (OD600 of 0.3) was diluted with distilled water to one-tenth and the diluted Xoo was incubated with different concentrations of Perosan diluted with distilled water (0, 0.0002, 0.0004, and 0.0006%) for 1 h. Then, Xoo were plated on the PSA media containing 7.5 mg/L cephalexin. Plates were incubated at 28 °C for 2 days, then colony forming units (CFU) were counted. The experiments were repeated three times with similar results. The statistical analysis was performed using the Tukey’s HSD test.
Antibacterial activity of Perosan in planta
Perosan effects on disease responses was conducted as described previously (Kim et al. 2016). Eight-week-old rice plants in a greenhouse were inoculated with Xoo using the scissors-dip method (Song et al. 1995). Tips of autoclaved scissors were dipped into Xoo suspensions (OD600 of 0.5) and leaf tips were cut away from the leaf. Only the top two fully expanded leaves of main tiller were inoculated. Twenty minutes after inoculation, 10 milliliters of different concentrations of Perosan (0, 2, and 5%) were sprayed on three Xoo-inoculated plants in a pot. The inoculated plants were kept in a greenhouse for 4 days, and the length from the cut leaf tip to the longest water-soaked symptom per leaf was measured as a lesion length (Song et al. 1995). Antibacterial activity experiments were repeated three times with similar results. The statistical analysis was performed using the Tukey’s HSD test.
Antifungal disc diffusion
Antifungal activity was examined in a solid media containing PDA media by disc diffusion method (Arikan 2007; Paik et al. 1998). Mycelial agar discs, which had a diameter of 6.5 mm, were cut from the margin of actively growing R. solani AG-1 colony and placed in the center of new PDA media. At 14 h after incubation at 28 °C, 8 mm paper discs impregnated with 20 μl of different concentrations of Perosan (0, 2, 4, and 8%) were placed 2 cm apart on actively growing edges of the R. solani AG-1. The plates were incubated at 28 °C over 12 h and the inhibition zones were measured. Digital pictures of each plate were obtained using a digital gel documentation system LSG1000, model GDS-200D (Korea Lab Tech). The experiments were repeated three times with similar results. The statistical analysis was performed using the Tukey’s HSD test.
Antifungal agar dilution
Agar dilution method was conducted as previously described (Stalons and Thornsberry 1975; Wiegand et al. 2008). Mycelial agar discs of 6.5 mm in diameter were cut from the margins of actively growing R. solani AG-1 and carefully placed in the center of new PDA media containing different concentrations of Perosan (0, 0.05, 0.07, 0.1, 0.15, and 0.2%). The plates were incubated at 28 °C for 2 days and fungal growth was measured. Digital pictures of each plate were obtained using a digital gel documentation system LSG1000, model GDS-200D (Korea Lab Tech). Antifungal activity experiments were repeated four times with similar results. The statistical analysis was performed using the Tukey’s HSD test.
Antifungal activity of Perosan in planta
For R. solani inoculation, rice leaves were detached from eight-week-old rice plants in a greenhouse and inoculated using the detached leaf method (Delventhal et al. 2016; Jia et al. 2013). Six detached leaves were placed on a humidity incubation box (Silicook). Mycelial agar discs that had diameters of 6.5 mm were cut from the margin of actively growing R. solani AG-1 in PDA media, and they were placed in the middle of the detached leaves. The inoculated leaves were sprayed one or three times with 5 ml/humidity incubation box of 0.5% Perosan. For a three-time treatment, the Perosan was applied 8 h after the first spraying and 24 h after the second spraying. The rice leaves were incubated in a plant growth chamber, set on a 16 h light and 8 h dark photoperiod at 23 °C, for 4 days. Digital pictures of inoculated leaves in a humidity incubation box were obtained using a digital gel documentation system LSG1000, model GDS-200D (Korea Lab Tech). The entire lesion lengths were measured using ImageJ 1.52e (Schindelin et al. 2012). The experiments were repeated four times with similar results. The statistical analysis was performed using the Tukey’s HSD test.
Results and discussion
Phytotoxicity of peroxyacetic acid mixture, Perosan, on rice plants
Most chemicals possess phytotoxic potential on plant growth. Before applying the peroxyacetic acid mixture, Perosan, as a green chemical, we examined if it causes phytotoxicity to rice plants. To maintain the physical contact of the Perosan solution on rice leaf surfaces, paper discs impregnated with Perosan were placed on detached rice leaves (Fig. 1a). Severe chlorosis and wilting were observed on the leaves that were treated with 25% Perosan. When high concentrations of Perosan were applied to rice leaves directly, the rice developed chlorosis rapidly developing pale, yellow, or yellow-white leaves. We quantified the phytotoxicity of Perosan by measuring the ion conductivity of floated leaf pieces after Perosan treatment (Fig. 1b). Increase in electrical conductivity, an indicator of ion leakage, was barely observed when the leaf pieces were treated with 0 and 1% Perosan. The conductivity values were increased at 5% and higher concentrations of Perosan. These results suggested that Perosan exhibited a concentration-dependent phytotoxicity, and less than 1% barely caused damage to the cellular structures and the ion leakage on the rice leaf discs.
Phytotoxicity and effects of Perosan treatment. a Phytotoxicity of Perosan on rice leaves. Rice leaves were photographed 18 h after Perosan treatment. The arrows indicate where the paper discs were placed. b Ion conductivity of rice leaf discs after Perosan treatments. The rice leaf pieces were floated on distilled water containing different concentrations of Perosan and ion leakage was monitored over time. c Growth of Perosan-treated rice plants. Rice seedlings were raised in dishes with distilled water for 2 weeks. After Perosan was applied to the rice seedlings, their biomasses were monitored for 6 days. d Effect of pH changes in a rice-growing tub after Perosan treatment. Perosan was added into a plastic tub containing rice-growing pots and the pH changes were monitored using a pH meter
It has been reported that peroxyacetic acid mixtures exhibited a concentration-dependent reduction in growth of all vegetative organs in hydroponic tomato plants (Hong et al. 2018; Vines et al. 2003). Therefore, we investigated the growth reduction of rice plants in a hydroponic system after the Perosan treatment (Fig. 1c). We monitored the fresh weight of rapidly growing two-week-old young seedlings, including the root and shoot systems, for 6 days. It seemed that the young seedlings in a hydroponic system were more sensitive to the phytotoxicity of Perosan, displaying a growth reduction 6 days after a 0.005% Perosan treatment. There was no significant difference in the growth reduction among the 0.005% and other higher concentrations, which were 0.01, 0.03, and 0.05%.
To examine the possible pH changes in irrigated rice fields with a Perosan treatment, 18 eight-week-old rice plants growing in six pots were prepared in a plastic tub containing 10 L of top-water. Under conditions that mimicked an irrigated rice field, the pH of water in the tub was 6.74 (Fig. 1d). After 10 ml of Perosan was added into the tub, resulting in 0.1% Perosan, the pH decreased to 5.16, which was a statistically significant decrease. As expected, these results indicated that the Perosan exhibited a weak effect on the environmental pH at relatively high doses.
In vitro antibacterial activity of Perosan
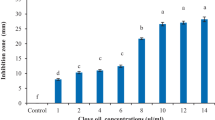
In vitro antimicrobial effectiveness of peroxyacetic acid has been reported in Enterococcus faecalis, which is a pathogenic bacterium that is normally found in human and animal intestines, (Guerreiro-Tanomaru et al. 2011) and non-pathogenic Bacillus stearothermophilus, which is found in soil, hot springs, and spoiled food products (Alasri et al. 1993; Bounoure et al. 2006). A few studies have also shown that peroxyacetic acid displays antimicrobial activity against plant pathogens. To investigate whether Perosan shows antibacterial activity, we applied Perosan to the Gram negative bacterium, Xanthomonas oryzae pv. oryzae (Xoo), which causes bacterial leaf blight of rice worldwide (Nino-Liu et al. 2006). The antibacterial test was carried out using the disc diffusion method with 100 μl of Xoo suspension (OD600 of 0.8) (Fig. 2). Xoo was spread on PSA media and paper discs containing diverse concentrations of Perosan were placed on the agar surface. The paper discs containing 1% Perosan exhibited antibacterial activity, with an inhibition zone of approximately 1.3 cm, and paper discs containing 10% Perosan almost completely inhibited the Xoo growth on the agar plates, which had inhibition zone of approximately 6.9 cm (Fig. 2a). The inhibition effect of the Xoo growth by Perosan exhibited a concentration-dependent manner (Fig. 2b). However, a relatively high concentration of Perosan, higher than 1%, was necessary for an obvious inhibition zone in our experiments. An inhibition zone in the disc diffusion method was influenced by the diffusion of antibiotics within an agar medium (Balouiri et al. 2016). We believe that a very limited amount of the Perosan was diffused in the PSA media because the Perosan was very reactive and quickly decomposed (Beuchat et al. 2004; Carrasco et al. 2011).
Antibacterial activity of Perosan using disc diffusion method. One hundred microliters of Xanthomonas oryzea pv. oryzea (Xoo) suspensions were spread on PSA Petri plates. Sterilized paper discs were impregnated with 20 μl of different concentrations of Perosan. The inhibition zones were photographed (a) and measured (b) 4 days after Perosan treatment
To determine the direct inhibitory effect on Xoo growth, broth dilution method was performed using an optical density measurement (Fig. 3a). Although 0.003% of Perosan significantly suppressed the Xoo growth at 12 h after treatment, the growth of the Xoo had fully recovered after 36 h. In contrast, 0.007% of Perosan produced no visible increase of the OD value for 36 h, which suggests that treatment with 0.007% or higher of Perosan completely inhibits Xoo growth.
Inhibition of Xoo growth and survival after Perosan treatment. a One milliliter of Xoo suspension (OD600 of 1.0) was inoculated in a peptone sucrose broth in the presence of different concentrations of Perosan and an optical density at 600 nm was monitored for 36 h. bXoo suspension was incubated in the presence of different concentrations of Perosan for 1 h, and then they were plated on PSA media. After 2 days of incubation at 28 °C, the Xoo colonies (CFU/ml) were counted as Xoo survival in the Perosan solution
Because the growth inhibition of Xoo does not indicate Xoo death, an optical density measurement cannot distinguish the bactericidal and the bacteriostatic effects. Therefore, we performed a colony counting assay in PSA media to count the live Xoo cells after incubating with Perosan diluted with distilled water for 1 h (Fig. 3b). In the absence of the Perosan, Xoo population was approximately 3.25 × 108 CFU/ml. However, a 0.0004% Perosan treatment decreased the population to 2.50 × 104 CFU/ml. None of the Xoo survival was observed after one-hour treatment with a dose of Perosan higher than 0.0006%, which indicates that the Perosan possesses strong bactericidal activity.
In vitro antifungal activity of Perosan
To further examine the antimicrobial activity of Perosan, we applied the Perosan to the plant pathogenic fungus, Rhizoctonia solani, which is responsible for sheath blight in rice. The antifungal activity was examined using the disc diffusion method (Fig. 4a). A mycelial agar disc of R. solani was placed in the center of PDA media and paper discs containing 20 μl of diverse concentrations of Perosan (0, 2, 4, and 8%) were placed on the agar’s surface. After 12 h incubation, the paper discs containing 2% Perosan exhibited antifungal activity, with inhibition zone of approximately 0.76 cm, and ones containing 20 μl of 8% Perosan significantly inhibited Xoo growth on the agar plates, with an inhibition zone of approximately 1.45 cm (Fig. 4b). Because the Perosan was very reactive and quickly decomposed (Beuchat et al. 2004; Carrasco et al. 2011), the diffusion of the Perosan within an agar medium was likely very limited, which resulted that relatively high concentrations of Perosan (2 to 8%) were necessary to observe an inhibition zone.
In agar dilution method, the front segment of the mycelia of R. solani AG-1 reached the edge of the PDA Petri dish in the absence of Perosan in 2 days (Fig. 5a). On the contrary, the fungal growth of R. solani was slightly and completely inhibited on the PDA containing 0.05% and 0.2% of Perosan, respectively. Fungal growth was inhibited in a dose-dependent manner, suggesting that Perosan is a potential antifungal agent (Fig. 5b).
In planta antimicrobial activity of Perosan
To determine the disease control efficacy of Perosan in planta, the antibacterial and the antifungal bioassay of Perosan was examined against Xoo PXO99 and R. solani AG-1 inoculated on rice leaves. Four days after spraying, leaves sprayed with 5% Perosan showed Xoo lesions of approximately 0.2 cm, shorter than the non-treated control which displayed lesions of approximately 1.6 cm (Fig. 6a and b). Using less than 5% Perosan did not show significant differences in the lesion lengths compared with the non-treated control. Considering that a very low concentration of Perosan displayed significant antibacterial activity in vitro (Fig. 3), these results suggest that the exogenously sprayed Perosan is not effectively able to come in contact the Xoo cells within the xylem vessels (Gonzalez et al. 2012).
In planta antibacterial assay of Perosan on Xoo-inoculated rice leaves. Fully-expanded rice leaves were inoculated with Xoo using the scissors-dip method. Twenty minutes after inoculation, 10 milliliters of different concentrations of Perosan (0, 2, and 5%) were sprayed at three inoculated plants in a pot. Four days after inoculation, rice leaves were harvested for photographs (a) and measurements (b). Arrows indicate bottoms of lesions
In planta antifungal activity of Perosan against R. solani was assayed on detached rice leaves using the detached leaf method (Delventhal et al. 2016; Jia et al. 2013). At 2 days after five milliliters of 0.5% Perosan treatments, the size of gray and brown necrotic lesions characteristic of Rhizoctonia infection was comparatively smaller than control leaves. After 4 days, all the control leaves displayed severe hyphal growths and lesion developments, whereas the Perosan-treated infection was restricted to small lesions (Fig. 7a and b). Repetitive treatments of Perosan, at 8 h after the first, and 24 h after the second spraying, increased the inhibition effect (Fig. 7c and d).
In planta antifungal assay of Perosan against R. solani on rice leaves. Detached rice leaves were inoculated with mycelial agar discs and then sprayed with 5 ml of 0.5% Perosan once at 0 h (a and b) or three times at 0, 8, and 24 h after inoculation (c and d). The symbol ‘-’ corresponds to control plants that were sprayed with distilled water, ‘+’ corresponds to plants that were sprayed once with Perosan, and ‘+++’ corresponds to plants that were sprayed three times with Perosan. Hyphal growth and the lesion formation were photographed (a and c) and quantified (b and d) at 0, 2, and 4 days after incubation
Conclusion
In summary, the peroxyacetic acid mixture, Perosan, exhibited in vitro and in planta antimicrobial activity against rice bacterial leaf blight and fungal sheath blight caused by Xoo and R. solani, respectively. However, as a chemical to control plant pathogens, Perosan also has limits; relatively high concentration and multiple applications were necessary. To overcome these limitations, various application methods need to be studied in the future. For example, recent study revealed that combined application of peroxyacetic acid mixture and commercially available fungicides displayed enhanced antifungal activity against Botrytis cinerea in vitro (Ayoub et al. 2017). To the best of our knowledge, this paper is the first one that describes the treatment of a peroxyacetic acid mixture on rice plants against these pathogens, which could reduce the negative impact of agricultural chemicals on the environment. It is possible that future in planta studies on the combination effect of Perosan with commercially available agricultural chemicals may allow farmers to minimize the total amount of toxic chemicals that are applied to crops.
References
Alasri A, Valverde M, Roques C, Michel G, Cabassud C, Aptel P (1993) Sporocidal properties of peracetic acid and hydrogen peroxide, alone and in combination, in comparison with chlorine and formaldehyde for ultrafiltration membrane disinfection. Can J Microbiol 39:52–60
Arikan S (2007) Current status of antifungal susceptibility testing methods. Med Mycol 45:569–587. https://doi.org/10.1080/13693780701436794
Arturo-Schaan M, Sauvager F, Mamez C, Gougeon A, Cormier M (1996) Use of peracetic acid as a disinfectant in a water-treatment plant: effect on the plasmid contents of Escherichia coli strains. Curr Microbiol 32:43–47
Ayoub F, Ben Oujji N, Chebli B, Ayoub M, Hafidi A, Salghi R, Jodeh S (2017) Antifungal effectiveness of fungicide and peroxyacetic acid mixture on the growth of Botrytis cinerea. Microb Pathog 105:74–80
Bai J, Choi SH, Ponciano G, Leung H, Leach JE (2000) Xanthomonas oryzae pv. oryzae avirulence genes contribute differently and specifically to pathogen aggressiveness. Mol Plant-Microbe Interact 13:1322–1329
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79
Beuchat LR, Adler BB, Lang MM (2004) Efficacy of chlorine and a peroxyacetic acid sanitizer in killing Listeria monocytogenes on iceberg and Romaine lettuce using simulated commercial processing conditions. J Food Prot 67:1238–1242
Bonadonna L et al (1999) Reduction of microorganisms in sewage effluent using hypochlorite and peracetic acid as disinfectants. Cent Eur J Public Health 7:130–132
Bouman B (2009) How much water does rice use. Rice Today 8:28–29
Bounoure F, Fiquet H, Arnaud P (2006) Comparison of hydrogen peroxide and peracetic acid as isolator sterilization agents in a hospital pharmacy. Am J Health Syst Pharm 63:451–455
Carrasco G, Urrestarazu M (2010) Green chemistry in protected horticulture: the use of peroxyacetic acid as a sustainable strategy. Int J Mol Sci 11:1999–2009
Carrasco G, Moggia C, Osses IJ, Alvaro JE, Urrestarazu M (2011) Use of peroxyacetic acid as green chemical on yield and sensorial quality in watercress (Nasturtium officinale R. Br.) under soilless culture. Int J Mol Sci 12:9463–9470
Cinquerrui A, Abriano S, Dimartino MA, Myrta A, Vitale A, Cirvilleri G, Polizzi G (2014) Efficacy of peracetic acid and hydrogen peroxide in controlling bacterial spot and pith necrosis caused by Xanthomonas perforans on tomato. Atti Giornate Fitopatologiche 2:443–446
Delventhal R, Loehrer M, Weidenbach D, Schaffrath U (2016) Inoculation of rice with different pathogens: sheath blight (Rhizoctonia solani), damping off disease (Pythium graminicola) and barley powdery mildew (Blumeria graminis f. sp. hordei). Bio-protocol 6:e2070
Gonzalez JF, Degrassi G, Devescovi G, De Vleesschauwer D, Hofte M, Myers MP, Venturi V (2012) A proteomic study of Xanthomonas oryzae pv. oryzae in rice xylem sap. J Proteome 75:5911–5919
Guerreiro-Tanomaru JM, Morgental RD, Faria-Junior NB, Berbert FL, Tanomaru-Filho M (2011) Antibacterial effectiveness of peracetic acid and conventional endodontic irrigants. Braz Dent J 22:285–287
Hong JK et al (2018) Reduced bacterial wilt in tomato plants by bactericidal Peroxyacetic acid mixture treatment. Plant Pathol J 34:78–84
Hopkins DL, Thompson CM, Hilgren J, Lovic B (2003) Wet seed treatment with peroxyacetic acid for the control of bacterial fruit blotch and other seedborne diseases of watermelon. Plant Dis 87:1495–1499
Jia Y, Liu G, Park DS, Yang Y (2013) Inoculation and scoring methods for rice sheath blight disease. Methods Mol Biol 956:257–268. https://doi.org/10.1007/978-1-62703-194-3_19
Kenyon WH, Duke SO, Vaughn KC (1985) Sequence of effects of acifluorfen on physiological and ultrastructural parameters in cucumber cotyledon discs. Pestic Biochem Physiol 24:240–250
Keskinen LA, Annous BA (2011) Efficacy of adding detergents to sanitizer solutions for inactivation of Escherichia coli O157:H7 on Romaine lettuce. Int J Food Microbiol 147:157–161
Kim SI, Song JT, Jeong JY, Seo HS (2016) Niclosamide inhibits leaf blight caused by Xanthomonas oryzae in rice. Sci Rep 6:21209
Liu J, Zhang L, Zhang Y, Deng H (2015) Trade-off between water pollution prevention, agriculture profit, and farmer practice--an optimization methodology for discussion on land-use adjustment in China. Environ Monit Assess 187:4104
Meinelt T, Phan TM, Behrens S, Wienke A, Pedersen LF, Liu D, Straus DL (2015) Growth inhibition of Aeromonas salmonicida and Yersinia ruckeri by disinfectants containing peracetic acid. Dis Aquat Org 113:207–213
Mestres R (2005) Green chemistry--views and strategies. Environ Sci Pollut Res Int 12:128–132
Meyer E (1976) Disinfection of sewage waters from rendering plants by means by peracetic acid. J Hyg Epidemiol Microbiol Immunol 21:266–273
Moss B (2008) Water pollution by agriculture. Philos Trans R Soc Lond Ser B Biol Sci 363:659–666
Nadarajah K, Mat Razali N, Cheah BH, Sahruna NS, Ismail I, Tathode M, Bankar K (2017) Draft genome sequence of Rhizoctonia solani anastomosis group 1 subgroup 1A strain 1802/KB isolated from rice. Genome Announc 5. https://doi.org/10.1128/genomeA.01188-17
Nino-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7:303–324
Norton GJ, Travis AJ, Danku JMC, Salt DE, Hossain M, Islam MR, Price AH (2017) Biomass and elemental concentrations of 22 rice cultivars grown under alternate wetting and drying conditions at three field sites in Bangladesh. Food Energy Secur 6:98–112
Paik SB, Sim SC, Ku HM, Yoe WG (1998) Screening for antifungal medicinal plants against brown patch and large patch diseases of turfgrass. Korean Turf Sci 12:183–194
Picon-Camacho SM, Marcos-Lopez M, Bron JE, Shinn AP (2012) An assessment of the use of drug and non-drug interventions in the treatment of Ichthyophthirius multifiliis Fouquet, 1876, a protozoan parasite of freshwater fish. Parasitology 139:149–190
Salzberg SL et al (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics 9:204
Schindelin J et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Sha J, Zhang C (2016) Antibacterial activity identification of pCM19 and pCM12 derived from hGlyrichin. Springerplus 5:1382. https://doi.org/10.1186/s40064-016-3025-4
Song WY et al (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270:1804–1806
Stalons DR, Thornsberry C (1975) Broth-dilution method for determining the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother 7:15–21
Su S, Zhou X, Liao G, Qi P, Jin L (2016) Synthesis and antibacterial evaluation of new sulfone derivatives containing 2-Aroxymethyl-1,3,4-oxadiazole/thiadiazole moiety. Molecules 22. https://doi.org/10.3390/molecules22010064
Taheri P, Gnanamanickam S, Hofte M (2007) Characterization, genetic structure, and pathogenicity of Rhizoctonia spp. associated with rice sheath diseases in India. Phytopathology 97:373–383
Vines JRL, Jenkins PD, Foyer CH, French MS, Scott IM (2003) Physiological effects of peracetic acid on hydroponic tomato plants. Ann Appl Biol 143:153–159
Wiegand I, Hilpert K, Hancock RE (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175
Acknowledgments
We thank Mr. Todd Tate (Sejong University, Korea) for critically reading this manuscript.
Funding
This study was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries through the Agri-Bio industry Technology Development Program (315091–03 and 316087–4) and the Advanced Production Technology Development Program (115051–2), funded by MAFRA, Korea.
Author information
Authors and Affiliations
Contributions
HJ, HM, HJK, JKH, and CJP conceived and designed the experiments. HJ and HM performed the experiments and analyzed the data. HJ, HM, JKH, and CJP wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors HJ, HM, HJK, JKH, and CJP declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jo, H., Moon, H., Kim, H.J. et al. Effect of a peroxyacetic acid mixture as green chemical on rice bacterial and fungal pathogens. J Plant Pathol 101, 661–669 (2019). https://doi.org/10.1007/s42161-019-00260-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-019-00260-3