Abstract
Jackfruit seeds are an underestimate residue having important biological activity such as anti-inflammatory, cytotoxicity and antimicrobial effects. However few researches have been done for this material using alternative extraction technologies, so this study aimed to evaluate the extraction of triterpenes and sterols from jackfruit seed by applying high- and low-pressure techniques. Response surface methodology (RSM) was used to determine the best conditions of pressure, temperature and CO2 flow rate for extraction with supercritical CO2. The yield and profile of these compounds were compared with the low pressure technique, which was considered as a reference. In vitro biological tests of anti-inflammatory activity and cytotoxicity in L929 and RAW 264.7 cells were also performed. The best extraction conditions in SFE for sterols were 40 °C/20 MPa/4 mL min−1 (0.832 ± 0.007 mgSR g−1sample) and 40 °C/20 MPa/3 mL min−1 (0.800 ± 0.009 mgSR g−1sample), for triterpenes were 50 °C/12 MPa/4 mL min−1 (1.501 ± 0.004 mgTT g−1sample) and 45 °C/9.3 MPa/3.5 mL min−1 (1.485 ± 0.004 mgTT g−1sample). No cytotoxic activity was detected in L929 cells in the extracts obtained from ethanol up to concentration of 100 μg mL−1 of extract. The Pearson's coefficient indicated that the reduction in cell viability was related to the concentration of triterpenes. Anti-inflammatory assays showed that some extracts could inhibit the inflammatory action induced in RAW 264.7 cells at concentration of 30 μg mL−1 of extract. Our results justify the further exploration of these characteristics to obtain natural products for the pharmaceutical and food industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Efforts have increased in recent years to characterize and utilize compounds of vegetal origin that have biological activities able to replace or improve upon the characteristics of synthetic compounds currently used in industrial applications (Baldino and Reverchon 2018; Pandey and Kaur 2018). Supercritical fluid extraction (SFE) technology has strong potential for obtaining molecules with biological activity, because it is possible to selectively solubilize the compounds of interest without the use of organic solvents and has been explored in several studies that indicate the high selectivity and purity of its extracts (Brunner 1994; Reverchon and De Marco 2006; Taylor 1996).
The selectivity of SFE can be improved by limiting co-extracted compounds if the mechanisms acting on the plant matrix and the appropriate choice of pressure and temperature are defined (Baldino and Reverchon 2018). In addition to improving selectivity, SFE allows to separate/isolate compounds by fractional extraction (multistep extraction at increasing pressures) or fractional separation (multistep separation at increasing pressures) and these strategies may represent a possibility to improve the quality of the extracted products (Baldino and Reverchon 2018; Brunner 1994; Taylor 1996).
The Response Surface Methodology (RSM) is a tool that contributes to process optimization, and highlights the most efficient conditions and possible interactions between experimental variables. It has been used to optimize pharmaceutical, microbiological processes (production of enzymes and metabolites) and has become important to improving system performance (Oludemi et al. 2018; Pandey and Kaur 2018). In addition, it has been used by several authors to determine the extraction of bioactive compounds, including in SFE (Domingues et al. 2013; Martins et al. 2016; Nyam et al. 2009; Oludemi et al. 2018; Pandey and Kaur 2018).
The genus Artocarpus belongs to the Moraceae family, which consists of 37 genera and about 1100 species (Pereira and Kaplan 2013). Artocarpus heterophyllus (jackfruit) is a plant native to Southeast Asia, and it is now distributed throughout many tropical countries. Jagtap and Bapat (2010) conducted a very complete review of the studies and main uses of plants of the genus Artocarpus, while Swami et al. (2012) and Baliga et al. (2011) presented a more specific review of Artocarpus heterophyllus, highlighting the various parts of this plant and its main opportunities for use.
However, few studies on the seeds of this plant have been performed, and those found in the literature generally explore conventional extraction techniques, such as Soxhlet (Nagala et al. 2015, 2013; Nagala and Tamanam 2017; Patel and Patel 2011) and maceration (Zzaman 2012) with such solvents as ethanol, hexane, methanol and petroleum ether. Apart from physicochemical characterization, these studies evaluated extraction yields, antioxidant activity, antimicrobial activity and chemical profiles of the extracts. The only report on use of a high-pressure technique is by Tramontin et al. (2019) who used SFE with CO2 as solvent and evaluated the yield of extracts and their in vitro properties, such as antioxidant activity, antimicrobial activity and photoprotective activity.
Extracts from Artocarpus heterophyllus are rich in sterols and triterpenos (Sharma et al. 2015; Srinivasan and Kumaravel 2016). The differentiation between the formation of sterols and triterpenes occurs through squalene biosynthesis in relation to conformation of carbon chains and enzymatic reactions that occur during the process (Dewick 2009). Phytosterols, which have been reported to minimize the risk of coronary heart diseases, are also helpful in preventing several types of cancer, including ovarian, prostate and breast cancers (Woyengo et al. 2009). Triterpenic acids are classified as useful for the development of new multi-targeting bioactive agents. They are reported to have anti-inflammatory, antimicrobial, antiviral, anti-HIV, antitumor, antimalarial, anticancer, and cytotoxic properties, as well as cardiovascular effects (Domingues et al. 2013; Pandey and Kaur 2018).
Due to the aforementioned, the compounds present in jackfruit seeds may have important biological activities. However, there is little research applying alternative extraction techniques to obtain extracts from this raw material, therefore, the objective of this research was to apply SFE to Artocarpus heterophyllus seeds, using RSM to determine the best pressure and temperature binomial to maximize total extraction yield (ηTotal), total sterol extraction yield (ηTotalSterols) and total triterpene extraction yield (ηTotalTriterpenes) comparing high and low pressure extraction methods to evaluate variations in the activities of the extracts obtained under different conditions and extraction techniques.
Material and methods
Raw material and sample preparation
The Artocarpus heterophyllus fruits used in this study came from farmers in the region of Curitiba, PR, Brazil. The variety employed was “soft type”, which consists of a smaller fruit with fibrous, soft and sweet pods (Baliga et al. 2011; Swami et al. 2012).
The seeds were dried in a laboratory dryer (CE 220/216, Cienlab, Campinas, SP, Brazil) at a temperature of 45 ºC for 96 h. The dried jackfruit seeds were ground in a knife mill (MA580, Marconi, Piracicaba, SP, Brazil), and the mean particle size was estimated by particle size distribution in a Tyler series set of sieves (45–140 mesh) (A Bronzinox, São Paulo, SP, Brazil). The samples were stored in the freezer compartment of a domestic refrigerator (BRM39, Brastemp, São Bernardo do Campo, SP, Brazil) until the extractions were performed. The moisture was determined according to the AOAC Official Method (2005) (method 925.10) whereby a sample mass of 2 g was dried in a forced convection dryer (CE 220/216, Cienlab, Campinas, SP, Brazil) at 103 °C until a constant mass was obtained.
Low-pressure extraction (LPE)
Soxhlet extraction (SOX) was performed following the AOAC Official Method (2005) (method 920.39). A sample:solvent ratio of 1:30 w/w was used, and extraction was conducted in a Sohxlet apparatus for 6 h.
Maceration extraction (MAC) was performed according Mazzutti et al. (2012), using a sample:solvent ratio of 1:5 w/v for 196 h with daily manual stirring at room temperature (25 °C).
Ultrasound-assisted extraction (UAE) was performed according to the method adapted from Luque-García and Luque De Castro (2003), using a sample:solvent ratio of 1:30 w/w. The ultrasound equipment with a probe (DES5000, Unique, São Paulo, SP, Brazil) was used for 4 min at 70% power (maximum power—500 W) and a frequency of 20 kHz.
The low-pressure extraction (LPE) was performed in triplicate using the solvents hexane (HEX) and ethanol (EtOH) with Rohrschneider polarity indexes of 0 and 5.2, respectively (Kumoro et al. 2009).
Supercritical fluid extraction (SFE)
The SFE experiments were performed at laboratory scale using a SFE and chromatography unit (LC 2000, Jasco, Hachioji, Tokyo, Japan) described by Tramontin et al. (2019). The volumetric flow rate of liquid CO2, pressure and temperature were kept constant during the extraction time. It was then pressurized with a positive displacement HPLC pump (PU-2087/2087 Plus, Jasco, Hachioji, Tokyo, Japan). About 7 g of the sample were placed in a column to form the fixed particle bed. The extraction time was defined from preliminary tests and by evaluation of the extraction curve, and extraction was maintained until the diffusive phase (150 min). CO2 was used as a solvent (99.99%, White Martins, Florianópolis, SC, Brazil). The sample collection of extracts was performed according to Taylor (1996), consisting of an offline collection system or “trapping”.
Post-processing of samples
The extracts obtained using the LPE techniques were separated from the solvent with a rotary evaporator (R-100, BUCHI, Flawil, St. Gallen, Switzerland). According to Martins et al. (2016) the total extraction yield (ηTotal), as expressed in mg of extract per g of sample (mgextract g−1sample). The same concept was used to calculate the total yield of extracted sterols (ηTotalSterols), as expressed in mg of sterols per g of sample (mgSR g−1sample), and total yield of extracted triterpenes (ηTotalTriterpenes), as expressed in mg of triterpenes per g of sample (mgTT g−1sample), according to Eqs. 1–3.
where wextract represents the mass of extract in mg, wSterols represents the total mass of sterols extracted in mg, wTriterpenes represents the mass of total triterpenes extracted in mg, and wsample represents the mass of the dried sample used in the experiment expressed in g. The results for total extraction yield for LPE were previously presented by Tramontin et al. (2019) and were included in the tables and text to improve the discussion.
Chemical profile
The method used for the characterization of the chemical profile of the extracts was described by Tramontin et al. (2019). The analysis was performed on an Agilent GC (7890A, Agilent, Santa Clara, CA, USA) instrument coupled with the Agilent MS detector (5975C MS, Agilent, Santa Clara, CA, USA). The capillary column (HP-5MS, Agilent, Santa Clara, CA, USA) of fused silica (30 m length × 0.250 mm i.d. × 0.25 µm film thickness) composed of 5% phenyl and 95% polydimethylsiloxane was connected to a quadrupole detector operating in EI mode at 70 eV. Helium was used as the carrier gas at a flow rate of 1 mL min−1. The injector and interface (G6502B, Agilent, Santa Clara, CA, USA) temperatures were both 250 ºC with a split ratio of 1:50. The injection volume was 1 µL. The oven temperature program consisted of ramping up to 80 °C for 1 min, then increasing at a rate of 10 °C min−1 to 190 °C, and then at 5 °C min−1 to 300 °C for 15 min. Each sample was analyzed in its extraction solvent, and the SFE extracts were diluted in ethanol (HPLC grade) at a concentration of 6500 ppm.
The identification of compounds was performed by comparison with a library of spectral data NIST 11 (Standard Reference Data Series of the National Institute of Standard and Technology—Mass Spectral Library) (NIST 2018). For quantitative analysis, the internal standard method described by Sparkman et al. (2011), bornyl acetate (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was used as a reference to obtain the response factor for quantification of the peak areas.
Biological activity in vitro
Cell cultures
A L929 cell line (mouse fibroblast) was purchased from Rio de Janeiro Cell Bank (BCRJ, Rio de Janeiro, RJ, Brazil) and maintained in Dulbecco’s Minimal Essential Media (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 100 μg L−1 streptomycin and 100 IU mL−1 penicillin at 37 °C in a 5% CO2 atmosphere. Cells were seeded in 24-well plates at a density of 1 × 105 cells well−1, and when confluency was achieved, the extracts were added to the plate wells at concentrations of 50 and 100 µg mL−1 culture medium. The following extracts were evaluated with these cells by the MTT method: SOX-EtOH, MAC-EtOH, UAE-EtOH, SOX-HEX, MAC-HEX, UAE-HEX, SFE1, SFE8 and SFE16. Only 3 SFE experiments were evaluated, considering the lower and upper limits and the center point.
The murine RAW 264.7 macrophage cell line was purchased from Rio de Janeiro Cell Bank (BCRJ, Rio de Janeiro, RJ, Brazil) and maintained in DMEM supplemented with 10% FBS, 100 μg L−1 streptomycin and 100 IU mL−1 penicillin at 37 °C in a 5% CO2 atmosphere. Cells were seeded in 96-well plates at a density of 5 × 104 cells well−1, and when confluency was achieved, the extracts were added to the plate wells at a concentration of 30 µg mL−1 culture medium. These cells were submitted to a MTT cell viability assay and an assay to determine nitric oxide production (NO) in order to get an index of their potential anti-inflammatory activity. All extracts were tested with these cells under the specified conditions.
MTT assay for cell viability
This assay was conducted as described by Van de Loosdrecht et al. (1991). After 24 h of contact between extracts and cells, MTT was added to a final concentration of 0.5 mg mL−1 culture medium, and the cells were incubated for 4 h at 37 °C and 5% CO2. The medium was then removed, and the precipitate was solubilized in DMSO (dimethyl sulfoxide). The absorbance was measured at 540 nm (Infinity M-200, Tecan, Männedorf, Zurich, Switzerland). The absorbance of the control wells was referred to as 100% viability, and the optical density from the other groups was calculated as a percentage of viability. Since all extracts were solubilized in DMSO, the control cells medium was supplemented with DMSO 2.5%.
Determination of nitric oxide (NO) levels
RAW 264.7 cells were treated with 30 μg mL−1 of each extract prepared in FBS-free DMEM. After a 1 h treatment, cells were stimulated with 5 μg mL−1 of lipopolysaccharide (LPS) for 24 h. After 24 h, the supernatant was removed to measure nitrite, one of the stable metabolites of nitric oxide.
Nitrite levels were assessed using the assay described by Grisham et al. (1996). To summarize, 100 μL of culture medium were incubated for 15 min with Griess reagent (equal parts of 1% sulfanilamide in 10% phosphoric acid in 0.1% naphtyl-ethylenediamine in water). Absorbance was read at 540 nm. The amount of nitrite was calculated using a sodium nitrite (NaNO2) standard curve. The results of inhibition of nitric oxide were expressed in μmol NO mL−1 ± standard deviation.
Response surface methodology (RSM)
A central composite design (CCD) was used to evaluate the extraction of compounds, and consisted of 14 experiments and 2 center points. All runs were done in duplicate, and the value presented in the results was the mean. The statistical analysis was performed using all experimental values. The independent variables were defined by screening, as follows: temperature (36.6–53.4 °C), pressure (9.3–22.7 MPa) and CO2 flow rate (2.7–4.3 mL min−1). In this study, the RSM was applied to ηTotal, ηTotalSterols and ηTotalTriterpenes responses in order to evaluate the significance of each factor studied and to quantify and describe the impact of the combined effects of the independent variables on the dependent variables. Determination of coefficients R2 and their adjusted version, R2adj, were used to evaluate the suitability of the fit of the regression model. The factors and levels of correspondence of each independent variable are presented in Table 1.
Statistical analysis
Total extraction yield (ηTotal), total sterols extraction yield (ηTotalSterols), total triterpenes extraction yield (ηTotalTriterpenes) and the biological activities were statistically evaluated by analysis of variance (ANOVA) to detect significant differences between the results. Significant differences (p < 0.05) were analyzed with the Tukey test. The Tukey test was applied twice to evaluate the results. The first consisted in comparing the results separately, that is, the LPE results were compared only with themselves and the SFE results were compared only with themselves. The second consisted in comparing the LPE and SFE results simultaneously, that is, LPE and SFE results were compared to each other. To evaluate the relationship between the triterpene and sterol profiles and the results of biological activity, Pearson's coefficient was used. All statistical analyses were evaluated using Statistica for Windows 7.0 software (Statsoft Inc., Tulsa, OK, USA).
Results and discussion
Characterization of raw material
The granulometry distribution from sample resulted in mean particle diameter of 0.340 ± 0.116 mm and the moisture content of the sample was 10.17 ± 0.14%. This particle diameter and water content in the sample were found in all assays.
Model fitting and data analysis using RSM
The compounds of more interest in our extractions were sterols and triterpenes. However, the following compounds were also co-extracted: monoterpenes, ketones, phenylpropene, sesquiterpenes and fatty acids.
Sterols were considered as the sum of the masses of the following compounds: campesterol, stigmasterol, β-sitosterol, 9,19-cyclolanost-24-en-3-ol,(3β) and 9,19-cycloergost-24(28)-en-3-ol,4,14-dimethyl-,acetate,(3β, 4α,5α)-. Triterpenes were considered as the sum of the masses of the following compounds: squalene, tirucalol, lanosterol, lupeol, 13,27-cycloursan-3-one, and 24-methylenecycloartanone. Figure 1 shows the response surface for ηTotal, ηTotalSterols and ηTotalTriterpenes. The results obtained for the experimental planning for each experimental run are presented in Table 2. The regression coefficients obtained using coded variables for the developed model, as well as the adjusted determination coefficients (R2adj) and the determination coefficients (R2), are shown in Table 3.
An implicit factor included in the experimental design was the solvent/sample ratio, which increased the total yield since it increased the number of CO2 molecules per unit volume in the bed. This, in turn, increased the molecular interaction between CO2 and solute (Nyam et al. 2009). For example, for a flow of 2.7 mL min−1 during a 150 min extraction, 0.406 kg CO2 were used, resulting in a consumption ratio of 57.98 kg CO2 kg−1 of sample. For a solvent flow of 4.3 mL min−1, during a 150 min extraction, 0.646 kg CO2 were used with a consumption ratio of 92.33 kg CO2 kg−1 of sample. This led to a difference of 37.2% in the mass of solvent used. Consequently, we can see that the increase of the relationship between raw material and solvent can represent a positive effect on global yield.
Total extraction yield (η Total)
Table 2 presents the values obtained for the total extraction yield for LPE and SFE. The results obtained for LPE ranged from 173.13 ± 5.06 to 4.76 ± 1.71 mgextract g−1 sample and indicate that the total extraction yield was strongly influenced by the increase of extraction temperature and solvent polarity. The increase in temperature reduces the viscosity of the solvent, thereby increasing mass transfer and diffusion. The polarity of the solvent is related with dielectric constant that increases the forces of attraction between solute and solvent (Mazzutti et al. 2012; Tramontin et al. 2019).
In order to understand the relationship between the independent variables and the total yield of extractions, it was necessary to evaluate the response surface shown in Fig. 1a. The response surface indicated that maximum ηTotal was reached in 2 different situations. The first was at low temperature (35 °C) and high pressure (23 MPa). The second was at high temperature (55 °C) and low pressure (9 MPa). The lowest total yield point was at low temperature (35 °C) and low pressure (9 MPa). The ηTotal increase was present at 2 different times, owing to better extraction conditions for the triterpenes and sterols, which occurred at the extremes of pressure and will be described in the following discussion.
Equation 4 represents the mathematical model that describes how the independent variables act on the dependent variable ηTotal, considering the factors and interactions identified as statistically relevant (p < 0.05).
The mathematical model proposed to describe the total extraction yield showed that the largest positive contributions were related to the linear coefficients of CO2 flow rate, followed by pressure, temperature and quadratic temperature coefficient. The linear interaction between temperature and pressure coefficients, as well as the quadratic flow rate, had a negative effect on the ηTotal variable. The other factors and interactions were not significant, considering the confidence level of the analysis and the ranges used in the independent variables. The graphical analysis of regression model residues indicated that their range is close to expected normal values. The determination coefficient (R2) and the adjusted determination coefficient (R2adj) values for the model were 0.891 and 0.809, respectively, indicating that the values calculated for the model correctly predicted the values obtained experimentally for ηTotal and are presented in Table 3, with the regression coefficients and their p-values.
Total sterols extraction yield ( η TotalSterols )
Table 2 shows results for sterol extraction for LPE and SFE. For LPE, the SOX-HEX technique presented the highest value of ηTotalSterols at 0.913 ± 0.005 mgSR g−1sample. The extractions with HEX had better results than those with EtOH.
The response surface analysis of ηTotalSterols in Fig. 1b indicated an increase in sterol profile with increasing pressure (23 MPa) at low temperature (35 °C). The highest sterols content was 0.832 ± 0.007 mgSR g−1sample at the extraction condition of 40 °C/20 MPa/4 mL min−1, followed by the value of 0.800 ± 0.009 mgSR g−1sample at the condition of 40 °C/20 MPa/3 mL min−1, which were statistically similar and obtained under the highest CO2 pressure and density conditions. To compare the extraction efficiency of sterols, the SOX-HEX technique was used as standard, thereby in SFE it was possible to recover from 53.23%–91.13%, i.e. almost the entire content of these compounds, which was a highly satisfactory result, considering the extraction time, and the absence of organic solvents.
These results are in agreement with the studies of Nyam et al. (2009) that evaluated sterol extraction by SFE and found that the optimal point for extraction of these compounds was at pressures between 22.8 and 40.0 MPa and temperatures ranging from 30 to 42 °C. On the other hand, Sajfrtová et al. (2010) describes that the best extraction condition for β-sitosterol was 15 MPa and 40 °C. This difference in temperature and pressure may be related to the fact that the authors considered a single sterol, while the present study evaluated the extraction conditions for a variety of sterols.
Equation 5 represents the mathematical model established for the dependent variable ηTotalSterols as a function of the independent variables, considering the factors and interactions identified as statistically relevant (p < 0.05).
The mathematical model describes the extraction of sterols, it shows that temperature had a negative effect on sterol extraction for the linear model, while pressure had a positive effect and the interaction of temperature and pressure had a negative effect. The other factors and interactions were not significant, considering the confidence level of the analysis. The graphical analysis of regression model residues indicated that they were normally distributed. The determination coefficient (R2) and the adjusted determination coefficient (R2adj) values for the model were 0.857 and 0.804, respectively, indicating that the values calculated for the model correctly predicted the values obtained experimentally for ηTotalSterols and are presented in Table 3, along with the regression coefficients and their p-values.
Total triterpenes extraction yield ( η TotalTriterpenes )
Table 2 shows results for triterpenes extraction for LPE and SFE. For LPE, the MAC-HEX technique presented the highest value for triterpenes extraction at 1.413 ± 0.004 mgTT g−1sample. Extractions with HEX had better results than those with EtOH, which was probably related to the solvent/solute affinity that occurs during extraction.
For SFE, the response surface analysis of ηTotalTriterpenes in the Fig. 1c indicated that the best conditions for extraction of triterpenes obtained in this study were at higher temperature (55 °C) and lower pressure (9 MPa). The highest value obtained was 1.501 ± 0.004 mgTT g−1sample at the condition of 50 °C/12 MPa/4 mL min−1, which was statistically similar to the 1.485 ± 0.004 mgTT g−1sample obtained at the condition of 45 °C/10.4 MPa/3.5 mL min−1, both obtained under the lowest pressure conditions and at low CO2 density. This may be explained by the increased solubility of triterpenes since increasing temperature results in increasing vapour pressure of the solute. Although low CO2 density conditions were used, the effect of higher temperature extraction had more influence than pressure; consequently, higher temperatures yield higher triterpenes content (Domingues et al. 2013). When using the results of the MAC-HEX technique, to compare with SFE regarding the efficiency of triterpenes extraction, the recovery varied from 59.02 to 106.00%. This was a positive triterpene recovery rate, considering the low extraction time and that CO2 was the only solvent. The difference in the recovery may be associated with the extraction conditions and characteristics of the extracted compounds.
The results obtained are in agreement with the study of Oludemi et al. (2018) who evaluated the extraction of triterpenoids from Ganoderma lucidum by ultrasound-assisted extraction and heat-assisted extraction and determined that the best extraction temperature was 90 °C. Pandey and Kaur (2018) studied the extraction of pentacyclic triterpenoids by heat reflux and obtained the best extraction condition at 65 °C. Meanwhile, Domingues et al. (2013) evaluated the extraction of terpenic acids from Eucalyptus globulus by SFE and determined that the best extraction condition was 40 °C and 20 MPa using 5% EtOH. The difference attributed to lower temperatures and higher pressures may be related to the fact that the author defined terpenic acids as those compounds not found in jackfruit seed extract by GC/MS.
Equation 6 represents the mathematical model proposed to represent the extraction of triterpenes as a function of the independent variables, considering the factors and interactions that were identified as statistically relevant (p < 0.05).
The mathematical model presented for extraction of triterpenes showed that the linear coefficient of pressure had a negative effect, while the linear coefficient of flow rate, the quadratic coefficient of pressure and the linear coefficient of temperature had positive effects on triterpene extraction. The graphical analysis indicated that residues were normally distributed. The determination coefficient (R2) and the adjusted determination coefficient (R2adj) values for the model were 0.957 and 0.925, respectively, indicating that the values calculated for the model correctly predicted the values obtained experimentally for ηTotalTriterpenes and are presented in Table 3, together with the regression coefficients and theirs p-values.
Cell toxicity assays
Artocarpus heterophyllus has been studied for its toxicity to various cell types (Burci et al. 2018; Haryoto and Widowati 2018; Matsuo et al. 2005; Patel and Patel 2011), however the toxicity of extracts from SFE has never been evaluated.
Figure 2 shows the results for tests of the cytotoxicity of the extracts to the mouse fibroblast cell line (L929) using the MTT method. LPE extracts with EtOH, such as SOX-EtOH, UAE-EtOH and MAC-EtOH, were not toxic to L929 cells at concentrations of 50 and 100 µg mL−1 of extract. Similarly, LPE (HEX and EtOH) and SFE at a concentration of 30 µg mL−1 of extract to RAW 264.7 cells, were not toxic for these cells.
However, SOX-HEX, UAE-HEX, MAC-HEX and SFE extracts caused a reduction in cell viability with a higher concentration of extract for L929 cells. For LPE extracts with HEX, a similar reduction in cell viability occurred with an increase in the extract concentration for SOX-HEX (100–88.12%) and UAE-HEX (100–84.73%). The highest reduction in cell viability was found in MAC-HEX (84.47–25.69%). For SFE extracts, the reduction in viability occurred at SFE1 (98.52–73.66%), SFE8 (100–83.72%) and SFE16 (100–75.65%). Depending on the use of the solvent and its polarity, these findings show that different compounds were extracted. The use of HEX and SFE techniques resulted in reduced cell viability with increasing extract concentration, indicating a dose-dependent phenomenon (Haryoto and Widowati 2018).
Evaluating the results obtained by Pearson's coefficient, it was possible to observe the correlation between triterpene concentration and cytotoxicity, depending on the extract concentration tested (50–100 μg mL−1 of extract) for the LPE extracts. For the concentration of 50 µg mL−1, a correlation of −0.61 was obtained, and for the concentration of 100 µg mL−1, a correlation of −0.75 was obtained. These results are in agreement with the reports in literature on cytotoxicity of triterpenes in some cell types (Domingues et al. 2013; Pandey and Kaur 2018). Patel and Patel (2011) and Burci et al. (2018) studied the cytotoxicity of jackfruit seed extract obtained using EtOH and methanol, respectively, in normal mouse fibroblast cells (L929) and normal human embryonic kidney (HEK293) cells, but detected no toxicity. These results are in agreement with those obtained in the present study for EtOH.
The results of our study showed that the EtOH extract of jackfruit seeds had no toxicity to L929 cells up to concentrations of 100 µg mL−1 of extract; nor was toxicity a factor for RAW 264.7 cell macrophages at 30 µg mL−1 of extract. However, the literature indicates that EtOH extracts from jackfruit seeds were effective against some types cells. Therefore, they may have promising applications, but more specific studies must be performed to define the mechanisms of action of the extracts.
Determination of nitric oxide (NO) levels
The main purpose of anti-inflammatory analysis is to quantify NO inhibition because more NO is produced during immune reaction to inflammation, as has been experimentally demonstrated in humans and animals (Grisham et al. 1996).
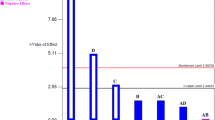
Figure 3 shows the results of NO levels for different LPE and SFE extracts obtained from jackfruit seed. To investigate the anti-inflammatory effects of Artocarpus heterophyllus, NO production was determined in the presence of the extracts, at concentration of 30 µg mL−1, in LPS-induced RAW 264.7 cells. When LPS was incubated with macrophages, nitrite generation increased from the basal level after 24 h of incubation. LPS-induced nitrite generation was, however, significantly attenuated in all Artocarpus heterophyllus extracts, except for MAC-EtOH, MAC-HEX and SFE11.
Evaluating the results obtained by Pearson's coefficient, was not observed correlation between the concentration of triterpenes, sterols and the inhibition of NO production for RAW 264.7 cells at the concentration of 30 μg mL−1 of extract. Absence of correlation might be due to the fact that compounds responsible for inhibiting NO production could be a compound or a class of compounds that were not considered in this study and were not present in MAC-EtOH, MAC-HEX and SFE11 extracts.
Fang et al. (2008) evaluated the in vitro anti-inflammatory effect of Artocarpus heterophyllus on LPS-induced RAW 264.7. The authors concluded that the inhibition of NO occurred satisfactorily, as did the results obtained by the present study. Fang et al. (2008) also concluded that the isolated bioactive compound that presented the best result was artocarpesin, a secondary metabolite, which belongs to the flavonoid class. All of the studies cited report that the anti-inflammatory effect was dependent on the dose of the concentrated extract.
Conclusion
The best extraction conditions for sterols in SFE were low temperature and high pressure (40 °C/20 MPa), which obtained values of 0.832 ± 0.007 mgSR g−1sample and 0.800 ± 0.009 mgSR g−1sample. For triterpenes, the best extraction conditions were high temperature and low pressure (50 °C/12 MPa and 45 °C/10.4 MPa), which obtained values of 1.501 ± 0.004 mgTT g−1sample and 1.485 ± 0.004 mgTT g−1sample.
The model obtained by response surface indicated that for the extraction of sterols, the CO2 flow was not a significant parameter, while for the extraction of triterpenes it had a positive influence. According to the statistical model, the variables that most contributed to the increase of ηTotal in the SFE were linear coefficients of CO2 flow rate, pressure, and temperature (linear and quadratic). The linear interaction coefficient for temperature and pressure, as well as the quadratic flow rate, had a negative effect. The sterol profile was affected negatively by temperature (linear), while pressure had a positive effect and the interaction between temperature and pressure had a negative effect. The triterpene extraction was affected negatively by the linear coefficient of pressure, while positively by the linear coefficient of flow rate. The quadratic coefficient of pressure and the linear coefficient of temperature had positive effects on triterpene extraction.
LPE extracts obtained with EtOH showed no cytotoxicity in L929 cells up to concentration of 100 μg mL−1 of extract, while those obtained with HEX had reduced cell viability at higher concentrations. Values varied between 88.12–25.69%. SFE with CO2 extracts reduced cell viability by 83.72–73.66%. The Pearson's coefficient showed that the reduction in cell viability was dependent on the concentration of triterpenes in the extracts. When evaluating the inhibition of NO in RAW 264.7 cells only the samples MAC-ETOH, MAC-HEX and SFE11 were not able to reduce levels of NO production when compared to the standard induced by LPS.
The jackfruit has numerous properties and the results obtained in this study contribute to refine and add even more information to the already existing knowledge about this species, since the extract obtained with supercritical CO2 had never been evaluated for these characteristics and open the way for countless possibilities in pharmaceutical, nutraceutical, cosmetic and food industry.
References
AOAC (2005) Association of official analytical chemist, official methods of analysis, 18, a. AOAC International, Maryland
Baldino L, Reverchon E (2018) Challenges in the production of pharmaceutical and food related compounds by SC-CO2 processing of vegetable matter. J Supercrit Fluids. https://doi.org/10.1016/j.supflu.2017.11.034
Baliga MS, Shivashankara AR, Haniadka R, Dsouza J, Bhat HP (2011) Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus lam (jackfruit): a review. Food Res Int. https://doi.org/10.1016/j.foodres.2011.02.035
Brunner G (1994) Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes, 2nd edn. Springer-Verlag, Berlin
Burci LM, da Silva CB, Rondon JN, da Silva LM, de Andrade SF, Miguel OG, de Dias Josiane FG, Miguel MD (2018) Acute and subacute (28 days) toxicity, hemolytic and cytotoxic effect of Artocarpus heterophyllus seed extracts. Toxicol Rep. https://doi.org/10.1016/j.toxrep.2018.02.006
Dewick PM (2009) Medicinal natural products - a biosynthetic approach, 3rd edn. Wiley, New York
Domingues RMA, De MMMR, Oliveira ELG, Neto CP, Silvestre AJD, Silva CM (2013) Optimization of the supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark using experimental design. J Supercrit Fluids. https://doi.org/10.1016/j.supflu.2012.12.005
Fang SC, Hsu CL, Yen GC (2008) Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus heterophyllus. J Agric Food Chem. https://doi.org/10.1021/jf800444g
Grisham MB, Johnson GG, Lancaster JR Jr (1996) Quantitation of nitrate and nitrite in extracellular fluids. Method Enzymol 268:237–246
Haryoto H, Widowati P (2018) Cytotoxicity of methanol leaf extract of Artocarpus altilis, Artocarpus heterophyllus and Artocarpus camansi against MCF7 breast cancer cells. J Nutraceuticals Herb Med 1:16–23
Jagtap UB, Bapat VA (2010) Artocarpus: a review of its traditional uses, phytochemistry and pharmacology. Int J Pharma Bio Sci. https://doi.org/10.1016/j.jep.2010.03.031
Kumoro AC, Hasan M, Singh H (2009) Effects of solvent properties on the soxhlet extraction of diterpenoid lactones from Andrographis paniculata leaves. Sci Asia 35:306–309. https://doi.org/10.2306/scienceasia1513-1874.2009.35.306
Luque-García JL, Luque De Castro MD (2003) Ultrasound: a powerful tool for leaching. TrAC - Trends Anal Chem 22:41–47. https://doi.org/10.1016/S0165-9936(03)00102-X
Martins PF, De MMMR, Sarmento P, Silva CM (2016) Supercritical fluid extraction of sterols from Eichhornia crassipes biomass using pure and modified carbon dioxide. enhancement of stigmasterol yield and extract concentration. J Supercrit Fluids 107:441–449. https://doi.org/10.1016/j.supflu.2015.09.027
Matsuo M, Sasaki N, Saga K, Kaneko T (2005) Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull 28:253–259
Mazzutti S, Ferreira SRS, Riehl CAS, Smania A, Smania FA, Martínez J (2012) Supercritical fluid extraction of Agaricus brasiliensis: antioxidant and antimicrobial activities. J Supercrit Fluids 70:48–56. https://doi.org/10.1016/j.supflu.2012.06.010
Nagala S, Tamanam RR (2017) Artocarpus methanol extract seed oils - a comparative study. Int J Pharm Sci Res 8:1781–1789. https://doi.org/10.13040/IJPSR.0975-8232.8(4).1781-89
Nagala S, Yekula M, Tamanam RR (2013) Antioxidant and gas chromatographic analysis of five varieties of jackfruit (Artocarpus) seed oils. Drug Invent Today. https://doi.org/10.1016/j.dit.2013.08.001
Nagala S, Rapaka G, Tamanam RR (2015) A comparative study of the antimicrobial activities of five varieties of essential oils from the seeds of artocarpus. J Pharm Biol Sci 10:2319–7676. https://doi.org/10.9790/3008-10611725
NIST (2018) National Institute of Standars and Technology – NIST-webbook.
Nyam KL, Tan CP, Lai OM, Long K, Man YBC (2009) Optimization of supercritical fluid extraction of phytosterol from roselle seeds with a central composite design model. Food Bioprod Process 88:239–246. https://doi.org/10.1016/j.fbp.2009.11.002
Oludemi T, Barros L, Prieto MA, Heleno SA, Barreiro MF, Ferreira ICFR (2018) Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: optimization study using the response methodology. Food Funct 9:209–226. https://doi.org/10.1039/c7fo01601h
Pandey DK, Kaur P (2018) Optimization of extraction parameters of pentacyclic triterpenoids from Swertia chirata stem using response surface methodology. 3 Biotech. https://doi.org/10.1007/s13205-018-1174-6
Patel RM, Patel SK (2011) Cytotoxic activity of methanolic extract of Artocarpus heterophyllus against A549, hela and MCF-7 cell lines. J Appl Pharm Sci 1:167–171
Pereira VDJ, Kaplan MAC (2013) Artocarpus: Um gênero exótico de grande bioatividade. Floresta e Ambient 20:1–15
Reverchon E, De Marco I (2006) Supercritical fluid extraction and fractionation of natural matter. J Supercrit Fluids 38:146–166. https://doi.org/10.1016/j.supflu.2006.03.020
Sajfrtová M, Ličková I, Wimmerová M, Sovová H, Wimmer Z (2010) β-Sitosterol: supercritical carbon dioxide extraction from sea buckthorn (Hippophae rhamnoides L.) seeds. Int J Mol Sci 11:1842–1850. https://doi.org/10.3390/ijms11041842
Sharma A, Gupta P, Verma AK (2015) Preliminary nutritional and biological potential of Artocarpus heterophyllus L. shell powder. J Food Sci Technol 52:1339–1349. https://doi.org/10.1007/s13197-013-1130-8
Sparkman DO, Penton ZE, Kitson FG (2011) Gas chromatography and mass spectrometry: a practical guide. Academic Press, Cambridge
Srinivasan K, Kumaravel S (2016) Mass spectrometry analysis of volatile constituints of jackfruit powder. Indo Am J Pharm Sci 3:331–339
Swami SB, Thakor NJ, Haldankar PM, Kalse SB (2012) Jackfruit and its many functional components as related to human health: a review. Compr Rev Food Sci Food Saf 11:565–576. https://doi.org/10.1111/j.1541-4337.2012.00210.x
Taylor LT (1996) Supercritical fluid extraction. Wiley, New York
Tramontin DP, Cadena-Carrera SE, Bella-Cruz A, Bella-Cruz CC, Bolzan A, Quadri MB (2019) Biological activity and chemical profile of Brazilian jackfruit seed extracts obtained by supercritical CO2 and low pressure techniques. J Supercrit Fluids 152:104551. https://doi.org/10.1016/j.supflu.2019.104551
Van de Loosdrecht AA, Nennie E, Ossenkoppele GJ, Beelen RHJ, Langenhuijsen MMAC (1991) Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. J Immunol Methods 141:15–22
Woyengo T, Ramprasat V, Jones P (2009) Anticancer effects of phytosterols. Eur J Clin Nutr 63:813–820. https://doi.org/10.1038/ejcn.2009.29
Zzaman W (2012) Optimization of antioxidant extraction from jackfruit (Artocarpus Heterophyllus Lam.) seeds using response surface methodology. Faculty of Bioscience Engineering Academic Year 2011–2012, Ghent University
Acknowledgements
The authors wish to thank UFSC (Federal University of Santa Catarina, Brazil), Center for Analysis of the Department of Chemical Engineering and Food Engineering (UFSC), and CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil) for the financial support and scholarships that have contributed to the realization of this ongoing project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tramontin, D., Cadena-Carrera, S.E., Assreuy, J. et al. Response surface methodology (RSM) to evaluate both the extraction of triterpenes and sterols from jackfruit seed with supercritical CO2 and the biological activity of the extracts. J Food Sci Technol 58, 3303–3313 (2021). https://doi.org/10.1007/s13197-020-04876-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04876-7