Abstract
In this study, an eco-friendly supercritical carbon dioxide (SC-CO2) extraction of polyphenolic compounds from Hippophae salicifolia leaf was optimized to achieve the highest extraction yield with maximum total phenolic content (TPC) and minimum IC50. The central composite design was used to establish an experimental design for RSM. The effect of the pressure, temperature, carbon dioxide flow rate, and co-solvent amount was scrutinized using variance analysis (ANOVA). Under optimized condition (25.13 MPa, 47.53 °C, 14.47 g/min, and 2.43%), the experimental data (yield of extraction: 4.38%, TPC: 84.31 mg GAE/g, and IC50: 41.94 µg/mL) showed good agreement with the predicted values (yield of extraction: 4.53%, TPC: 83.37 mg GAE/g, and IC50: 40.2 µg/mL). Nine polyphenolic compounds: gallic acid, caffeic acid, ferulic acid, vanillic acid, p-coumaric acid, quercetin, myricetin, kaempferol, and rutin were analyzed in SC-CO2 extract using HPLC. SC-CO2 extraction was more selective for ferulic acid, myricetin, and quercetin extraction. The study results revealed that SC-CO2 extract had significant antibacterial activity against eight bacterial strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sea buckthorn (SBT) is a dioecious thorny willow-like shrub with distinct male and female plants that is economically important. Different vegetative parts of the male and female SBT plants contain compounds with varying biological activities, with the leaves having the highest phenolic content and antioxidant and antibacterial activities (Górnaś et al. 2016). SBT leaves contain large quantities of phenolics, flavonoids, tannins, carotenoids, tocopherols, free and esterified sterols, and triterpenols that have various health benefits such as antioxidant, cytoprotective, antibacterial (Upadhyay et al. 2010), antitumor (Zhamanbaeva et al. 2014), antiviral, anticancer (Enkhtaivan et al. 2017), immunomodulatory, anti-inflammatory (Tanwar et al. 2018), radioprotective (Bala et al. 2009), adaptogenic (Anbarasu et al. 2015), and anti-cardiovascular activity (Padwad et al. 2006). Phenolic compounds are described to be the major contributor to the biological activity of SBT leaf (Kumar et al. 2013; Upadhyay et al. 2010). Despite the manifestation of nutritionally and pharmaceutically valuable bioactive compounds, most SBT leaves left as agricultural wastes after the berry harvest. SBT leaves are rarely employed in food production because they are yet to be widely recognized as a food and food supplement. However, attempts have been made to introduce the scope and procedure of Novel Food Regulation to use sea buckthorn leaves as food, food supplement, or spice. Therefore, it is worthwhile to extract, measure, and identify bioactive compounds from the unused leaves and promote consequent applications based on the antioxidant, antimicrobial, and anticancer properties.
Since most of the phytochemicals are scarcely soluble in water, biologically unstable, and delicate to high temperatures, selecting an appropriate extraction method is a crucial step. Supercritical fluid extraction with SC-CO2, a compound with a low critical temperature of 31.1 °C and pressure of 7.28 MPa, has been widely used to extract natural products. SC-CO2 is a non-toxic and eco-friendly alternative approach that allows quicker extraction at a reduced temperature and provides solvent-free extract. Nevertheless, pure CO2 is innately non-polar and not useful for extracting polar and high molecular mass compounds. Employing co-solvent as a modifier and/or entrainer to moisten/soak makes the extraction of polar compounds possible by augmenting the solvation power of SC-CO2 (Herrero et al. 2006). The most commonly utilized organic co-solvents are ethanol and methanol. Since it is generally regarded as safe, ethanol was chosen as a co-solvent and entrainer in 53% of studies on SC-CO2 extraction from vegetable matrices. Water has recently received a lot of interest as a co-solvent. However, ethanol is more soluble in SC-CO2 than water, avoiding the formation of a biphasic solvent combination at low pressure (Solana et al. 2014).
SC-CO2 extraction of isorhamnetin (Jayashankar et al. 2014), oleoresin, fat-soluble vitamins, carotenoids (Sajfrtova and Sovova 2012), and flavonoids (Ghatnur et al. 2012) from the leaves of H. rhamnoides have been studied. SC-CO2 extract of SBT leaves exhibits adjuvant activity by effectively enhancing antibody and cell-mediated immunity in response to inactivated rabies antigen (Jayashankar et al. 2016; 2017). Sajfrtova and Sovova (2012) have optimized parameters for SC-CO2 extraction of oleoresin, fat-soluble vitamins, and carotenoids from the leaves H. rhamnoides L. Various researchers have optimized the SC-CO2 extraction of polyphenolic compounds from the leaves and bark of plants in the range of 50–80 °C temperature, 18–46 MPa pressure, and 1–5% co-solvent (Domingues et al. 2013; Liu et al. 2009; Maran et al. 2015; Rodrigues et al. 2018; Sajfrtova and Sovova 2012). H. salicifolia grown in the Himalayan regions of northeast India is an underutilized variety, and no research has been reported on the extraction of polyphenol from leaves using SC-CO2.
The objective of this study was to optimize polyphenols extraction from H. salicifolia leaf using SC-CO2 for the highest yield of extraction with maximum TPC and best IC50 and to understand the effect of extraction parameters. Extraction was accomplished at process parameters ranging from 40–60 °C, 18–32 MPa, 10–20 g/min (CO2 flow rate), and 1–3% (amount of co-solvent). Further, the extract obtained under optimized conditions was characterized by its phenolic and flavonoid content using HPLC. In addition, in vitro antibacterial activity of the extracts was evaluated and compared with conventional Soxhlet extract.
Materials and methods
Plant materials and chemicals
Leaves of the male and female plant of Hippophae salicifolia D. Don were collected from Arunachal Pradesh, Northeast India, in November during its fruiting season when male and female plants can morphologically be distinguished. The leaves were dried at ambient temperature and milled to the average particle size between 60 and 40 µm mesh size. The moisture content of the powdered leaf was recorded as 12.5%. The carbon dioxide required for experiments (99.9%) was procured from Assam Air Products, India.
HPLC grade methanol, ethanol, acetonitrile, and other solvents and chemicals including glacial acetic acid, phosphoric acid, gallic acid, caffeic acid, ferulic acid, vanillic acid, and p-coumaric acid, quercetin, myricetin, kaempferol, and rutin used were of analytical grade and were purchased from Merck India Pvt. Ltd. and Himedia. Bacterial cultures of Staphylococcus aureus (MTCC 9886), Micrococcus luteus (MTCC 2848), Bacillus subtilis (MTCC 1133), Staphylococcus epidermidis (MTCC 9040), Enterobacter aerogenes (MTCC 8558), Klebsiella pneumonia (MTCC 4030), Pseudomonas aeruginosa (MTCC 8727), and Escherichia coli (MTCC 1687) were purchased from MTCC, Chandigarh, India.
Preliminary studies
Comparison between leaves from the male and female plant
To select the best sample for the SC-CO2 extraction study, a comparison between the yield of extraction, TPC, and IC50 of Soxhlet extracts from female (FL) and male leaf (ML) of SBT plant was made. Conventional Soxhlet extraction of FL and ML of H. salicifolia was performed under reflux conditions using ethanol. FL extract had a higher extraction yield and higher TPC, TFC, and antioxidant activity than ML extract (Table S1). Thus, FL was chosen for the optimization study due to the higher yield of extraction, TPC, and antioxidant activity.
Effect of leaf powder-to-entrainer ratio
SC-CO2 extraction of FL leaves was carried out at the minimal extraction variables utilized in the optimization study (18 MPa, 40 °C, 10 g/min CO2 flow rate, and 1% co-solvent). SC-CO2 extract yielded much lower extraction yield, TPC, and antioxidant activity than Soxhlet extract. As a result, an attempt has been made to improve the yield of extraction, TPC, and the antioxidant activity of SC-CO2 extract by soaking the powdered FL in ethanol for 24 h at room temperature (25 ± 1 °C) before extraction. Ethanol was chosen as a co-solvent and entrainer for SC-CO2 extraction since it is generally regarded as safe and readily soluble in SC-CO2. In this study, co-solvent is defined as a solvent mixed with SC-CO2, whereas an entrainer is used to soak the sample. Consequently, the solubility of the solute, TPC, and antioxidant activity of the extracts as a function of powder-to-entrainer ratios of 1:0, 1:0.5, 1:1, and 1:1.5 (w/v) were studied. The extraction time was 1 h.
As the leaf-to-entrainer ratio varied from (1:0) to (1:1), the extraction yield, the TPC, and the antioxidant activity of extracts were progressively increased, as shown in Table S1. FL(1:1.5) extract resulted in the highest extraction yield, while the TPC, TFC, and antioxidant activity were lower than the FL(1:1) extract. Further, it was also noticed that the use of a higher entrainer (1:1.5) causes the accumulation of liquid in the extraction vessel. The powder-to-entrainer ratio (FL(1:1)), which gives a higher yield of extraction with higher TPC, TFC, and lower IC50 value, was selected for the optimization study.
Effect of extraction time
Following the determination of the best powder-to-entrainer ratios, the influence of extraction time on extraction yield, TPC, TFC, and IC50 was examined at 1 h, 2 h, and 3 h (Table S1). When extraction time varied from 1 to 3 h, the extraction yield increased from 1.16% to 3.82%, while TPC, TFC, and antioxidant activity of the extract decreased as extraction time was extended from 2 to 3 h. Finally, due to the higher yield of extraction with maximum TPC and minimum IC50, a 2-h extraction time was chosen for the optimization study.
SC-CO2 extraction procedure
The SC-CO2 extraction was performed using a laboratory-scale supercritical fluid extractor: SFE 500 Systems (Waters, USA). The extractor was equipped with a 500-mL extraction vessel and separator, pumps, heat exchangers, and an automated back pressure regulator (ABPR—to maintain the CO2 in the supercritical state).
The sample for SC-CO2 extraction was prepared by soaking 50 g powdered leaves in 50 mL ethanol [FL1:1 (w/v)] for 24 h. The soaked sample was packed in double-layered muslin fabric and placed into a high-pressure SC-CO2 extraction vessel. The CO2 cylinder's shut-off valve was open, allowing CO2 to flow through the chiller to the CO2 pump. The CO2 and co-solvent were mixed and preheated before being transferred to the extraction vessel, kept at a temperature and pressure that maintained the mixture at a supercritical state. Inside the extraction vessel, the mixture of solvents (SC-CO2 and co-solvent) meet the plant and breaks the tissues of the plant matrix, allowing it to dissolve part of the plant material. The solvent and the extract flow through the ABPR and while exiting the system, the pressure was reduced, and the extract was continuously precipitated in the separator (cyclone) held at 30 °C. The extracts were collected in amber-colored vials, and the extraction yield was calculated using Eq. (1):
Extraction experimental design
Four highly responsible variables: X1, pressure (MPa); X2, temperature (°C); X3, CO2 flow rate (g/min); and X4, amount of co-solvent (%) were chosen to study their effect on extraction. The range of independent variables was: temperature (X1), 40–60 °C, pressure (X2), 18–32 MPa, CO2 flow rate (X3), 10–20 g/min, and amount of co-solvent (X4), 1–3%. The lower and upper limits of variables were decided based on published literature (Ghatnur et al. 2012; Maran et al. 2015; Rodrigues et al. 2018; Sajfrtova and Sovova 2012). A full factorial CCD technique was used to optimize extraction yield, TPC, and IC50. A 24 full factorial design CCD of four independent variables coded at five levels (− 1.682, − 1, 0, + 1, + 1.682) was applied and gives 30 (2n + 2n + 6) experiments (Table 1).
ANOVA and model fitting
The response behavior as a function of independent variables was studied based on the experimental runs (Table S2). Analysis of variance (ANOVA) was used to estimate the effect of model coefficients and their interaction on the extraction yield (%), TPC (mg GAE/g), and IC50 (µg/mL) in the response surface multiple regression analysis. The independent variables were mathematically related to dependent variables using the following second-order polynomial equation:
where Y stands for response variables [yield of extraction (%), TPC (mg GAE/g), or IC50 (µg/mL)]; βi, βii, and βij are linear, quadratic, and interaction coefficients, respectively, and Xi and Xj are independent extraction variables. A regression coefficient R2 estimated the best fit and goodness of the model. The response surface and contour plots were obtained from the fitted quadratic equation generated from regression analysis by varying two of the independent variables at a time while keeping the other two at their central point (0).
Total phenolic and flavonoid content
The total phenolic content (TPC) and total flavonoid content (TFC) were evaluated using the procedure outlined by Olayinka and Anthony (2010). The gallic acid calibration curve ranging from 10 to 50 µg/mL was used to calculate the total phenolic content present as milligram gallic acid equivalents per gram of dried extract (mg GAE/g). Total flavonoid content in each extract was quantified from the standard curve of quercetin (10–50 µg/mL), and results represented as milligram quercetin equivalents per gram of dried extract (mg QE/g).
HPLC analysis
Quantification of phenolic (gallic acid, caffeic acid, ferulic acid, vanillic acid, and p-coumaric acid) and flavonoid (quercetin, myricetin, kaempferol, and rutin) compounds was carried out using HPLC (Shimadzu Corporation, Kyoto, Japan) equipped with a UV–Vis detector and C18, 250 × 4.6 ID column kept at 25 ± 2 °C (Moges et al. 2021). The detection wavelengths were 280 nm for gallic acid, 270 nm for vanillic acid, 296 nm for caffeic acid and ferulic acid, and 310 nm for p-coumaric acid. Quercetin, myricetin, kaempferol, and rutin were detected at 368 nm. The mobile phases for phenolic compound analysis were Milli-Q water (63%) and acetonitrile (37%) (v/v), each with 1% phosphoric acid (flow rate 1 mL/min), while for flavonoids it was a mixture of methanol (40%): acetonitrile (15%): Milli-Q water (45%) (v/v/v) with 1% glacial acetic acid (flow rate 0.5 mL/min). Extracts were dissolved in HPLC-grade methanol and filtered through a 0.45-µm filter (Axiva). The injection volume was 20 µL. The standard compounds were prepared in the range of 10–100 μg/mL, and calibration curves (R2 ≥ 0.99) were established.
Limit of detection (LOD) and limit of quantification (LOQ)
LOD (lowest concentration in a sample that can be detected) and LOQ (lowest concentration of analyte that can be determined) were calculated using the formula LOD = 3.3 × SD/S and LOQ = 10 × SD/S, where SD = standard deviation of response and S = slope of the calibration curve (Alquadeib 2019).
Antioxidant activity
The DPPH assay was performed using the method described by Sen et al. (2013). Various concentrations of extract solution (3 mL) were vigorously mixed with 0.1 mM DPPH (1 mL) reagent. After 30-min incubation, absorbance was measured at 517 nm. The percentage scavenged was calculated using Eq. 3:
where Acontrol = absorbance of control and Asample = absorbance of sample.
As a control, the extract was replaced by 3 mL of methanol. The antioxidant activity of extracts was presented in IC50 obtained from the plot of percentage scavenged versus extract concentration.
Antibacterial activity
Minimum inhibitory concentration (MIC)
MIC was determined through the procedure outlined by Sarker et al. (2007). Extracts were dissolved in 10% DMSO and sterilized with a 0.22-µm filter. A volume of 30 µL of 3.3X broth media, 50 µL sample, and 10 µL (5 × 106 CFU/mL) of each bacterium was pipetted into 96 well plates. The plate was kept in an incubator at the appropriate bacterium growth temperature for 8 h. After the incubation period, 10 µL resazurin blue (1 mg/mL) was added to each well, and the suspension was again kept in an incubator until the color changed from blue to pink. The lowest concentration at which the blue color did not change to pink was the MIC.
Zone of inhibition (ZoI)
Agar well diffusion assay was adopted to determine the ZoI (Purohit et al. 2021). The culture of the bacterium was spread on the agar surface. After the spread had been absorbed, three equidistant wells (0.5 mm in diameter) were bored on the agar plate. 50 µL of 1 mg/mL sterilized solutions of extracts were drained into the wells and kept in the laminar airflow until the solution was well diffused into the solidified agar. Control experiments comprised 10% DMSO and inoculums. The plates were incubated at respective incubation temperatures (30 °C and 37 °C), and ZoI was measured after 12 to 14 h.
Results and discussion
Fitting the models and analysis of ANOVA
The individual and combined effect of four independent variables X1, pressure (MPa); X2, temperature (°C); X3, CO2 flow rate (g/min); and X4, amount of co-solvent (%) for SC-CO2 extraction from FL of SBT was examined. Evaluation of yield of extraction, TPC, and IC50 of all experimental runs was performed (Table S2), and the results were analyzed by multiple regression analysis. Sources of variances were used to determine optimum extraction conditions (Tables 2, 3). This study aimed to attain the maximum yield of extraction with maximum TPC and a minimum IC50. The quadratic model equations fitted to the experimental data to predict the yield of extraction, TPC, and IC50 are given in Eqs. (4–6):
The results of second-order response surface models were thoroughly analyzed with ANOVA to ensure the model fit and estimate individual model coefficients test for lack-of-fit (Table 2). The greater F values of the models for the yield of extraction, TPC, and IC50 (453.55, 1887.07, and 3958.52, respectively) with very fewer P-values (< 0.0001) indicate that the three models are extremely important to represent the relationship between the independent variables and responses. All the linear terms (X1, X2, X3, and X4); all the quadratic terms (X12, X22, X32, X42); and the majority of the cross-terms (X1X2, X1X4, X2X3, X3X4) are significantly important parameters with lower P-values. On the other hand, the interaction between X1X3, X2X4 on the yield of extraction, X1X4 on TPC, and X1X3 on IC50 are not significant. The lack of fit for each response was insignificant, with F-value and P-value of 0.5324 and 0.8147 (for extraction yield); 1.04 and 0.5145 (for TPC); and 0.9759 and 0.547 (for IC50), indicating that the developed model was suitable for the experimental data and showed good correlations between the reproducible results.
Regression coefficients, standard deviation (Std. dev.), and coefficient of the variation (CV %) for responses obtained from CCD are shown in Table 3. The model's accuracy was judged by the regression coefficient (R2), and the suitability of the model was confirmed by its value. The values of R2 implied that 99.76%, 99.94%, and 99.97% of the model behavior could be interpreted for the extraction yield, TPC, and IC50, respectively. The predicted R2 agreed with the adjusted R2, which signifies the noticeable statistical correlation between each response's actual and predicted values. The plot of model-predicted values against experimental data of the yield of extraction, TPC, and IC50 envisioned the fitting qualities of the developed model equations, as shown in Fig. 1a–c.
Influence of extraction variables on the yield of extraction, TPC, and IC50
Effect of pressure and temperature
The effect of extraction variables on the yield of extraction, TPC, and IC50 is presented in Fig. 2a–l, and Fig. S1a–d, respectively. The regression analysis of the source of variances of the yield of extraction and TPC and IC50 of leaf extract data revealed that the extraction yield was significantly (P < 0.05) affected by pressure, temperature, and the interaction between pressure and temperature. Increasing pressure at lower temperatures reduces SC-CO2 diffusivity, initiates solid matrix compaction, lowers the void fraction, minimizes solvent interaction with matrix pores, and hence hinders solute dissolution and solubility. Because of this reason, at a lower temperature (40 °C) the increase in pressure from 25 to 32 MPa resulted in a gradual reduction in extraction yield (Fig. 2a). At higher temperatures (60 °C), however, the opposite phenomenon was observed, since the increase in pressure at elevated temperatures upsurges the density of the solvent and the solvating power. The extraction yield improved with an increase in temperature up to 55 °C at higher pressure (32 MPa), while the extraction yield was best around 50 °C at lower pressure (18 MPa). At 25 MPa and 50 °C, the maximum extraction yield achieved was 4.35%. Gadkari et al. (2013) observed similar behavior while studying SC-CO2 extraction from fresh frozen tea leaves.
The effect of pressure and temperature on TPC of extracts is related to alterations in the selectivity of SC-CO2 to solutes, which depends on amendments in the polarity and density of SC-CO2. TPC was highest at higher pressures and lower temperatures and decreased with temperature (Fig. 2g). In the temperature range from 40 to 50 °C, the increase in pressure resulted in a slight increase in TPC, and beyond 50 °C, TPC decreased with increased pressure. Even though an increase in temperature favors the extraction of phenolic compounds from plant tissues, the TPC of extracts was limited because of the thermal degradation that occurs above 50 °C. Zulkafli et al. (2014) have reported that the phenolic compound extraction was reduced by increasing the temperature at reduced pressure and vice versa.
The concentration of phenolic compounds contained in the extracts usually ascribed to the antioxidant activity. Therefore, the IC50 value reached its highest point at the higher pressure and lower temperature, where TPC was lowest, as shown in Fig. S1a–d. At lower pressure, the IC50 value decreased when the temperature increased from 45 to 60 °C. A similar phenomenon was reported on the antioxidant activity of SC-CO2 rosemary extract by Genena et al. (2008).
Effect of pressure and CO2 flow rate
The interaction of pressure and CO2 flow rate significantly impacted TPC but not extraction yield. The best yield was achieved at around 25 MPa and 14.5 g/min CO2 flow rate. The RSM plateau of pressure versus CO2 flow rate indicated that an increase in the pressure at a lower CO2 flow rate (10 g/min) improved TPC extraction (Fig. 2h). As reported by Roberto et al. (2010), the increase in pressure at a constant temperature increases the CO2 density, resulting in increased solvation power and hence solubility of polyphenolic compounds, whereas variation in the pressure at a higher CO2 flow rate (20 g/min) did not affect the TPC.
Effect of pressure and amount of co-solvent
The interaction between pressure and amount of co-solvent significantly (P < 0.05) affected the yield of extraction and IC50. The increase in co-solvent concentration resulted in a greater extraction yield across all pressure ranges, as presented in Fig. 2c. Co-solvent had a maximum effect on the yield of extraction (4.5%) toward the maximum percentage (3%) and minimum pressure (18–20 MPa). The extent of yield increment as a function of the amount of the co-solvent was faster at a lower pressure. This means co-solvent at lower pressure improves extraction efficiency, while an increase in the pressure decreases the diffusivity of co-solvent into the matrix and deteriorates the extraction efficiency. This could be considered an economic benefit of using co-solvent in the SC-CO2 extraction. On the other hand, the interaction between pressure and co-solvent amount did not significantly affect TPC. TPC was at its peak at approximately 2.4% co-solvent, as presented in Fig. 2i. The co-solvent was found to be the most important factor in extracting biologically active phenolic compounds from SBT leaf. The addition of co-solvent influences the extraction by distorting the matrix–analyte diffusion process and increasing the analyte’s solubility and desorption. However, the incorporation of surplus co-solvent might alter the state of SC-CO2 and lead to a decrease in extraction selectivity and efficiency. That might be the reason for a slight decrease in TPC after 2.4% co-solvent. Amount of co-solvent showed the most substantial affirmative effect on the radical scavenging activity. These results granted the findings of Floch et al. (1998) and Sajfrtova and Sovova (2012) on the SC-CO2 extraction yield of a polar component from leaves of Olive and SBT, respectively.
Effect of temperature and CO2 flow rate
The effect of the CO2 flow rate on the extraction yield is depicted in Fig. 2d. As the CO2 flow rate increased up to 16 g/min, the extraction yield increased. This could be attributed to the enhancement of mass transfer of the system by SC-CO2. However, a further increase in CO2 flow rate moderated the yield of extraction. This could be due to a reduction in the required contact time between CO2 and the plant matrix for the equilibrium separation process. Yet, at higher temperatures, the extraction yield decreased due to the reduced density of SC-CO2.
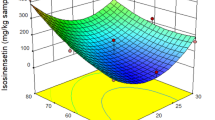
The effect of temperature and the CO2 flow rate was one of the most critical factors for the extraction of phenolic compounds. TPC was at its highest around 47 °C, with a CO2 flow rate of 14 g/min, as presented in Fig. 2j. The identical effect of temperature on the extraction of bioactive compounds from Camellia sinensis L. leaves was reported by Maran et al. (2015). A rise in temperature results in an upsurge of solute vapor pressure which eventually increases the solubility of phenolic compounds with the increase in vapor pressure. Though the solvating power of SC-CO2 decreased with density, an increase in temperature improved the extraction rate, perhaps due to the dominance effect of vapor pressure over density.
Effect of temperature and co-solvent
The yield of extraction was highest at around 47 °C and 2.4% co-solvent (Fig. 2k, l). An increase in the co-solvent up to around 2.4% augmented the TPC of extracts in all ranges of extraction temperatures. Though the increase in co-solvent intensifies the extraction yield, TPC was not eternally in an affirmative proportion to the yield. As ethanol is polar, it is expected that the solubility of polyphenols increases with the increase in the density of the binary mixture. The increase in the density of the binary mixture enhances co-solvent–solute interactions, assists matrix swelling, improves the osmotic diffusion process, and the solubilization of polar compounds (Veggi et al. 2014). Hence, extraction with better TPC and antioxidant activity could be achieved. Our findings on the effect of co-solvent are in favor of reports of various researchers on the extraction of bioactive compounds (Maran et al. 2015; Rodrigues et al. 2018; Solana et al. 2014; Song et al. 2019; Valadez-Carmona et al. 2018; Zulkafli et al. 2014).
Effect of CO2 flow rate and co-solvent
Figure 2f shows that a higher percentage of extraction yield was viewed when the amount of co-solvent reached its highest level at the lower ranges of CO2 flow rate. This is because, at a lower CO2 flow rate, the lipid can efficiently be removed from the sample matrix. The inner matrix containing desired polyphenolic compounds can be extracted with co-solvent. Figure 2l shows that a continuous rise in the co-solvent percentage increased the extraction yield but not the content of polyphenolic compounds. Ariff et al. (2018) have reported the effect of CO2, and co-solvent flow rate in the range from 15 to 35 g/min and 5–30%, respectively, for the extraction yield from Mariposa Christia Vespertilionis leaves. They reported that extraction yield decreased above 25 g/min CO2 flow rate and increased with co-solvent flow rate from 5 to 30%.
Process optimization for higher extraction yield, TPC, and lower IC50
Among the 30 experimental sets, maximum and the minimum yield, TPC and IC50 achieved were (4.53% and 1.92%), (83.06 ± 3.8 mg GAE/g and 47.58 ± 8.1 mg GAE/g), and (115.17 ± 0.3 µg/mL and 42.11 ± 1.3 µg/mL), respectively. The desirability function tool chose the optimum extraction conditions that gave the highest yield of extraction with maximum TPC and minimum IC50. The optimum SC-CO2 extraction conditions were pressure: 25.13 MPa, temperature: 47.53 °C, CO2 flow rate: 14.47 g/min, and amount of co-solvent: 2.43%. At this condition, the predicted yield of extraction, TPC, and IC50 was 4.53%, 83.37 mg GAE/g, and 40.2 µg/mL, respectively.
Validation of SC-CO2 extraction parameters
Following optimization, a verification experiment was conducted under optimal extraction conditions to confirm the experimental and theoretical values of the yield of extraction, TPC, and IC50. Validation experiments at the optimum condition revealed that extraction yield, TPC, and IC50 of 4.38% and 84.31 ± 3.98 mg GAE/g, and 41.94 ± 3.13 µg/mL, respectively (Table 4). These results were very close to the projected results. This suggests that the formulated models were supposed to be precise and reliable. The percentage recovery described the contrast between SC-CO2 extract under optimum conditions and Soxhlet extract. The ratio of the targeted component in SC-CO2 extract to Soxhlet extract is the SC-CO2 extract recovery. At optimum conditions, SC-CO2 extraction from FL led to the recovery of 48.83%-yield of extraction and 38.1%-TPC.
Characterization of extract obtained at optimum SC-CO2 extraction conditions
FL extract obtained at optimum SC-CO2 extraction conditions was further characterized for its total flavonoid content (TFC), quantitative HPLC analysis of phenolic acids and flavonoids (Table 4), and antibacterial properties (Table 6). FL's optimal SC-CO2 extraction setup was also used to extract ML and a 50–50% blend of male and female leaves (MIX). The TFC of FL SC-CO2 extracts was 77.95 ± 1.53 mg QE/g, encompassing 64.8% of the conventional extract. The HPLC chromatograms of phenolic acids and flavonoids in the extract obtained by Soxhlet and the optimum SC-CO2 extraction process from FL, ML, and mixture of leaves (MIX) are given in Fig. S2 and Fig. S3. The linearity of the established HPLC method was determined with the standard solutions with the concentration range of 10–100 μg/mL at five concentration levels. The retention rime, regression equation, the limit of detection (LOD), and the limit of quantification (LOQ) values of investigated compounds are listed in Table 5. The standard curves revealed good linear regression (R2 > 0.99) within test ranges. The flavonoids method was more sensitive than phenolic acids, as indicated by the lower values of the LOD and LOQ values.
Gallic acid (61.95 ± 0.24 mg/g) was found to be the most abundant phenolic acid in SC-CO2 extracts, followed by ferulic acid (0.19 ± 0.01 mg/g), p-coumaric acid (0.03 ± 0.01 mg/g), and caffeic acid (0.02 mg/g). SC-CO2 extracted ferulic acid better than Soxhlet, with a 146.2%t recovery. This prove the benefit of SC-CO2 selectivity and the drawback of thermal degradation using Soxhlet. Gallic acid, p-coumaric acid, and rutin were, in fact, better extracted using Soxhlet extraction. SC-CO2 extraction, however, remains a safer option than Soxhlet extraction because of its shorter extraction time, lower extraction temperature, and higher selectivity for extracting specific compounds. Rutin was identified only in Soxhlet extract.
The most abundant flavonoids in FL extract were myricetin (5.05 ± 0.97), followed by quercetin (0.13 mg/g). SC-CO2 extract had significantly greater myricetin contents than Soxhlet extract, with a recovery rate of 112.72%. Only SC-CO2 extracts contained quercetin. HPLC analysis confirmed that SC-CO2 extraction was more selective for the extraction of flavonoids than phenolic compounds. This demonstrates that SC-CO2 is a promising alternative over conventional solvent extraction for obtaining flavonoid-rich extracts from SBT leaves. Vanillic acid and kaempferol were not detected in all extracts. Similar investigations were reported by researchers (Pereira et al. 2016; Song et al. 2019).
Antibacterial activity of extract obtained at optimum SC-CO2 extraction conditions
The antibacterial activities of Soxhlet extracts from the leaves of H. salicifolia are given in Table 6. The FL extract obtained at optimized SC-CO2 extraction condition showed significant antibacterial activity against eight bacterial strains as evaluated by MIC (125–500 µg/mL) and ZoI (3 to 12.1 ± 0.6 mm) assays. SC-CO2 extract showed the highest activity against E. aerogenes at 125 MIC. There were no substantial differences in the MIC of the extract against other bacterial strains. Soxhlet extract of FL showed the highest antibacterial efficacy with the lowest MIC of 62.5 µg/mL against S. aureus, E. aerogenes, and B. subtilis, eightfold lower than SC-CO2 extract of FL. The difference in antibacterial activity of SC-CO2 and Soxhlet extracts is attributed to the variation in the content of polyphenol compounds, especially gallic acid and caffeic acid (Ouattara et al. 2011).
The ZoI trend bears consistent with the MIC. SC-CO2 extract showed the highest activity against S. epidermidis with the highest ZoI (12.1 ± 0.6 mm) and the smallest activity against E. aerogenes with the lowest ZoI (3 mm). Gram-negative bacteria were most resistant to the extract, and lower values of ZoI were obtained for these bacteria. Gram-positive bacteria lack basic lipopolysaccharide components on their outer membranes and were more vulnerable to antibacterial agents than Gram-negative. Information regarding the polyphenolic content, antioxidant and antibacterial properties of SBT leaf extract using SC-CO2 in the literature is rather limited.
Comparison between SC-CO2 extracts of FL, ML, and the mixture of leaves
The yield and TPC of ML extract were 3.9% and 70.47 ± 4.41 mg GAE/G, respectively, which was lower than the FL extract, whereas TFC of ML, 59.46 ± 0.86 mg QE/g; and MIX, 62.27 ± 1.96 mg QE/g were slightly higher than FL. The TFC, antioxidant activity, and the gallic acid content of MIX extract lie between the extracts from ML and FL. No difference was observed in the MIC of FL, ML, and MIX extracts. The ZoI of MIX was found to be slightly higher than FL and ML extracts for B. subtilis. Cumulatively, the extracts from MIX showed higher antibacterial activity than ML in ZoI assay. This may be due to the benefit of the synergistic effect of bioactive compounds from the leaves of female and male plants.
Conclusions
Optimization of SC-CO2 extraction of polyphenol from Hippophae salicifolia D. Don leaves collected from northeast India is reported. The optimum condition for maximum yield of extraction with maximum TPC and minimum IC50 occurred at 25.13 MPa, 47.53 °C, 14.47 g/min CO2 flow rate, and 2.43% co-solvent. The validation experiment at this condition resulted in 4.38% yield of extraction, 84.31 ± 3.9 mg GAE/g TPC, and 41.94 ± 3.13 µg/mL IC50. Soxhlet extraction performed better to yield of extraction, TPC, IC50, and antibacterial activity than SC-CO2 extraction. However, SC-CO2 extraction was more selective for ferulic acid, myricetin, and quercetin extraction. Further research needs to be done on the selectivity of SC-CO2 extraction for flavonoids. The research results indicate the status of utilizing SC-CO2 extract of Hippophae salicifolia leaf as functional foods and ingredients, food supplements, and food preservatives.
References
Alquadeib BT (2019) Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets. Saudi Pharm J 27(1):66–70. https://doi.org/10.1016/j.jsps.2018.07.020
Anbarasu S, Radhakrishnan M, Suresh A, Joseph J (2015) Phytochemical, ethnomedicinal and pharmacological potentials of seabuckthorn—a mini review. Int J Pharma Bio Sci 6(3):263–272
Ariff MAM, Yusri AM, Razak NAA, Jaapar J (2018) Effect of CO2 flow rate, co-solvent and pressure behavior to yield by supercritical CO2 extraction of Mariposa Christia Vespertilionis leaves. AIP Conf Proc 020072:2–7. https://doi.org/10.1063/1.5080885
Bala M, Prasad J, Singh S, Tiwari S, Sawhney RC (2009) Whole-body radioprotective effects of SBL-1: a preparation from leaves of hippophae rhamnoides. J Herbs Spices Med Plants 15(2):203–215. https://doi.org/10.1080/10496470903139496
Domingues RMA, de Melo MMR, Oliveira ELG, Neto CP, Silvestre AJD, Silva CM (2013) Optimization of the supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark using experimental design. J Supercrit Fluids 74:105–114. https://doi.org/10.1016/j.supflu.2012.12.005
Enkhtaivan G, John KMM, Pandurangan M, Hur JH, Leutou AS, Kim DH (2017) Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi J Biol Sci 24(7):1646–1656. https://doi.org/10.1016/j.sjbs.2016.01.004
Floch FL, Tena MT, Rios A, Valcarcel M (1998) Supercritical fluid extraction of phenol compounds from olive leaves. Talanta 46:1123–1130
Gadkari PV, Balarman M, Kadimi US (2013) Polyphenols from fresh frozen tea leaves (Camellia assamica L.,) by supercritical carbon dioxide extraction with ethanol entrainer—application of response surface methodology. J Food Sci Technol 52(2):720–730. https://doi.org/10.1007/s13197-013-1085-9
Genena AK, Hense H, Junior AS, de Souza SM (2008) Rosemary (Rosmarinus officinalis)—a study of the composition, antioxidant and antimicrobial activities of extracts obtained with supercritical carbon dioxide. Ciencia e Tecnologia De Alimentos 28(2):463–469. https://doi.org/10.1590/S0101-20612008000200030
Ghatnur SM, Sonale RS, Balaraman M, Kadimi US (2012) Engineering liposomes of leaf extract of seabuckthorn (SBT) by supercritical carbon dioxide (SCCO2)-mediated process. J Liposome Res 22(3):215–223. https://doi.org/10.3109/08982104.2012.658576
Górnaś P, Šnē E, Siger A, Segliņa D (2016) Sea buckthorn (Hippophaerhamnoides L.) vegetative parts as an unconventional source of lipophilic antioxidants. Saudi J Biol Sci 23(4):512–516. https://doi.org/10.1016/j.sjbs.2015.05.015
Herrero M, Cifuentes A, Ibañez E (2006) Sub- and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae—a review. Food Chem 98(1):136–148. https://doi.org/10.1016/j.foodchem.2005.05.058
Jayashankar B, Mishra KP, Ganju L, Singh SB (2014) Supercritical extract of Seabuckthorn Leaves (SCE200ET) inhibited endotoxemia by reducing inflammatory cytokines and nitric oxide synthase 2 expression. Int Immunopharmacol 20(1):89–94. https://doi.org/10.1016/j.intimp.2014.02.022
Jayashankar B, Singh D, Mishra K, Madhusudana S, Singh S, Ganju L (2016) Supercritical carbon dioxide extract of seabuckthorn leaves enhances rabies virus neutralizing antibody titers and CTL response in Swiss albino mice. J Vacc Immunol 2(1):004–009. https://doi.org/10.17352/jvi.000013
Jayashankar B, Singh D, Tanwar H, Mishra KP, Murthy S, Chanda S, Mishra J, Tulswani R, Misra K, Singh SB, Ganju L (2017) Augmentation of humoral and cellular immunity in response to Tetanus and Diphtheria toxoids by supercritical carbon dioxide extracts of Hippophae rhamnoides L. leaves. Int Immunopharmacol 44:123–136. https://doi.org/10.1016/j.intimp.2017.01.012
Kumar MSY, Tirpude RJ, Maheshwari DT, Bansal A, Misra K (2013) Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophaerhamnoides L.) leaves in vitro. Food Chem 141(4):3443–3450. https://doi.org/10.1016/j.foodchem.2013.06.057
Liu S, Yang F, Zhang C, Ji H, Hong P, Deng C (2009) Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J Supercrit Fluids 48(1):9–14. https://doi.org/10.1016/j.supflu.2008.09.013
Maran JP, Manikandan S, Priya B, Gurumoorthi P (2015) Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. J Food Sci Technol 52:92–104. https://doi.org/10.1007/s13197-013-0985-z
Olayinka AA, Anthony IO (2010) Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complem Altern Med 10:21
Ouattara L, Koudou J, Karou DS, Giacò L, Capelli G, Simpore J, Fraziano M, Colizzi V, Traore AS (2011) In vitro anti Mycobacterium tubersculosis H37Rv activity of Lannea acida A. Rich. from Burkina Faso. Pak J Biol Sci 14:47–52
Padwad Y, Ganju L, Jain M, Chanda S, Karan D, Banerjee PK, Sawhney RC (2006) Effect of leaf extract of Seabuckthorn on lipopolysaccharide induced inflammatory response in murine macrophages. Int Immunopharmacol 6(1):46–52. https://doi.org/10.1016/j.intimp.2005.07.015
Pereira P, Cebola M-J, Oliveira MC, Bernardo-Gil MG (2016) Supercritical fluid extraction vs conventional extraction of myrtle leaves and berries: comparison of antioxidant activity and identification of bioactive compounds. J Supercrit Fluids 113:1–9. https://doi.org/10.1016/j.supflu.2015.09.006
Purohit S, Barik CR, Kalita D, Sahoo L, Goud VV (2021) Exploration of nutritional, antioxidant and antibacterial properties of unutilized rind and seed of passion fruit from Northeast India. J Food Meas Charact. https://doi.org/10.1007/s11694-021-00899-6
Roberto M, Junior M, Leite AV, Romanelli N, Dragano V (2010) Supercritical fluid extraction and stabilization of phenolic compounds from natural sources—review (supercritical extraction and stabilization of phenolic compounds). Chem Eng J 4:51–60
Rodrigues VH, de Melo MMR, Portugal I, Silva CM (2018) Supercritical fluid extraction of Eucalyptus globulus leaves. Experimental and modelling studies of the influence of operating conditions and biomass pretreatment upon yields and kinetics. Sep Purif Technol 191:173–181. https://doi.org/10.1016/j.seppur.2017.09.026
Sajfrtova M, Sovova H (2012) Solute-matrix and solute-solute interactions during supercritical fluid extraction of sea buckthorn leaves. Procedia Eng 42:1682–1691. https://doi.org/10.1016/j.proeng.2012.07.561
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42(4):321–324. https://doi.org/10.1016/j.ymeth.2007.01.006
Sen S, De B, Devanna N, Chakraborty R (2013) Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb., an Indian medicinal plant. Chin J Nat Med 11(2):149–157. https://doi.org/10.1016/S1875-5364(13)60042-4
Solana M, Boschiero I, Dall’Acqua S, Bertucco A (2014) Extraction of bioactive enriched fractions from Eruca sativa leaves by supercritical CO2 technology using different co-solvents. J Supercrit Fluids 94:245–251. https://doi.org/10.1016/j.supflu.2014.08.022
Song L, Liu P, Yan Y, Huang Y, Bai B, Hou X, Zhang L (2019) Supercritical CO2 fluid extraction of flavonoid compounds from Xinjiang jujube (Ziziphus jujuba Mill.) leaves and associated biological activities and flavonoid compositions. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.111508
Tanwar H, Shweta SD, SinghGanju SBL (2018) Anti-inflammatory activity of the functional groups present in Hippophae rhamnoides (Seabuckthorn) leaf extract. Inflammopharmacology 26(1):303. https://doi.org/10.1007/s10787-017-0353-0
Upadhyay NK, Kumar MSY, Gupta A (2010) Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophaerhamnoides L.) leaves. Food Chem Toxicol 48(12):3443–3448. https://doi.org/10.1016/j.fct.2010.09.019
Valadez-Carmona L, Ortiz-Moreno A, Ceballos-Reyes G, Mendiola JA, Ibáñez E (2018) Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J Supercrit Fluids 131:99–105. https://doi.org/10.1016/j.supflu.2017.09.011
Veggi PC, Prado JM, Bataglion GA, Eberlin MN, Meireles MAA (2014) Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction. J Supercrit Fluids 89:68–77. https://doi.org/10.1016/j.supflu.2014.02.016
Zhamanbaeva GT, Murzakhmetova MK, Tuleukhanov ST, Danilenko MP (2014) Antitumor activity of ethanol extract from Hippophaerhamnoides L. leaves towards human acute myeloid leukemia cells in vitro. Bull Exp Biol Med 158(2):252–255. https://doi.org/10.1007/s10517-014-2734-3
Zulkafli ZD, Wang H, Miyashita F, Utsumi N, Tamura K (2014) Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasapalmata). J Supercrit Fluids 94:123–129. https://doi.org/10.1016/j.supflu.2014.07.008
Moges A, Barik CR, Purohit S, Goud VV (2021). Dietary and bioactive properties of the berries and leaves from the underutilized Hippophae salicifolia D. Don grown in Northeast India. Food Sci Biotechnol 30(10):1555–1569. https://doi.org/10.1007/s10068-021-00988-8
Acknowledgements
Mr. Abebe Moges would like to acknowledge sponsorship from Haramaya University under the Ethiopian Ministry of Science and Higher Education, Government of Ethiopia. The authors gratefully acknowledge to Department of Biotechnology (DBT) (Grant no. BT/311/NE/TBP/2012), Government of India, for their fund and support to conduct this study.
Author information
Authors and Affiliations
Contributions
Mr. AM was involved in conceptualization, has performed the experiments, methodology, data curation, wrote the original draft, reviewed and edit the manuscript; Mr. CRB: was involved in conceptualization, resources, methodology, and data curation. Dr. LS: was involved supervision, and funding acquisition. Dr. VVG: was responsible in conceptualization, supervision, funding acquisition, and project administration, and reviewed and edit the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moges, A., Barik, C.R., Sahoo, L. et al. Optimization of polyphenol extraction from Hippophae salicifolia D. Don leaf using supercritical CO2 by response surface methodology. 3 Biotech 12, 292 (2022). https://doi.org/10.1007/s13205-022-03358-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03358-1