Abstract
Ultrafiltration and Diafiltration processes are used to concentrate proteins present in defatted milk in order to manufacture milk protein concentrate (MPC) powders. Selective passage of the water-soluble components causes retention as well as concentration of colloidal milk components in these processes. Increase in calcium and casein contents decreases the stability of milk proteins present in ultrafiltered retentates and negatively influence properties of manufactured MPC powders. Homogenization, diafiltration and disodium phosphate induced changes in properties of low-protein MPC powders were targeted in this study. Applied treatments significantly (P < 0.05) improved foaming and emulsification, solubility, viscosity, heat stability, dispersibility, specific surface area and buffer index of resultant MPC powders over control. Fresh, treated low-protein MPC powders showed significantly higher (< 0.05) solubility values over control sample, which remains higher even after 60 days of storage at 25 ± 1 °C. The rheological behaviour of reconstituted low-protein MPC solutions was also studied. It was best explained as Herschel–Bulkley rheological behaviour. Low-protein MPC powders with improved functional properties may find better use as a protein ingredient in different dairy and food applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk protein concentrate (MPC) powders are rich in milk proteins. The casein to whey protein (4:1) ratio in MPC powders is similar to that present in natural milk (Meena et al. 2017). Similar to whey protein concentrate (WPC) powders, the protein content of a particular type of MPC powder is denoted by a numeric value. Thus, the protein content of MPC powders ranges between 42 and 89% on dry matter (DM) basis. Beyond this protein level (≥ 90%), they are known as milk protein isolates (MPI). The most common types of MPC powders are MPC42, MPC60, MPC70, MPC80 and MPC85. MPC powders containing ~ 50% protein content on DM basis are known as low-protein powders. Either defatted (skim) or micro-filtered milks are used as raw material for the production of MPC powders. Low-protein MPC powders are produced using ultrafiltration (UF) and spray drying.
Based on protein content, MPC powders find various uses in different food applications. As a quality protein ingredient, their demand is continuously rising in food and pharma sector (Patil et al. 2018). Low-protein MPC powders are majorly used as skim milk powder (SMP) replacer and in cheese and yogurt manufacturing (Patel and Patel 2014). Particularly in cheese production, incorporation of low-protein MPC powders decreases casein losses, increases whey proteins retention and subsequently enhances cheese yield (Mistry and Maubois 2004).
MPC powders are criticized for their poor techno-functional properties (like calcium co-precipitates) such as solubility because of their higher protein, calcium and low lactose contents (Meena et al. 2017). During concentration of skim milk in UF, a part of lactose, minerals and vitamins (water soluble milk components) selectively passes into permeate which led to increase in TS, protein, calcium and fat contents in retentate. Such fractionation of milk components during UF causes alteration in chemical composition of UF retentates that disturbs their sensitive salt balance and ultimately reduces the stability of the milk proteins present in UF retentates (Meena et al. 2016). According to Alexander et al. (2011), such changes adversely affect the functionality of milk proteins. Ultrafiltration of milk enables higher transfer of κ-casein and calcium into serum phase that led to swelling of casein micelles and results in dissociation of colloidal calcium phosphate (CCP) nanoclusters and increases total calcium contents (Alexander et al. 2011). Migration of κ-casein further lead to aggregation, interaction and denaturation of calcium-depleted proteins during spray drying of UF retentates. Thus, all these unavoidable changes collectively exhibit adverse effect on solubility (most important) of MPC powders. It is well-established that poor solubility hinders the complete expression of other functional (such as foaming, heat stability, gelation, viscosity, emulsification, oil binding and water binding) properties of MPC powders in different food applications.
Most of the research conducted so far on production, characterization and solubility improvement using chemical, physical and enzymatic interventions was dedicated to high (≥ 80% protein) or medium (60–70% protein) protein MPC powders. The solubility of MPC powders decrease with increase in their protein and calcium contents (Meena et al. 2017). Due to difference in chemical composition, outcomes of studies conducted on high-protein and medium-protein MPC powders cannot be applied as such on low-protein MPC powders. Our previous study demonstrated that stability of milk proteins in UF retentates (studied in terms of change in zeta potential and heat coagulation time) decreased with increase in degree of protein concentration during ultrafiltration of pasteurized cow skim milk. However, addition of stabilizing salts induced promising improvement in HCT values of liquid 5 × UF retentates (Meena et al. 2016). However, adopting such interventions, low-protein MPC powders have not been manufactured and characterized so far. Therefore, present investigation was undertaken to manufacture, characterize and evaluate the effects of (i) Disodium phosphate (DSP, Na2HPO4) addition with and without homogenization in ultra-filtered retentate and (ii) diafiltration of ultra-filtered retentate with DSP and NaCl salts on changes in various properties of low-protein MPC powders.
Materials and methods
Material
For each trial, fresh cow milk was procured from experimental dairy located at ICAR-National Dairy Research Institute, Karnal, India. Thereafter, it was preheated, separated and pasteurized (73 ± 1 °C) to obtain pasteurized cow skim milk (PCSM). The total solid (TS), calcium, ash, protein contents and pH of PCSM (n = 3) were 8.34, 0.12, 0.67, 3.17% and 6.60, respectively. Sigma-Aldrich (St. Louis, MO, US) chemicals were used in this investigation.
Methods
Manufacturing of low-protein MPC powders
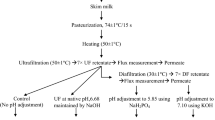
Total 400 kg PCSM was heated at 50 ± 1 °C and then concentrated up to 4.50 × concentration factor (CF) using a pilot scale UF Plant (Techsep, France, membrane area 1.8m2, 50 kDa) in ~ 7 h to obtain 0.55 protein to TS ratio in UF retentate. This 4.50 × UF retentate (TS-19.63%, pH-6.65) was then equally divided into five parts and subjected to following treatments:
-
Control sample (C): No treatment was given to first part, it was treated as control. Its TS and pH were 19.63 and 6.54, respectively.
-
Treatment 1 (T1): Second part of UF retentate was diafiltered with 40 kg reverse osmosis (RO) water containing 150 mM Na2HPO4 in the same UF plant.
-
Treatment 2 (T2): Third part of UF retentate was diafiltered with 40 kg reverse osmosis (RO) water containing 150 mM NaCl in the same UF plant.
-
Treatment 3 (T3): Forth part of UF retentate was homogenized (2500 and 500 psi pressure) followed by its pH was adjusted to 6.60 using Na2HPO4 solution.
-
Treatment 4 (T4): The pH of the fifth part of UF retentate was adjusted to 6.60 using Na2HPO4 solution, but this retentate was not homogenized.
All 4.50 × UF (control and treated) retentates were subjected to spray drying (185/85 ± 5 °C) to obtain MPC55 powders such as control (MPC-C), and treated (MPC-T1, MPC-T2, MPC-T3 and MPC-T4) powders. Production flow diagram of these powders is depicted in Fig. 1. A single stage spray drier (Jektrons Engineers Pvt. Ltd., Bhosari, Maharashtra) equipped with a rotary/ disk type atomizer and having feed rate of 110 kg/h was used for drying of these retentates. Feed samples were atomized at 20,000 rpm.
Low-density polyethene (LDPE) laminate pouches were used to pack and store these powder samples at 25 ± 1 °C for two months. Production and analysis of these low-protein MPC powders were carried out three times. Solubilities of these samples were determined at zero (fresh) and after 60 days of storage.
Low-protein MPC powders: evaluation of different properties
Chemical composition such as TS, fat, ash, protein, and calcium contents of low-protein MPC powders were determined using standard methods (AOAC 1998). A method described by Sjollema (1963) was used to estimate the loose bulk density (LBD, g mL−1), packed bulk density (PBD, g mL−1) and flowability (as angle of repose, \(\theta\)°) of these samples. Dispersibility (%) was measured adopting the method described by the American Dry Milk Institute (ADMI 1965). Wettability (s) of these powders were measured using the method reported by Muers and House (1962). Particle size distribution (d10, d50 and d90), specific surface area (SSA, m2 kg−1), Sauter (volume) mean diameter (D3,2 and D4,3), dispersion index were determined adopting the method reported by Patil et al. (2018). Hunter colorimeter (Colour Flex®, Hunter Associates Laboratory Inc., VA, U.S.A.) and Aqua lab (U.S.A.) equipment’s were used to determine colour (L*, a* and b*) and water activity (aw) values of low-protein MPC powders, respectively.
Methods described by Meena et al. (2018) were used to determine the solubility (%), viscosity (mPa.s), heat coagulation time (HCT), foaming capacity and stability (%), emulsion capacity (%) and stability (min) of reconstituted low-protein MPC solutions. Buffering capacity of reconstituted low-protein MPC solutions were determined using a method described by Van Slyke (1922) while heat coagulation time (HCT) of these solutions were determined at 140 °C adopting the method reported by Crowley et al. (2014).
Viscosity measurement and rheological modelling
To determine apparent viscosity (at 50 s−1), low-protein MPC powders were reconstituted to a 10% w/v solutions. A Rheometer (MCR 52, Anton Paar, Germany) equipped with a cone plate (CP) 75–1° geometry made of stainless steel was used for this purpose. The rheological data obtained in 1 to 100 s−1 range of shear rate at 20 ± 1 °C were fitted to Power law, Herschel–Bulkley and Bingham rheological models. Mathematically these models are expressed as follows:
where σ, σo, \(\dot{\gamma }, K, n \;{\text{and}} \;\eta o \) are shear stress (Pa), yield stress, shear rate (s−1), consistency index (Pa-sn), flow behaviour index and viscosity with no yield stress at zero shear rate.
Statistical analysis
Data (n = 9) obtained was statistically analysed for one-way analysis of variance, ANOVA using SAS Enterprise guide (version 5.1, 2012) as reported by Patil et al. (2018). For the comparison of mean values, Tukey's HSD test was employed.
Result and discussion
Effect of treatments on chemical composition of low-protein MPC powders
Applied approaches induced variation in chemical composition of treated low-protein MPC powders (Table 1). According to Crowley et al. (2014), protein, lactose, water and ash contents (% w/w) of MPC50 and MPC60 powders were 49.9, 35.8, 3.8, 7.8 and 60.8, 24.5, 4.0, 7.7%, respectively. Chemical composition of manufactured control and treated MPC55 (low-protein) powders showed variation with the values reported by Crowley et al. (2014) for MPC50 and MPC60 powders. Particularly, ash contents of the samples evaluated in this study were higher. This was due to addition of Na2HPO4 and sodium chloride salts either in DF or for pH adjustment. Lactose and calcium contents were significantly higher (P < 0.05), while ash content was significantly lower (P < 0.05) in control powder compared to other treated powders (Table 1). Diafiltration of 4.50 × retentate (as mentioned in Treatment 2 and 3) and adjustment of retentate pH with Na2HPO4 (Treatment 4 and 5) caused the significant decrease (P < 0.05) in lactose and calcium contents (data not shown) of the resultant low-protein production powders. Relatively higher passage of lactose during applied diafiltration and possible dilution, resulted from DSP addition in 4.50 × retentate were responsible for such decrease in their lactose contents. Further, these treatments are also responsible for significant increase (P < 0.05) in ash contents of MPC-T1, MPC-T2, MPC-T3 and MPC-T4 powders and pH of their solutions than that of control powder (Table 1). Ash content of MPC powder produced applying T1 treatment was markedly higher and it was attributed to DF of UF retentate with Na2HPO4 which associated with its components and restricted higher passage of minerals into permeate.
Properties of MPC powders are also influenced by their calcium content (Meena et al. 2018). It was observed that applied treatments caused significant decrease (P < 0.05) in calcium contents of MPC-T1, MPC-T2, MPC-T3 and MPC-T4 powders compared to control powder (Table 1). Such decrease in their calcium contents were attributed to diafiltration of 4.50 × retentate with DSP and NaCl salts. Diafiltration process is known to enhance the protein purity of UF retentate via its dilution as this process facilitates higher mineral migration into permeate which is otherwise not possible in UF alone (Meena et al. 2016). Apart this, diafiltartion of 4.50 × retentates with DSP and NaCl containing RO water might have inter-changed calcium and sodium ions present in casein micelles. Such replacement of calcium with sodium ions in 4.50 × retentates might have led to higher migration of soluble calcium into permeate and decreased the total calcium content of MPC-T1 and MPC-T2 powders (Table 1). Bhaskar et al. (2003), Mao et al. (2012) and Sikand et al. (2013) also reported reduction in total calcium of MPC80 powders through NaCl/ KCl assisted diafiltration of UF retentates. However, the extent of diafiltration used in this investigation is low (UF retentate to RO water ratio was about 1:2 only) that resulted lower decrease in calcium contents compared to above cited studies. Furthermore, applied treatments are also responsible for the observed difference in pH of the reconstituted solutions of these powders (Table 1).
Effect of treatments on physical properties of low-protein MPC powders
The observed differences in various physical properties of manufactured low-protein powders are shown in Table 2. The LBD and PBD values are measures of random loose packing and random dense packing of powders particles. The LBD and PBD values of these powders showed a significant difference (P < 0.05) with each other. Tapped density of MPC50 and MPC60 powders were 0.59 g cm−3 and 0.54 g cm−3 (Crowley et al. 2014). The PBD values obtained in this investigation for low-protein MPC powders were in line with above mentioned tapped density values. The LBD and PBD values of MPC-T2 and MPC-T3 powders were lowest and statistically at par (P > 0.05) with each other. The observed variation in LBD and PBD values of low-protein MPC powders could be explained by difference in their particle size distribution and surface-volume mean particle diameter (Table 2). Bulk density of dried milk powders have been experimentally demonstrated and reported in the literature to relate well with LBD and PBD values. Further, LBD and PBD values of powders are being a complex property depends on several factors related to feed characteristics and processing parameters.
Water activity (aw) has a major influence on quality parameters of dried milk powders during their storage. To minimize the nutritional losses and also to retard the crystallization of amorphous lactose, storage of dried powders at proper temperature and aw conditions (preferably below the glass transition of lactose) is critical (Pugliese et al. 2016). Mistry and Pulgar (1996) reported that deterioration of reconstitution properties and problems of lumping and caking in MPC powders during storage is favoured by irreversible conversion of the amorphous lactose to its crystalline form. A significant difference (P < 0.05) was observed in aw values of low-protein MPC powders. However, aw values of control and MPC-T2 powders were statistically at par (P > 0.05) with each other. The observed differences in aw values of these powders were maily attributed to difference in their moisture content as a function of applied treatments that caused variation in their chemical composition (Table 1).
In general, high-protein MPC powders are greyish-white in color while skim milk powder is yellowish-white in color (Mistry and Pulgar 1996). It is one of the important physical property of milk powders that decide their compatibility and use in different food formulations. The effect of applied treatments on color values of low-protein MPC powders is shown in Table 2. The b* values of these powders were significantly difference (P < 0.05) with each other. The observed variation in their color values might be attributed to extent of browning (Maillard reaction) during spray drying at constant temperature as a function of their chemical constituents.
Flowability measures the free-flowing characteristics of high-protein powders. It plays significant role in handling and packaging of powder samples. Free-flowing powders are generally preferred by end users. According to Carr (1965) classification, MPC-C, MPC-T2 and MPC-T4 powders were observed as fairly free-flowing (\(\theta\) = 35–45°), while MPC-T1 and MPC-T3 having even lower \(\theta\) values (i.e. 31.94° and 34.28°) exhibited better (easy) flow. Small variation in flowability of low-protein MPC powders could be explained by the difference in their specific surface area and particle size values.
The ability to absorb water by milk powders through their surface is called wettability (Meena et al. 2018). Indeed, it is the time required for a given amount of powder to get wet. This depends on various factors such as surface activity, charge, area, porosity, density and size of milk powder particles. Aggregated (such as MPC and casein) powders are criticized for their poor wettability (> 120 s) because of their different surface composition (Schuck et al. 2013) compared to better wettability values (< 30 s) of skim milk powder, whole milk powders and dairy whiteners. The low-protein MPC powders also exhibited poor wettability (Table 2) because of their higher specific surface area and smaller size of powder particles. In general, powders with smaller particles, needs higher time for the completion of wetting process.
Dispersibility of milk powders is an important physical property that has paramount importance in reconstitution of powder particles. Huppertz and Gazi (2015) analysed 32 commercial (MPC35 to MPC90) samples of MPC powders and observed a wide variation (38 to 100%) in their dispersibility values. In present investigation, applied treatments significantly increased (P < 0.05) the dispersibility of treated (MPC-T1, MPC-T2, MPC-T3 and MPC-T4) powders over control powder (Table 2). Singh and Newstead (1992) have reported that presence of larger particle size in powders led to improvement in their dispersibility values. However, increase in percentage of fine particles (< 90 μm) decreses dispersibility of powders. Maximum specific surface area or lower particle size could explain the lowest dispersibility values of MPC-C (control) powder. Contrary to this, the applied treatments might have prevented the severe casein-casein interactions during spray drying. Furthermore, reduction in calcium contents, increase in pH (as a result of Na2HPO4 addition) and homogenization could explain their higher dispersibility values (Table 2).
Specific surface area, average particle size, volume mean diameter, and particle size distribution of low-protein MPC powers are shown in Table 2. A clear difference was observed in these parameters for the low-protein powders manufactured in this investigation. Feed viscosity had a major role on atomization of spray drier feed and particle size of final powders. It was established through our earlier investigation that diafiltration, Na2HPO4 and trisodium citrate addition increased, but homogenization decreased the viscosity values of five fold UF retentate (Meena et al. 2016). Variation in these parameters of low-protein MPC powders were attributed to changes induced in feed properties (data not shown) as a function of applied treatments (T1, T2, T3 and T4). Homogenization reduced the particle size in reconstituted MPC80 solution (Sikand et al. 2012). Similar findings were reported by Mao et al. (2012) for MPC80 that was produced from retentate previously diafiltration with 150 mM NaCl solution.
Effect on functional properties of low-protein MPC powders
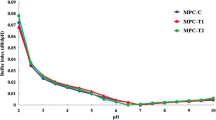
Among different functional properties of dried milk powders, solubility is of prime importance as it governs the expression of other functional properties in various product applications. Solubility of low-protein (MPC55-MPC60) powders were reported in the range of 68–88% by Floris et al. (2007). For 32 commercial (MPC35 to MPC90) samples of MPC powders, Huppertz and Gazi (2015) reported even lower (27 to 81%) solubility values. Applied treatments significantly increased (P < 0.05) the solubility values of the treated low-protein MPC powders over control (Fig. 2). These solubilities were also higher than the values reported by Floris et al. (2007) for MPC55-MPC60 powders. Higher dispersibility of treated powders also advocates their better solubility than control. Solubility of MPC powders had inverse relation with their calcium and protein contents. Such increase in solubility is related to applied treatments which caused reduction in calcium contents (Table 1). During MPC production, decrease in calcium contents of ultrafiltered retentate led to weaker casein-casein and casein-mineral interactions which favours increase in solubility. Apart this, solubility of milk proteins increases with increase in pH. Homogenization also caused shear induced physical changes in casein micelles that prevents severe casein-casein interactions during spray drying of treated ultrafiltered retentate and contributed towards better solubility in treated powder. Thus, applied treatments were responsible for better solubility of fresh low-protein MPC powders. Furthermore, applied treatments also found effective in retaining higher solubility of treated powders during storage over control powder (as shown in Fig. 2). Such improvement in solubility of high-protein (MPC80) powders via different interventions such as addition of calcium chelators (Fox et al. 1998), emulsifying salts (Meena et al. 2018), increase in pH (Broyard and Gaucheron 2015) and, diafiltration with NaCl and KCl salts (Mao et al. 2012) and homogenization (Sikand et al. 2012) have been earlier reported in the literature.
Thermal stability or heat coagulation time (HCT) of reconstituted MPC solutions is of great importance during their utilization as a high protein ingredient in formulations which needs processing at relatively higher temperatures. Huppertz and Gazi (2015) determined the HCT of 32 commercial, reconstituted MPC (MPC35 to MPC90) solutions. Their HCT values were ranged between 0–40 min. Crowley et al. (2014) reported that at 6.3, 6.7, 7 and 7.3 pH, HCT of reconstituted solutions of MPC60 were 1, 3, 13 and 18 min only. The HCT of reconstituted control and treated low-protein MPC solutions are given in Table 2. Reconstituted MPC-T1, MPC-T3 and MPC-T4 solutions exhibited maximum HCT (120 min) values which were statistically at par (P > 0.05) with each other. These values were significantly higher (P < 0.05) than that observed for reconstituted MPC-C (16 min, minimum) and MPC-T2 (19 min) solutions. Presence of higher calcium content and lower pH were responsible for poor HCT of the reconstituted solution of control powder. Contrary to this, applied treatments caused an increase in pH and decrease in calcium contents of reconstituted solutions of treated powders which markedly improved their HCT values. Although, T2 sample had lower calcium content, but its pH was also lower compared to other treated samples that could explain its higher and lower HCT value than control and other treated samples. Le Ray et al. (1998) reported that added sodium citrate and sodium phosphate salts improved the heat stability of casein micelles dispersions due to decrease in micellar minerals and enhanced steric repulsions.
A significant difference (P < 0.05) was observed in the viscosities of reconstituted solutions of control and treated powders (Table 2). Such variation in their viscosities could be attributed to the difference in their chemical composition (Table 1), physical and reconstitution properties (Table 2) as a function of applied treatments. Viscosity of milk protein solutions are inversely proportional with the pH of the solution. Matheis et al. (1983) reported that addition of phosphate led to increase in viscosity of casein solution which could also explain higher viscosities of reconstituted solutions of MPC-T1 and MPC-T4 powders, lower pH can explain higher viscosity of T2 sample.
Foam capacity and foam stability of low-protein MPC solutions are shown in Table 2. The foam capacity and foam stability of all treated powder solutions were significantly (P < 0.05) higher compared to that of control. Foam stability of control and MPC-T4 solutions were statistically at par (P > 0.05) with each other. Improvement in foaming properties was attributed to increase in electronegativity of proteins in these solution due to their higher pH (Table 1) and specific surface area values (Table 2). A positive correlation between foaming properties and particle size of milk powders is well-established.
Table 2 shows the emulsion capacity and emulsion stability of low-protein MPC solutions. Emulsion capacity of control and MPC-T2 solutions were statistically par (P > 0.05) with each other, but significantly higher (P < 0.05) than that of MPC-T1, MPC-T3 and MPC-T4 solutions. Lower emulsion capacity of these solutions were attributed to disodium phosphate addition. Matheis et al. (1983) also reported that addition of 7.4 phosphate groups per mole of caseins led to decrease in emulsification capacity in casein solution. The MPC-T1, MPC-T2 and MPC-T4 solutions showed significantly higher (P < 0.05) emulsion stability than that of control and MPC-T3 solutions.
Effect on buffer index
Using 0.1 N HCl and 0.1 N NaOH solutions, the natural pH values of reconstituted (0.5 g 100 mL−1 protein) control and treated low-protein MPC powder solutions were increased (alkalization) and decreased (acidification) between 2 and 10 pH ranges to determine their buffering capacity or Buffer index. The amount of alkali and acid consumed during titration is shown in Fig. 3a, while subsequent change in Buffer index values of these reconstituted powder solutions are shown in Fig. 3b, respectively. Higher buffer index of reconstituted solutions of treated powders were mainly contributed by the added phosphate and sodium salts (disodium phosphate and sodium chloride). Broyard and Gaucheron (2015) reported that such groups bind the protons to resist the change in pH. Mizuno and Lucey (2005) reported that Na2HPO4 addition increased the buffer index of casein micelles (5% wt/wt) solution at 5.8 pH. Salaun et al. (2005) reported that total buffering capacity of milk powders receives 5%, 20%, 35% and 40% contribution from whey proteins, colloidal calcium phosphate, casein and soluble minerals, respectively. Thus, observed difference in buffer index of reconstituted control and treated MPC samples was mainly attributed to difference in their chemical-makeup.
Effect on rheological behaviour of reconstituted solutions of low-protein powders
Flow behaviour of all reconstituted MPC (10 g mL−1) solutions were evaluated. All these samples exhibited a non-Newtonian flow characteristics and their shear-thinning behaviour was best described by the Herschel–Bulkley model (Table 3). It suggested the requirement of yield stress to commence the flow. The flow behaviour index of all samples was less than unity, indicating the flow pattern similar to pseudo-plastic flow behaviour post-yield stress. This indicates the effects of higher protein content in gelation that tends to flow like condensed milk with increase in shear-rate. Addition of disodium phosphate reduced the yield stress in MPC-T1, MPC-T3 and MPC-T4 solutions in comparison to MPC-C (control) and MPC-T2 solutions. Further, disodium phosphate was found effective in reducing the gelation when employed as during diafiltration and along with homogenization. However, NaCl addition during diafiltration process increased the yield stress to the greater extent, which had a significant impact on the HCT and viscosity in MPC-T2 solutions. Its lower pH than other samples could also play important role in rheological behaviour. The addition of DSP results in lower yield stress as compared to NaCl.
Conclusion
Applied treatments induced significant changes in chemical composition and different properties of manufactured low-protein powders. Presence of higher calcium and protein (particularly) casein contents have detrimental effects on solubility and other functional properties of MPC powders. Extent of casein-casein and casein-calcium interactions taking place during spray drying of ultrafiltered retentates mainly determine the end properties of the resultant powders. Disodium phosphate addition led to increase in pH and decrease in calcium contents which ultimately reduced the severity of these interactions. This has been hypothesized as the key mechanism behind the marked improvement in solubility, dispersibility, specific surface area, viscosity, foam capacity and foam stability, buffer index, thermal stability and emulsion stability values of treated low-protein MPC solution over these properties of control powder solution. Herschel–Bulkley model efficiently described the rheology of reconstituted solutions of these powders. This investigation has been established the production, characterization of different powders manufactured using applied treatments which also improved their functional properties. Such powders can be used in different food formulation to tailor their nutritional potential and sensory attributes.
References
AOAC (1998) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Washington, DC
Alexander M, Nieh MP, Ferrer MA, Corredig M (2011) Changes in the calcium cluster distribution of ultrafiltered and diafiltered fresh skim milk as observed by small angle neutron scattering. J Dairy Res 78:349–356
American Dry Milk Institute, Standards for grades of dry milk including methods of analysis. Bull. 915 (1965) ADMI, USA
Bhaskar GV, Singh H, Blazey ND (2003) Milk protein products and processes. US Patent US 2003/0096036
Broyard C, Gaucheron F (2015) Modifications of structures and functions of caseins: a scientific and technological challenge. Dairy Sci Technol 95:831–862
Carr RL (1965) Evaluating flow properties of solids. Chem Eng 72(2):163–169
Crowley SV, Gazi I, Kelly AL, Huppertz T, O’Mahony JA (2014) Influence of protein concentration on the physical characteristics and flow properties of milk protein concentrate powders. J Food Eng 135:31–38
Floris R, Alting A, Slangen C, van der Meulen IE, Adamse M, Klok H, Verbeek M (2007) MPC functionality: a comparitive study of commercial samples. NIZO-Rep E 157:1–55
Fox PF, McSweeney PLH (1998) Water in milk and dairy products. In: Fox PF, McSweeney PLH (eds) Dairy chemistry and biochemistry. Blackie Academic & Professional, London, pp 308–309
Huppertz T, Gazi I (2015) Milk protein concentrate functionality through optimized product-process interactions. New Food 18(1):12–17
Mao XY, Tong PS, Gualco S, Vink S (2012) Effect of NaCl addition during diafiltration on the solubility, hydrophobicity, and disulfide bonds of 80% milk protein concentrate powder. J Dairy Sci 95:3481–3488
Matheis G, Penner MH, Feeney RE, Whitaker JR (1983) Phosphorylation of casein and lysozyme by phosphorus oxychloride. J Agric Food Chem 31:379–387
Meena GS, Singh AK, Borad S, Panjagari NR (2016) Effect of concentration, homogenization and stabilizing salts on heat stability and rheological properties of cow skim milk ultrafiltered retentate. J Food Sci Technol 53(11):3960–3968
Meena GS, Singh AK, Gupta VK, Borad S, Parmar PT (2018) Effect of change in pH of skim milk and ultrafiltered/diafiltered retentates on milk protein concentrate (MPC70) powder properties. J Food Sci Technol 55(9):3526–3537
Meena GS, Singh AK, Raju PN, Arora S (2017) Milk protein concentrates: opportunities and challenges—a review. J Food Sci Technol 54(10):3010–3024
Mistry VV, Maubois JL (2004) Application of membrane separation technology to cheese production. Cheese Chem Phys Microbiol 1:261–285
Mistry VV, Pulgar JB (1996) Physical and storage properties of high milk protein powder. Int Dairy J 6:195–203
Mizuno R, Lucey JA (2005) Effects of emulsifying salts on the turbidity and calcium-phosphate–protein interactions in casein micelles. J Dairy Sci 88:3070–3078
Muers MM, House TU (1962) A simple method for comparing wettability of instant spray dried sep-arated milk powders. In: XVI international dairy congress, Copenhagen, Denmark, vol 8, pp 299
Patel H, Patel S (2014) Milk protein concentrates: manufacturing & applications- technicalreport.https://www.usdairy.com/~/media/usd/public/mpc_tech_report_final.pdf. Accessed 8 Feb 2020
Patil AT, Meena GS, Upadhyay N, Khetra Y, Borad S, Singh AK (2018) Production and characterization of milk protein concentrates 60 (MPC60) from buffalo milk. LWT-Food Sci Technol 91:368–374
Pugliese A, Paciulli M, Chiavaro E, Mucchetti G (2016) Characterization of commercial dried milk and some of its derivatives by differential scanning calorimetry. J Therm Anal Calorim. https://doi.org/10.1007/s10973-016-5243-y
Le Ray C, Maubois JL, Gaucheron F, Brulé G, Pronnier P, Garnier F (1998) Heat stability of reconstituted casein micelle dispersions: changes induced by salt addition. Le Lait 78(4):375–390
Salaun F, Mietton B, Gaucheron F (2005) Buffering capacity of dairy products. Int Dairy J 15:95–109
Schuck P (2013) Dairy protein powders. In: Smithers GW, Augustin MA (eds) Advances in dairy ingredients and the institute of food technologists. Wiley, Hoboken, pp 1–29
Sikand V, Tong PS, Roy S, Rodriguez-Saona LE, Murray BA (2013) Effect of adding salt during the diafiltration step of milk protein concentrate powder manufacture on mineral and soluble protein composition. Dairy Sci Technol 93:401–413
Sikand V, Tong PS, Vink S, Walker J (2012) Effect of powder source and processing conditions on the solubility of milk protein concentrates 80. Milchwissenschaft 67(3):300–303
Singh H, Newstead DF (1992) Aspects of proteins in milk powder manufacture. In: Fox PF (ed) Advanced dairy chemistry, vol 1, proteins, 2nd edn. Elsevier, New York, pp 735–765
Sjollema A (1963) Some investigation on the free flowing properties and porosity of milk powders. Neth Milk Dairy J 17:245–253
Van Slyke DD (1922) On the measurement of buffer values and on the relationship of buffer value to the dissociation constant of the buffer and the concentration and reaction of the buffer solution. J Biol Chem 52(525570):20
Acknowledgements
The economic assistance and required facilities required to conduct this piece of work was provided by the Director, ICAR-National Dairy Research Institute, Karnal. Authors thankfully acknowledge the same.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meena, G.S., Singh, A.K. & Gupta, V.K. Production and characterization of cow milk based low-protein milk protein concentrate (MPC) powders. J Food Sci Technol 58, 3205–3214 (2021). https://doi.org/10.1007/s13197-020-04824-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04824-5