Abstract
Milk protein concentrate (MPC) powders offer a great potential for use in an array of food applications because of their nutritional and functional values. However, MPC powders with protein content ≥80% (MPC80) exhibit poor solubility and hence restrict their potential use. The objective of this study was to determine the impact of adding salt during the diafiltration stage of MPC80 manufacture on solubility, turbidity, and to compare minerals and protein content of supernatants of ultracentrifuged samples with control sample. Three types of samples were produced: MPC80-C (control) with or without salt treatment, MPC80-Na (150 mM NaCl), and MPC80-K (150 mM KCl). Lower solubility was observed in MPC80-C (53%) as compared to MPC80-Na or MPC80-K (100%). Higher turbidity was observed in MPC80-C (530 NTU) and lower turbidity was observed in samples of MPC80-Na (128 NTU) and MPC80-K (131 NTU). Furthermore, lower protein and calcium contents were observed in supernatants of ultracentrifuged samples of MPC80-C (2.3%; 0.35 mg.mL−1) as compared to MPC80-Na (3.8%; 0.63mg.mL−1) and MPC80-K (3.7%; 0.67 mg.mL−1). The opposite trend was found in reconstituted samples (5% TS). Our results showed that the addition of salt impacted the distribution of minerals and proteins in colloidal and soluble phases of MPC80-Na and MPC80-K. The results from this work will contribute to our understanding of the role that mineral-induced changes (depletion or addition) play in the functionality of MPC80.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Milk protein concentrate (MPC) powder is manufactured by ultrafiltration, diafiltration followed by spray drying (Mistry and Hassan 1991a). The ultrafiltration process removes smaller components such as lactose, water, minerals, and non-protein nitrogen compounds from milk while larger components such as caseins, whey proteins, and lipids are retained. Additionally, a portion of minerals such as calcium, magnesium, phosphate, and citrate may be retained because they are associated with casein micelles (Singh 2007). In the diafiltration process, additional lactose and minerals are removed. Depending on the degree of diafiltration, MPC with 80% (MPC80) or 85% (MPC85) protein content can be produced. Thus, the final product of high protein milk powder has higher protein (83.9%), ash (7.05%), and a lower lactose content (0.73%) (Mistry and Hassan 1991a).
Processing steps such as ultrafiltration, diafiltration, and spray drying used during the manufacture of MPC results in changes in salt equilibrium between the colloidal and soluble phases of this system, which may adversely affect the environment of milk proteins (Mistry and Hassan 1991b; Singh 2007). These changes may negatively impact functional properties such as solubility. High protein milk powders such as MPC80 and native phosphocaseinate have been reported to be poorly soluble when tested at 25 °C (Mistry and Hassan 1991b; Schuck et al. 2007). A subsequent study by NIZO group (Huppertz et al. 2010) conducted on 32 commercial MPCs collected globally indicated that the low solubility of MPC80 continued to be a problem. Solubility was found to be negatively correlated with protein content. Furthermore, low solubility of MPC85 was found to have increased with respect to increased time and temperature of storage (Anema et al. 2006; Havea 2006).
Various studies on ways to improve solubility of high protein milk powders have been conducted. The addition of minerals has been reported to play an important role in the water transfer during spray drying of milk (Schuck et al. 1999). Furthermore, these authors (Schuck et al. 2002) reported that quality of milk powder depends on when mineral salt is added such as before, during, or after spray drying. Additionally, they reported an improved water transfer in casein micelles upon addition of NaCl and attributed it to hygroscopic nature of NaCl. Enhanced solubility of MPC80 has been reported where calcium ions were exchanged with sodium ions (Bhaskar et al. 2001). Furthermore, Carr et al. (2002) disclosed that improved cold solubility of MPC can be achieved upon addition of monovalent salt prior to evaporation or spray drying of retentate. Similarly, improved solubility of micellar casein was reported by adding sodium caseinate before spray drying of milk concentrate (Schokker et al. 2011). Thus, improved solubility was attributed to reduced micellar interaction and increased release of non-micellar casein (Schokker et al. 2011), structural changes, and mineral composition (Famelart et al. 1999), resulting in modified protein–protein interactions (Schuck et al. 1999).
Many factors such as temperature and shear and ionic strength play an important role in imparting solubility of MPC or native micellar casein (Zwijgers 1992; Schuck et al. 2002; Hussain et al. 2011; Sikand et al. 2012). Minerals, especially monovalent salts such as NaCl or KCl, can be manipulated to increase ionic strength and may improve solubility of MPC80. Improved solubility was shown in our laboratory (Mao et al. 2012) when 50–150 mM NaCl was added during diafiltration stage of MPC80 manufacture. Enhanced solubility of MPC80 was associated with the modification of hydrophobicity and the reduction in the formation of disulfide bonds, which may reduce protein aggregation during concentration and drying.
Like milk, the proteins in MPC are made up of roughly 80% caseins and 20% whey proteins. The caseins, being the major component of milk protein, exist in colloidal dispersion and form complexes with inorganic phosphate and calcium (Schmidt 1982). The colloidal calcium phosphate plays an integral role in maintaining the structure of casein micelles. Besides colloidal calcium phosphate, other forces such as hydrophobic, electrostatic, and hydrogen bonding are important for structural integrity. Various factors such as mineral salts (Griffin et al. 1988; Ward et al. 1997), high pressure (Altuner et al. 2006), and alkalinization (Vaia et al. 2006) are responsible for disrupting casein micelle stability. As a result, many changes such as reduced turbidity, modified distribution of calcium phosphate in colloidal and soluble phase have been reported. Aoki et al. (1999) reported that an addition of NaCl decreased casein aggregates cross-linked by micellar calcium phosphate and thus, forming the loosened casein micelle structures. Upon the addition of NaCl to bovine milk, calcium and phosphate in casein micelles probably exchanged with sodium and chloride ions in the aqueous phase. As a result, different properties of casein micelles are exhibited, such as enhanced solubility.
The current study was conducted to measure the solubility of KCl-treated MPC80 samples and was compared with the solubility of NaCl-treated MPC80 samples, already available in our lab (Mao et al. 2012). There has not been much documentation about effect of adding salt during the diafiltration stage of MPC80 manufacture on minerals and protein distribution.
The objectives of this study were to: (1) evaluate and compare the solubility of MPC80 powders manufactured by adding 150 mM NaCl with 150 mM KCl during the diafiltration step as compared to control (untreated) and (2) evaluate and compare mineral and protein composition of reconstituted MPC80 powder samples treated with or without monovalent salts and their respective supernatants from ultracentrifuged samples (soluble phase). The results from this work will contribute to our understanding of the role of mineral-induced changes on MPC80 functionality.
2 Materials and methods
2.1 Samples preparation
MPC80 powder samples were manufactured at Dairy Products Technology Center, San Luis Obispo, CA. All chemicals used were reagent grade.
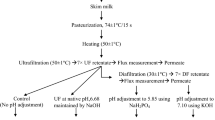
MPC80 powders were manufactured as described by Gualco (2010). Pasteurized skim milk (140 kg) was ultrafiltered by using a model R12 cross flow membrane re-circulatory pilot plant unit (Niro Inc, Hudson, WI, USA) equipped with dual 10 kDa cut-off spiral-wound polyethersulfone membranes (Snyder Filtration, Vacaville, CA, USA). Ultrafiltration (UF) was commenced at 5.9 ± 1.2 °C. During UF, the temperature was allowed to increase in such a way that by end of the UF process, the temperature was 19.7 ± 1.2 °C. Milk was concentrated up to 6×. UF milk was mixed with 117 kg of water containing either no salt or salt (150 mM NaCl or KCl). The product was diafiltrated until 6× concentration was achieved. A pre-mixed salt solution (150 mM NaCl or KCl) was used at each diafiltration stage for salt-treated samples. The ultrafiltered milk was collected after the third (final) diafiltration stage and spray-dried by using a Niro Filterlab (Hudson, WI) unit. Inlet temperature was 208.7 ± 19.7 °C and outlet temperature was 82.0 ± 0.8 °C. Thus, three types of MPC80 powders were produced. The control sample was named MPC80-C and had no salt added in water during the diafiltration stage of MPC80 manufacturing. The sample was named MPC80-Na when water was used for washing the retentate containing 150 mM sodium chloride (NaCl), and was named MPC80-K when water was used during the washing of retentate containing150 mM potassium chloride (KCl) during powder manufacturing. MPC80 powders were manufactured in three batches following a randomized complete block design and as a result, a total of nine samples were produced.
All of the freshly manufactured powders were placed in airtight Ziplock Mylar® bags and stored in airtight containers at 4 °C for future testing. The powder samples were tested within 6 months of manufacture.
2.2 Methods
2.2.1 Basic composition
Samples of MPC80 powders (MPC-C, MPC80-Na, and MPC80-K) were analyzed for basic composition analysis (AOAC 1995). Total nitrogen was determined by the Dumas method (AOAC 993.13) and multiplied by 6.38 to arrive at the protein content. Ash content was determined by ignition at 550 °C in an electric muffle furnace (AOAC 945.46; 33.2.10). Fat content was determined by the Mojonnier method (AOAC 989.05; 33.2.26) and free moisture content by oven-drying method (AOAC 990.20; 33.2.44). Lactose content was determined by the HPLC method as described by Amamcharla and Metzger (2011).
2.2.2 Nitrogen solubility index
Nitrogen solubility index (NSI) of MPC80 powders was measured at room temperature (21 ± 2 °C) by method of (Morr et al. 1985) with some modifications where 500 mg of dry MPC80 powder was mixed with a small aliquot of DI water to form a paste. Additional water was added to bring the total volume of the dispersion to about 40 mL. The pH of the dispersion was adjusted to 7.0 with a 0.1 N HCl solution. After stirring for an hour, the dispersion was then transferred into a 50-mL volumetric flask, diluted to the mark with additional water. An aliquot of the dispersion was centrifuged for 30 min at 20,000×g and resulting supernatant fraction was filtered through Whatman No. 1 filter paper. The Vario Max analyzer (Hanau, Germany) was used to determine the nitrogen content in the samples by the Dumas method. A conversion factor of 6.38 was used to convert nitrogen to protein content.
2.2.3 Preparation of reconstituted milk from MPC80 powder samples
Three types of MPC80 powder samples from three different trials were reconstituted in random order to contain 5% total solids (TS) content. These samples were stirred for 4 h at room temperature (21 ± 2 °C). After 4 h of stirring at 900 rpm with a laboratory stirrer (R010 Power, IKA Works, Wilmington, NC), samples were tested for pH and were kept in a refrigerator overnight and brought to room temperature (21 ± 2 °C) the next day. pH of all the samples was measured again to ensure no spoilage of samples had occurred. One part of each type of sample was kept for measuring turbidity, protein content, and mineral contents (Ca, Mg, Na, K, and P) while the other aliquot was subjected to ultracentrifugation for further analyses of protein and minerals.
2.2.4 Preparation of ultracentrifuged samples from MPC80 reconstituted samples
Samples were ultracentrifuged at 100,000×g for 1 h at 21 °C using a SW50.1 rotor (Beckman Coulter, Fullerton, CA). A firm pellet and a liquid supernatant were formed upon centrifugation. The supernatant was analyzed for minerals such as Ca, Mg, Na, K, and P as described in Section 2.2.6 and total nitrogen was measured by Dumas method.
2.2.5 Turbidity
The turbidity of all the reconstituted MPC80 samples (5% TS) was measured using a nephelometer (Micro 1000 IR, Fort Myers, FL). These reconstituted samples were diluted in water (1:25) and kept for an hour to equilibrate at room temperature (21 ± 2 °C) before measuring turbidity. Turbidity measurements are reported in nephelometric turbidity units (NTU) for each sample.
2.2.6 Mineral analyses
Mineral analysis for reconstituted MPC80 powder samples (5% TS) and ultracentrifuged samples was determined by inductively coupled plasma optical emission spectroscopy (PerkinElmer, Waltham, MA). The mineral content of the reconstituted and supernatants of ultracentrifuged samples was analyzed by the method of Sikand et al. (2011).
2.2.7 Protein content and SDS–polyacrylamide gel electrophoresis
The protein content (N x 6.38) was measured in the samples reconstituted to contain 5% TS and their respective supernatants after ultracentrifugation by Dumas method.
A qualitative protein composition analysis and level in the reconstituted samples and their respective ultracentrifuged samples was done by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (15%) using a Mini-PROTEAN cell electrophoresis system (Bio-Rad Laboratories, Hercules, CA) per Laemmli method (Laemmli 1970). The reconstituted MPC80 samples were diluted to contain 2 mg.mL−1 protein content. The ultracentrifuged samples were diluted ten times. Furthermore, these samples were mixed with a sample buffer in a 1:1 dilution under reducing conditions. Gels were run at 110 volts. Gels were stained with Coomassie R-250 for 8–12 h and then de-stained the following day.
2.2.8 Statistical analyses
All analyses were done using ANOVA in the statistics program JMP 9.0.2 (SAS Institute). The response variables in each ANOVA were NSI, turbidity, mineral content, and protein content. Because samples were produced from three separate batches of MPC80 powder, a random batch factor was included in the analysis along with the treatment factor. Differences between the three treatments (MPC80-C, MPC80-Na, and MPC80-K) were tested using Tukey’s HSD intervals with α = 0.01.
3 Results and discussion
3.1 Basic composition
Compositional analyses of the three types of MPC80 powders are shown in Table 1.
3.2 Nitrogen solubility index
NSI for MPC80 samples was measured for MPC80-C, MPC80-Na, and MPC80-K. The solubility for these samples ranged from 53 to 100% and is shown in Table 2. MPC-C sample had a solubility of 53%. These results confirm previously reported findings of poor solubility measured at 25 °C of high protein milk powders such as MPC80 and micellar casein (Mistry and Hassan 1991b; Schuck et al. 2007). These results are in agreement with Carr et al. (2002) who reported low solubility (<60%) of fresh MPC85 reconstituted at 22 °C (±2 °C). De Castro-Morel and Harper (2002) attributed low solubility of MPC to susceptibility of milk proteins specifically whey proteins to heat denaturation. Furthermore, low solubility was attributed to casein aggregation via non-covalent bonding (Havea 2006; Anema et al. 2006) and slow release of casein micelles during dispersion (Mimouni et al. 2010).
The solubility of high protein milk powders such as MPC80 and micellar casein has been reported to improve by addition of monovalent salts such as NaCl. The hygroscopic nature of NaCl was used to explain the enhanced reconstitution of micellar casein. Furthermore, improved rehydration due to minerals was associated with modified protein–protein interactions (Schuck et al. 1999) followed by modification in the casein micelle structure (Baldwin 2010). Carr et al. (2002) reported significantly improved solubility (>60%) for MPC85 samples treated with monovalent salt as compared to control sample. These samples were stored at 40 °C for 1 month. The improved solubility of salt-treated samples was observed upon powder reconstitution at either 40 or 50 °C as compared to the control samples. In our study, we observed improved solubility (100%) in MPC80-Na and MPC80-K samples when they were tested in fresh samples, and similar results were obtained when tested for solubility at various intervals over 1 year for samples stored at 4 °C and at 21 °C. The data included in this study is for the solubility measurement of MPC80-Na and MPC80-K samples at 4 °C within 6 months of storage. Furthermore, we observed that samples treated with salt were more than 90% soluble when kept at room temperature for 1 year (results not included). Similar results for enhanced solubility of MPC85 were previously reported where calcium was partially replaced with sodium (Bhaskar et al. 2001). This improved solubility after prolonged storage was attributed to the enhancement of electrostatic repulsive forces between the casein micelles (Havea 2006).
Besides electrostatic forces, hydrophobic forces play an important role in the solubility of high protein milk powders (Anema et al. 2006; Havea 2006; Mao et al. 2012). Enhanced solubility of MPC80 treated with monovalent salts likely modifies hydrophobicity sites and reduces disulfide bond formation. Thus, such changes could modify protein–protein interactions that may limit protein aggregate formation and contribute to the improved solubility (Mao et al. 2012).
3.3 Turbidity
The turbidity for all three types of samples is given in Table 3. A significant decrease in the turbidity was observed in the MPC80-Na and MPC-K as compared to MPC-C sample. Low turbidity can be attributed to casein micelles dissociation as turbidity is a function of light scattering by the suspended colloidal casein micelles in the milk.
Casein micelles are well-known stable entities under the environmental conditions of milk. Various forces such as hydrophobic, electrostatic, hydrogen bonding, and calcium phosphate linkages are responsible for their stability. Several factors such as calcium chelators (Griffin et al. 1988), reduced pH (Famelart et al. 1999), high pressure (Altuner et al. 2006), alkanization (Ahmad et al. 2009), and ionic strength (Huppertz and Fox 2006) are accountable for imbalanced forces, and thus, are responsible for the structural integrity of casein micelles. Considering differences in ionic strength between control and salt-treated samples, exhaustive dialysis (24 h) of MPC80-Na and MPC80-K samples against MPC80-C was conducted. Also, the turbidity of the MPC80-Na and MPC80-K dialyzed samples against MPC80-C was tested. The turbidity of dialyzed samples was noted to be slightly higher, although not much different, than salt-treated undialyzed samples but still was significantly lower than the control samples (results not shown). This decrease of turbidity probably corresponds to a modification of the casein micelle organization. Thus, our results indicate that even after dialysis of MPC80-Na and MPC80-K against MPC80-C, both dialyzed samples still had reduced light scattering properties, which may be attributed to irreversible casein micelles dissociation. Thus, casein micelles were unable to regain their original light scattering power even after exhaustive dialysis.
3.4 Mineral analyses
Table 4 shows ANOVA results, conducted on each of the five minerals to detect differences between the treatments (with and without salt treatments). Mineral analyses were conducted for MPC80-C, MPC80-Na, and MPC80-K of reconstituted powder containing 5% TS. The reconstituted MPC80-C samples contained significantly (P = 0.0004) increased total calcium (1.07 mg.mL−1) than the MPC80-Na (0.80 mg.mL−1) and MPC80-K (0.81 mg.mL−1) samples. No significant difference (P = 0.1256) was found in the total phosphate between the three treatment groups. Our results are in agreement with previous findings (Famelart et al. 1999) of depleted colloidal calcium upon addition of monovalent salt (NaCl) and thus, improving the rehydration of high protein milk powders due to minerals distribution. As expected, significantly higher sodium (1.11 mg.mL−1) in MPC80-Na and higher potassium (1.83 mg.mL−1) in MPC80-K was observed as compared to sodium and potassium content in MPC80-C (0.11 mg.mL−1 and 0.03 mg.mL−1, respectively).
The mineral content of the supernatant fractions from ultracentrifuged milks was measured. The ANOVA results comparing the ultracentrifuged samples are displayed in Table 5. MPC80-Na and MPC80-K both had higher concentrations of total calcium (P = 0.0069) and Pi (P = 0.0037) than the MPC-C samples. Our results are in agreement with previous findings of solubilization of calcium and phosphate upon addition of monovalent salt such as NaCl (Famelart et al. 1999). Similar observations of calcium and phosphate solubilization have been reported using high pressure (Schrader et al. 1997) or calcium chelator (Udabage et al. 2000), thus leading to changes in the mineral distribution and hence calcium dissociation from casein micelle. No significant difference was observed in Mg levels in samples with or without salt treatment (P = 0.0779). As expected, higher sodium and higher potassium content was observed in MPC80-Na (1.01 and 1.70 mg.mL−1) as compared to the control’s sodium and potassium content (0.096 and 0.028 mg.mL−1). It has been reported that the monovalent ions, mainly Na, K, and Cl ions are present in the aqueous phase while ions such as Ca, Pi, citrate, and Mg are associated with casein micelles and to some extent in the aqueous phase. Sikand et al. (2011) reported high solubility in two commercial MPI samples and enhanced solubility was attributed to increased levels of monovalent cations such as sodium and potassium and decreased levels of divalent cations such as calcium and magnesium. In our current study, a significant decrease in Ca and a slight decrease in Pi were observed in the reconstituted milk of salt-treated MPC80 samples. This decrease in Ca and Pi may be due to the displacement of calcium bound to the casein micelle through phosphoseryl residues with sodium or potassium during washing process (diafiltration stage) of MPC80 manufacturing. However, these minerals were found to increase in the supernatants of ultracentrifuged MPC80-Na and MPC80-K. This can be attributed to calcium dissociation from casein micelles because, upon ultracentrifugation, higher levels of calcium and phosphate are observed. Huppertz and Fox (2006) reported that the addition of increasing level of NaCl (0–600 mM) resulted in increased levels of calcium in the serum phase of concentrated milk (15% TS). These authors attributed increased levels of soluble calcium to the increased ionic strength resulting in an increased solubility and calcium phosphate dissociation due to lower ion activity coefficient. Similarly, we observed an increased level of calcium in supernatants of ultracentrifuged MPC80-Na and MPC80-K. Contrary to the findings of Huppertz and Fox (2006) our study shows an increase in the phosphate levels of MPC80-Na and MPC80-K.
Similar observations were made with respect to an increase in pH by alkalinization (Ahmad et al. 2009). Furthermore, these authors reported that with alkalinization, Pi probably changed from HPO4 −2 to PO4−3. The latter form of PO4 −3 has a greater affinity for Ca as compared to HPO4 −2 (Vaia et al. 2006). Ahmad et al. (2009) suggested that calcium specifically interacts with phosphoseryl residue of casein micelles and is surrounded by PO4 −3 to form calcium phosphate salt. These authors attributed high calcium and phosphate in the supernatants to newly formed calcium phosphate that remained non-sedimentable by ultracentrifugation.
3.5 Protein analyses
The protein content in reconstituted milk (5% TS) and the supernatant fractions of ultracentrifuged milks were measured. Table 6 shows the ANOVA results. The mean total protein content in the reconstituted MPC80-C, MPC80-Na, and MPC80-K was not significantly different (P = 0.4444). However, the mean total protein content of the ultracentrifuged supernatants of MPC80-Na and MPC80-K was higher (3.78 and 3.68%) than the MPC-C (2.34%; P < 0.0001). Thus, release of protein in the supernatant of ultracentrifuged samples indicates casein micelle dissociation. The soluble protein complex may be responsible for subunit or submicelle structure of casein micelles, through which they are attached to colloidal calcium phosphate. Similar results of depleted colloidal calcium phosphate upon using calcium-chelating agents resulting in micellar casein dissociation with an increase in protein components (α-CN, β-CN, and k-CN) in supernatant samples has been reported (Griffin et al. 1988). In the current study, SDS-PAGE analyses of three types of supernatants of ultracentrifuged samples showed differences with respect to band intensities when equal quantities of the samples were loaded. Figure 1 shows that the band intensities of α-CN and β-CN protein of the ultracentrifuged supernatants of MPC80-Na and MPC80-K were higher than the MPC-C. Our results indicate that MPC80-Na and MPC80-K induced solubilization of casein especially α-CN and β-CN protein when compared to MPC80-C sample.
Rose (1968) reported protein solubilization at both sides of milk’s neutral pH. More solubilization has been found to be on acidic side (pH 5.3) as compared to other side of neutral pH (pH 7.0 or more). For the current study, MPC-C samples had a pH of 7.53, MPC80-Na had a pH of 7.76, and MPC80-K had a pH of 7.93. The study revealed protein solubilization only in MPC80-Na and MPC80-K as compared to MPC80-C. Similar effects of increased casein protein content in the pasteurized ultracentrifuged samples in bovine milk (pH = 8.6) and buffalo’s milk (pH = 9.7) have been reported (Ahmad et al. 2009). These authors attributed these changes to modifications in mineral equilibrium and protein ionization, which induces changes in casein micelles. Furthermore, these authors stated that increased levels of nitrogen content in the supernatants of ultracentrifuged samples confirm casein micelles dissociation. Our results are in agreement with Farrell et al. (1988) who reported increased solubility of proteins by the addition of KCl. Furthermore, these authors reported that with increased levels of ionic strength (35 to 140 mM), increasing values of calcium-induced solubility in alpha-casein was observed. Increased solubility may be the result of interactions between charged proteins groups and salt (K+ or Na+ and Cl−). Our current study shows similar protein levels in the reconstituted samples while higher solubilization of casein proteins especially α-CN and β-CN in supernatants of ultracentrifuged samples of MPC80-Na and MPC80-K as compared to MPC80-C (Fig. 1).
4 Conclusions
The impact of salt addition during the diafiltration stage of MPC80 manufacturing on the solubility, minerals, and protein composition in reconstituted and in the soluble phase were investigated in the present study. This study has shown that both MPC80-Na and MPC80-K powder samples were readily soluble as compared to MPC80-C. Low calcium contents were observed in the reconstituted samples of MPC80-Na and MPC80-K powder samples as compared to MPC80-C. In contrast, higher calcium contents and protein contents (α-CN and β-CN) were observed in the soluble phase of MPC80-Na and MPC80-K as compared to MPC80-C. Our study notes an increase in pH in reconstituted samples (5% total solids) of MPC80-Na (7.76) and MPC80-K (7.93) as compared to MPC80-C (7.53) and this observation is in contrast to many previous studies due to reported decrease in pH. The results from previous studies indicate a binding of sodium with casein resulting in H+ release. The salt-induced changes in the minerals and protein composition are probably responsible for structural modifications of casein micelles and may account for higher solubility.
References
Ahmad S, Piot M, Rousseau F, Grongnet J, Gaucheron F (2009) Physico-chemical changes in casein micelles of buffalo and cow milks as a function of alkalinisation. Dairy Sci Technol 89:387–403. doi:10.1051/dst/2009020
Altuner E, Alpas H, Erdem Y, Bozoglu F (2006) Effect of high hydrostatic pressure on physicochemical and biochemical properties of milk. Eur Food Res Technol 222:392–396. doi:10.1007/s00217-005-0072-4
Amamcharla JK, Metzger LE (2011) Development of a rapid method for the measurement of lactose in milk using a blood glucose biosensor. J Dairy Sci 94:4800–4809. doi:10.3168/jds.2011-4416
Anema SG, Pinder DN, Hunter RJ, Hemar Y (2006) Effects of storage temperature on the solubility of milk protein concentrate (MPC85). Food Hydrocoll 20:386–393. doi:10.1016/j.foodhyd.2005.03.015
Aoki T, Umeda T, Nakano T (1999) Effect of sodium chloride on the properties of casein micelles. Milchwissenschaft 54:91–93
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis, 16th edn., Gaithersburg, MD, USA
Baldwin AJ (2010) Insolubility of milk powder products—a minireview. Dairy Sci Technol 90:169–179
Bhaskar G, Singh H, Blazey N (2001) Milk protein concentrate products and process. International Patent Specification Patent WO01/41578
Carr A, Bhaskar G, Ram S (2002) Monovalent salt enhances solubility of milk protein concentrate. US Patent 0208955
De Castro-Morel M, Harper W (2002) Basic functionality of commercial milk protein concentrates. Milchwissenschaft 57:367–370
Famelart M, Le Graet Y, Raulot K (1999) Casein micelle dispersions into water, NaCl and CaCl2: physicochemical characteristics of micelles and rennet coagulation. Int Dairy J 9:293–297
Farrell H, Kumosinski T, Pulaski P, Thompson M (1988) Calcium-induced associations of the caseins: a thermodynamic linkage approach to precipitation and resolubilization. Arch Biochem Biophys 265:146–158
Griffin M, Lyster R, Price J (1988) The disaggregation of calcium–depleted casein micelles. Eur J Biochem 174:339–343
Gualco SJ (2010) Effect of sodium chloride addition during diafiltration on the solubility of milk protein concentrate. M.Sc. Thesis, California Polytechnic State University, San Luis Obispo, USA
Havea P (2006) Protein interactions in milk protein concentrate powders. Int Dairy J 16:415–422
Huppertz T, Alting A, Slangen C, Floris R (2010) Milk protein concentrate functionality (oral presentation). http://www.wds2010.com/delegates/presentations/09tue/05-Session3_2-Thom%20Huppertz.pdf. Accessed Sept 9 2012
Huppertz T, Fox P (2006) Effect of NaCl on some physico-chemical properties of concentrated bovine milk. Int Dairy J 16:1142–1148
Hussain R, Gaiani C, Aberkane L, Scher J (2011) Characterization of high-milk-protein powders upon rehydration under various salt concentrations. J Dairy Sci 94:14–23. doi:10.3168/jds.2010-3323
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mao X, Tong P, Gualco S, Vink S (2012) Effect of NaCl addition during diafiltration on the solubility, hydrophobicity, and disulfide bonds of 80% milk protein concentrate powder. J Dairy Sci 95:3481–3488
Mimouni A, Deeth H, Whittaker A, Gidley M, Bhandari B (2010) Rehydration of high-protein-containing dairy powder: slow- and fast-dissolving components and storage effects. Dairy Sci Technol 90:335–344
Mistry V, Hassan H (1991a) Delactosed, high milk protein powder. 1. Manufacture and composition. J Dairy Sci 74:1163–1169
Mistry V, Hassan H (1991b) Delactosed, high milk protein powder. 2. Physical and functional properties. J Dairy Sci 74:3716–3723
Morr CV, German B, Kinsella JE, Regenstein JM, Buren JPV, Kilara A et al. (1985) A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J Food Sci 50:1715–1718. doi:10.1111/j.1365-2621.1985.tb10572.x
Rose D (1968) Relation between micellar and serum casein in bovine milk. J Dairy Sci 51:1897–1902
Schmidt DG (1982) Association of caseins and casein micelle structure. In: Fox P (ed) Developments in dairy chemistry, vol 1. Applied Science Publishers Ltd, London, pp 61–86
Schokker E, Church J, Mata J, Gilbert E, Puvanenthiran A, Udabage P (2011) Reconstitution properties of micellar casein powder: effects of composition and storage. Int Dairy J 21:877–886
Schrader K, Buchheim W, Morr CV (1997) High pressure effects on the colloidal calcium phosphate and the structural integrity of micellar casein in milk. Part 1. High pressure dissolution of colloidal calcium phosphate in heated milk systems. Food/Nahrung 41:133–138. doi:10.1002/food.19970410303
Schuck P, Briard V, Mejean S, Piot M, Famelart M, Maubois J (1999) Dehydration by desorption and by spray drying of dairy proteins: influence of the mineral environment. Dry Technol 17:1347–1357
Schuck P, Davenel A, Mariette F, Briard V, Méjean S, Piot M (2002) Rehydration of casein powders: effects of added mineral salts and salt addition methods on water transfer. Int Dairy J 12:51–57
Schuck P, Mejean S, Dolivet A, Gaiani C, Banon S, Scher J, Jeantet R (2007) Water transfer during rehydration of micellar casein powders. Lait 87:425–432
Sikand V, Tong P, Roy S, Rodriguez-Saona L, Murray B (2011) Solubility of commercial milk protein concentrates and milk protein isolates. J Dairy Sci 94:6194–6202
Sikand V, Tong P, Vink S, Walker J (2012) Effect of powder source and processing conditions on the solubility of milk protein concentrates 80. Milchwissenschaft 67:300–303
Singh H (2007) Interactions of milk proteins during the manufacture of milk powders. Lait 87:413–423
Udabage P, Mckinnon IR, Augustin MA (2000) Mineral and casein equilibria in milk: effects of added salts and calcium-chelating agents. J Dairy Res 67:361–370
Vaia B, Smiddy MA, Kelly AL, Huppertz T (2006) Solvent-mediated disruption of bovine casein micelles at alkaline pH. J Agric Food Chem 54:8288–8293
Ward BR, Goddard SJ, Augustin MANN, Mckinnon I (1997) EDTA-induced dissociation of casein micelles and its effect on foaming properties of milk. J Dairy Res 64:495–504
Zwijgers A (1992) Outline of milk protein concentrate. Int Food Ingred 3:18–23
Acknowledgments
Financial support of this work by Dairy Research Institute (Rosemont, IL) and California Dairy Research Foundation (Davis, CA) is appreciated. The authors would like to thank Dr. Lloyd Metzger, South Dakota State University, and Dr. Chao Wu, Hilmar Ingredients (Hilmar, CA) for technical lab analyses support. In addition, the authors would like to thank Dr. Frédéric Gaucheron, French National Institute for Agricultural Research (Rennes, France), and Dr. Harjinder Singh, Massey University (Palmerston North, New Zealand) for their helpful insight and comments on this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sikand, V., Tong, P.S. & Walker, J. Effect of adding salt during the diafiltration step of milk protein concentrate powder manufacture on mineral and soluble protein composition. Dairy Sci. & Technol. 93, 401–413 (2013). https://doi.org/10.1007/s13594-013-0110-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-013-0110-0