Abstract
In this work, different whey protein (WP) ratios (5, 10, 20, 30, 40 and 50% w/w) were added as stabilizers to high degree of polymerization Agave fructans (HDPAF) capsules to decrease the hygroscopicity. Results showed that the WP and HDPAF in 1:520:80 ratio (20/80 w/w) decreased significantly the hygroscopicity of capsules from 12.19 to 8.34%. Additionally, this polymeric mixture was assessed for the encapsulation of sea grape (Coccoloba uvifera L.) leaf extract was achieved by via electrospray, using this biopolymers mixture. Scanning electron microscopy (SEM) images exhibited spherical particles with sizes from 655 to 7250 nm. The thermal stability of encapsulated extract was demonstrated by via thermogravimetric analysis. The in vitro release study in simulated stomach (0–180 min) and intestine conditions (0–300 min) showed the controlled release of the controlled release of the encapsulated extract. The encapsulated extract and its bioavailability in simulating the stomach (0–180 min) and small intestine (0–300 min) Therefore, HDPAF–WP is a viable option as an encapsulating matrix susceptible to be used in the food, pharmaceutical, and cosmetic industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of High biological value compounds (HBVC) such as polyphenols, probiotics, vitamins, polyunsaturated fatty acids are mainly found in fruits and vegetables. The use of these compounds is increasing for the fortification enrichment of foods due to its beneficial effects on health. However, some HBVC has have low stability to environmental factors such as oxygen, moisture, light and heat; which can bring to isomerization or structural changes (Jacobsen et al. 2018; Ramos-Hernández et al. 2018a), leading to its degradation, and therefore loss of nutritional value and loss of bioavailability. Coccoloba uvifera L., commonly named as sea grape, is a tree that grows up to 15 m. Sea grape is located on the coasts across tropical America and the Caribbean and is an important source of compounds with great biological interest triterpenes such as lupeol, α- and β-amyrin (Ramos-Hernández et al. 2018b). Lupeol has pharmacological activities against several disease conditions such as antiinflammation, arthritis, diabetes, cardiovascular ailments, renal disorder, hepatic toxicity, microbial infections and cancer (Siddique and Saleem 2011). Further, α- and β-amyrin show different biological activity like pharmacological (Melo et al. 2011) and antimicrobial activity (Díaz-Ruiz et al. 2012). However, those compounds present low solubility in water, low bioavailability and are highly susceptible to oxidation (Saleem 2009). Therefore to guarantee the bioactivity obtain the desired activity of the HBVC sea grape leaf triterpenes in the human body, these compounds need to be efficiently protected to avoid their deterioration due to the environmental conditions during the transportation through the gastrointestinal (GI) tract, and acquire the targeted controlled controllably released in the digestive system gut. Biopolymers particles may be used to protect and deliver this HBVC in food systems (López-Rubio and Lagaron 2012). The encapsulation technology encloses the HBVC (core) within a biopolymer (wall material) to protect it from the environmental conditions (Jacobsen et al. 2018). Hence, a certain interest in the formation of biopolymer capsules from proteins and polysaccharides have been reported (Jones et al. 2010). New encapsulants with improved delivery properties can be developed by decreasing the particle size (Jacobsen et al. 2018). A wide variety of processes have been developed to obtain micro or nanoparticles, such as spray-drying, emulsifying–crosslinking or coacervation (Jacobsen et al. 2018). However, these techniques require heating or organic solvents, leading to decomposition of sensitive encapsulated compounds (López-Rubio and Lagaron 2012), or generate big particle sizes that affect the organoleptic characteristics of the final product.
In this regard, electrospray is a simple, versatile and non-thermal process used to produce capsules. This process is based on the application of an external electrostatic field between two electrodes to a polymer solution. As the electrostatic field increases, the electrical charges on the surface of the pendant drop overcome the surface tension and the shape of the drop changes from partially spherical to conical (so-called Taylor’s cone effect). As the charged jet is accelerated to regions of lower potential, the solvent is evaporated and the capsules are obtained in the collector (López-Rubio and Lagaron 2012). Electrospray device has four main components: (1) a high-voltage source (1–30 kV), (2) a stainless steel needle or capillary, (3) a syringe pump and (4) a grounded collector. The electrospraying process has been widely used to generate capsules of novel functional ingredients with applications in the food industry. This technology has been used for the encapsulation of several HBVC such as vitamins, probiotics, antioxidants and omega-3 fatty acids (López-Rubio and Lagaron 2012; Ramos-Hernández et al. 2018a, García-Moreno et al. 2018). The encapsulates obtained through electrospraying exhibit higher bioavailability compared with to traditional capsules, since this process generates particles with small particle size and high encapsulation efficiency, and the electrospraying process is carried out at ambient temperature (Jacobsen et al. 2018). Agave fructans from Agave are emerging as components for wall materials to encapsulate encapsulating materials and have properties such as prebiotic effect and anti-obesity properties (Arrizón et al. 2014). Fructans from Agave tequilana are a mixture of polymers mostly used in tequila production (Pinal et al. 2009). Agave fructansThey are a complex mixture of fructooligosaccharides with long branched structures formed by β-(2 → 1) and β-(2 → 6) fructosyl linkages with one internal or external glucosyl residue. Physico-chemical and functional properties of fructans are due to the polymerization degree (PD) as well as the presence of branches (Lopez et al. 2003). The native fructans contain a mixture of high, medium, and low polymerization degrees. Fructans of low degree of polymerization are the most hygroscopic (Jimenez-Sánchez et al. 2018). Recently, Ramos-Hernández et al. (2018a) encapsulated β-carotene by electrospraying, founding the potential of HDPAF as a new biopolymer in the fabrication of nanocapsules. The particles showed good photoprotection to β-carotene under UV light avoiding photoisomerization and a thermal protective effect (Ramos-Hernández et al. 2018a). Also, the use of HDPAF as encapsulating material allows developing a the targeted delivery of HBVC in the small intestine where most absorption occurs (Fathi et al. 2014). In addition to their proved protective and controlled release capacities, this material showed prebiotic effect and anti-obesity properties (Arrizón et al. 2014). Nevertheless, an optimization in the formulation of HDPAF is required to improve the hygroscopicity of the capsules.
Recently, Souza et al. (2017) and García-Moreno et al. (2018) used proteins to increase the hygroscopic stability of capsules made by carbohydrates such as maltodextrin and pectin. A mixture with WP as a stabilizer has been recently used (Souza et al. 2017; García-Moreno et al. 2018). This combination of proteins and carbohydrates can form covalent and hydrogen bonds (López-Rubio and Lagaron 2012) and therefore, prevent the interaction with environmental water.
An interesting approach to be evaluated is the use of WP as hygroscopic stabilizer, since in addition to the hygroscopic stability capacity, WP exhibits as well gelling, emulsifying and highly protective properties (López-Rubio and Lagaron 2012). Therefore, the aim of this work was to combine WP and HDPAF to enhance the hygroscopic and thermal stability of HDPAF the capsules produced by electrospray.using as a stabilizer to WP. Additionally, this polymeric mixture was assessed for the encapsulation of sea grape (Coccoloba uvifera L.) leaf extract using the electrospray technology. The capsules were characterized in terms of morphology, hygroscopic stability, thermal stability and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). Moreover, the in vitro controlled release potential of sea grape leaf extract was evaluated (Coccoloba uvifera L.).
Materials and methods
Materials
HDPAF were purchased from Campos Azules Co., (Mexico City, Mexico). WP from DAVISCO (Le Sueur, U.S.). TEGO SML (sorbitan fatty acid ester) was purchased from Evonik Inc., (Essen, Germany). Ethyl alcohol from CTR (Monterrey, Mexico), hexane from Jalmek (San Nicolás, N.L. Mexico) and chloroform from Fermont (Monterrey, Mexico). Deionized water was used throughout the study. Pancreatin and pepsin from Sigma (St. Louis, U.S.). Leaves of sea grape plant (Coccoloba uvifera L.) were collected along the coast of Tecolutla (Veracruz, Mexico).
Extraction of high value biological compounds (HVBC)
Freeze-dried samples at − 50 °C and 0.12 mbar (FreeZone 4.5, Labconco, Kansas City, Missouri MO, U.S.) were added with hexane (w/v) in ratio of 1:10 (g sample:ml solvent) at 25 °C and then placed in a Branson bath sonicator 1510 (Branson Ultrasonics Corp, Danbury, U.S.) for 30 min at constant frequency of 42 kHz. After The mixture was filtered through Whatman No. 1 filter paper (Whatman No. 1 11 μm) under vacuum (64 kPa) and the filtrate was evaporated to dryness. The extract obtained was stored in an amber vials at 25 °C until analysis.
Preparation of formulation
Hygroscopic stability of HDPAF was evaluated at different ratios HDPAF:WP ratios. A polymersic mixture was prepared at 50% (w/w) of solid concentration in a hydroalcoholic solution at 50% (w/w) was prepared according to the protocol reported by Ramos-Hernández et al. (2018a). The mixture of polymers were formulated at ratios of 95:5, 90:10, 80:20, 70:30, 60:40 and 50:50 (HDPAF:WP). The hydroalcoholic solution used as solvent 50% was prepared in a 90:10 ratio (water:ethanol)., 90:10) and was used as a solvent. and In case of encapsulation, Tthe leaf extract (0.1%) was added dissolved previously in the ethanol previously. TEGO SML (1%) was added as surfactant. Solutions were prepared under magnetic stirring (Agimatic-S 7000242, Barcelona, Spain) at 350 rpm for 5 min (Agimatic-S 7000242, Barcelona, Spain).
Characterization of solutions
Viscosity
Apparent viscosity (η) was determined using a rotational viscosimeter Visco Basic Plus L from Fungilab S.A. (San Feliu de Llobregat, Spain) with a Low Viscosity Adapter (LCP). All measurements were made at 25 °C in triplicate.
Surface tension
Surface tension was measured using the Wilhelmy plate method in an EasyDyne K20 tensiometer (Krüss GmbH, Hamburg, Germany). All measurements were made at 25 °C in triplicate.
Conductivity
Conductivity was measured using a conductivity meter XS Con6 (Labbox, Barcelona, Spain). All measurements were made at 25 °C in triplicate.
Preparation of capsules by electrospraying
Electrospray from Fluidnatek® LE-10 from Bioinicia S.L. (Valencia, Spain) basically involves the application of a high voltage electrical (0–30 kV) field to a polymer solution to be sprayed and the dry nano or microparticles are projected on a charged collector. It device equipped with a variable high-voltage (0–30 kV) power supply, was a from Fluidnatek® LE-10 from BioIinicia S.L. (Valencia, Spain). Solutions with the extract were introduced in a 12 mL plastic syringe and were electrospun under a steady flow rate using a stainless-steel needle of 700 µm diameter. The needle was connected through a polytetrafluoroethylene (PTFE) tube to the syringe. The syringe was lying on a digitally controlled syringe pump while the needle was horizontal towards the collector. Electrospraying conditions of the solutions for obtaining capsules were optimized and fixed at 0.1 mL/h of flow-rate, 17 kV of voltage and a tip-to-collector distance of 22 cm. Collected capsules were stored in a desiccator at 2–3% relative humidity (RH) and protected from light for subsequent analysis.
Scanning electron microscopy (SEM)
Morphology and size of the capsules were examined using SEM on a Hitachi microscope (Hitachi S-4100, Tokyo, Japan) after sputtered at an accelerating voltage of 10 kV and a working distance of 8 mm. 1–2 mg of sample were deposited over a double side carbon strip prior to sputtering with a gold–palladium mixture under vacuum for 3 min (SC7640, Polaron, Kent, UK). All SEM experiments were carried out with 1–2 mg of the sample in a sample holder with double-sided tape at 10 kV. Capsule diameters were measured by Adobe Photoshop CS3 software from the SEM micrographs in their original magnification, averaging at least 100 capsules per image. Capsules were measured at a magnification of ×20K.
Hygroscopicity of capsules
Hygroscopicity tests analysis were performed according to the methodology described by Al-Kahtani and Hassan (1990). 5 grams of sample were placed in a desiccator at 21 °C and 76% relative humidity (solution of 36 g of NaCl in 100 g of water) in a petri dish of 9 cm in diameter. Weight was registered for 15 min or constant weight. The results were reported in grams of water absorbed by 100 g of dried solids (g/100 g). The absorbed water percentage was calculated using Eq. (1).
where Δm (g) is the increase in powder weight after equilibrium, M is the initial mass of powder, and Mi (% db) is the free water content of the powder before exposure to the humid air environment.
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
ATR-FTIR spectra of HDPAF–WP capsules were recorded using a Spectrum 400 (Perkin Elmer, Beaconsfield, UK) equipped with a universal attenuated total reflectance accessory. Spectra were collected in transmission mode within the wavenumber range 4000–400 cm−1 and 4 cm−1 of resolution. Measurements were performed in triplicate.
Differential scanning calorimetry (DSC)
Glass transition temperature (Tg) was estimated using a differential scanning calorimeter (DSC, Q2000 TA-Instruments, New Castle, DE, USA). 3–6 mg of the sample at 25 °C was heated in hermetic aluminum crucibles and an empty hermetically sealed aluminum crucible was used as a reference. A heating ramp of 20 to 300 °C at 10 °C/min was used. The DSC was calibrated with metallic indium standard for temperature. The calorimeter was purged with nitrogen at a flow rate of 50 mL/min. The data measurements were analyzed using the Universal Analysis 2000 software, version 4.7a (TA Instruments, New Castle, U.S.); Tg was calculated by evaluating the midpoint of the inflection region in the heat flow signal. All measurements were made in triplicate.
Thermogravimetric analysis (TGA)
Thermogravimetric analyses of free extract and HDPAF–WP capsules with and without and with extract were done in triplicate using TGA 550 equipment (TA Instruments, New Castle, U.S.) and TRIOS 4.3.0.38388 was the analysis software used. Analyses were conducted under the following conditions: 3–6 mg of sample, heating from 25 to 500 °C, at a heating rate of 5 °C/min under nitrogen flow.
In vitro release
HDPAF–WP capsules with extract were dissolved (5.6 mg/mL) in phosphate-buffered saline at pH 1.5 and 6.9 (with pepsin and pancreatin respectively), emulating gastrointestinal conditions (Guerra et al. 2012). Samples were placed in a shaking bath (shaking hot tub, 290400 Boekel scientific, Pennsylvania, U.S.) at 37° C and 150 rpm and then, aAliquots were taken every 30 min during 3 and 5 h (stomach and small intestine, respectively) to analyze the terpenes release at 210 nm in a UV–vis spectrophotometer CARY-50 (VARIAN, Midelburgo, the Netherlands). A calibration curve of triterpenes was made. All measurements were made in triplicate.
Statistical analysis
Statistical analysis was performed with STATISTICA software version 10 for Windows (StatSoft, Inc). Data were analyzed using an ANOVA followed by an LSD test (p < 0.05).
Results and discussion
Solution properties
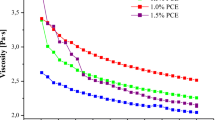
Properties of biopolymers solutions, such as viscosity, conductivity, and surface tension influence the electrospraying process. These solution properties are affected by the molecular weight of the used polymers (Härdelin et al. 2013), HBVC composition, surfactants and used solvents used (Ramos-Hernández et al. 2018a). The viscosity values increased as a function of WP concentration (p < 0.05). This could be attributed to the structure of the WP which is mainly conformed by α- and β-lactalbumin and bovine serum albumin (Alehosseini et al. 2019). The stable conformation of the folded β-sheet-structure of β-lactoglobulin (Yi et al. 2015), have globular proteins molecules, which result in high molecular weight. Moreover, the intra/inter molecular isopeptide bonds cause structural changes of proteins (Alehosseini et al. 2019). Kutzli et al. (2019) reported an increase of viscosity in mixtures of maltodextrin with WP and soy protein, when the proteins content increased. This could be, due to the higher molecular weight of the protein and the higher concentration of the polymers in the solution. Viscosity values obtained for solutions with WP at ratio of 80:20% and 70:30% in the solution at 50% polymers (Table 1) are in the range recommended to manufacture capsules by electrospraying, according to Ramos-Hernández et al. (2018a), who characterized solutions of HDPAF from 10 to 50% of HDPAF and reported viscosity values from 2.4 ± 0.1 to 162.2 ± 0.6 cP.
Similar values for surface tension were obtained (Table 1) for all mixtures with WP. For instance, The values of surface tension values (Table 1) for WP mixtures 5–30% were adequate for an electrospraying, since drying according to Ramos-Hernández et al. (2018a), solutions with surface tension above 50 mN/m cannot be electrosprayed. Variation of surface tension would not affect the electrospray process as it is below the critical value. These values obtained for surface tension were attributed to the presence of ethanol addition in the formulations, since because ethanol surface tension is lower than the water (Gómez-Mascaraque et al. 2017). On the other hand, the mixtures 40–50% WP could not be electrosprayed since it was not possible to stabilize the Taylor cone. A similar decrease in surface tension values was observed in maltodextrin-WP mixtures (Kutzli et al. 2019) and this behavior can could be due attributed to the closely-packed globular structure of WP that produce a slow diffusion of the proteins to the interface. Therefore, WP has reduced surface activity, even lower compared to plant proteins such as soy, pea, and rice (Fox et al. 2015; Kutzli et al. 2019; Fang et al. 2013). Conductivity values increased with increasedas the content of WP in solutions did (Table 1). This behavior is in agreement with the reported by Kutzli et al. (2019) that used mixtures of maltodextrin-WP and obtained an increase in the conductivity values when the concentration of WP increase. Conductivity values determined for the solutions were lower than the values reported as adequate for an electrospraying process < 2200.00 µS/cm (Librán et al. 2017) since electrical conductivity should not exceed this value to avoid the destabilization of the electrospraying jet.
Capsule morphology
The capsules have spherical morphology and sizes between 655 nm and 7250 nm, without cracks or deformations (Fig. 1). These results demonstrate the ability of mixtures of HDPAF with WP to form capsules with total polymer concentrations around 50% in mixtures in ratios HDPAF:WP 95:5, 90:10, 80:20 and 70:30 (w:w). Capsule size was bigger than the size of the capsules obtained by Ramos-Hernández et al. (2018a) for pure HDPAF, whose dimensions were between 440 nm and 880 nm. The WP addition could be the reason for the increment in size. López-Rubio and Lagaron (2012) and Kutzli et al. (2019) previously reported that the addition of WP can increase the particle size due to its high molecular weight. Morphology of electrospraying materials largely depends on the physical characteristics of the starting polymeric solution (López-Rubio and Lagaron 2012). Moreover, the increase of polymer concentration (> 70:30, w:w) can lead to higher diameters during electrospraying process, due to higher viscosity resistance against deformation and sprinkling (Kutzli et al. 2019). On the other hand, the capsules micrograph (80:20, w:w) did not show pores or cracks, limiting the oxygen permeability oxygen, and the heat transfer, as well as, increasing the protection against ultraviolet light (UV) Ramos-Hernández et al. (2018a).
Hygroscopicity of capsules
Ramos-Hernández et al. (2018a) had previously obtained nanocapsules using HDPAF; however, these capsules presented high hygroscopicity. Thus, the objective of this work was to evaluate the addition of WP toThen, WP was used mainly to reduce hygroscopicity. The effect of the WP addition to HDPAF in the hygroscopicity of the capsules is shown in Table 2.
Hygroscopicity values were significantly reduced (p < 0.05) from 5% of WP addition, then values between 8.3 and 9.7% were obtained (Table 2). These hygroscopicity values were adequate to preserve the capsules stability at at a relative humidity around 60%. This confirmed that WP is an efficient stabilizer in the reduction of the hygroscopicity of HDPAF capsules. However, when we increased the concentration of WP was increased (40 and 50%), it was not possible to obtain capsules by electrospraying since the viscosity was notably increased (Table 1). Similarly, high concentrations of HDPAF increase the physicochemical properties related to the yield, hygroscopicity, and glass transition temperature (Cervantes et al. 2016). The hygroscopicity of HDPAF might be related to the water absorption capacity due to its branched structure (Jimenez-Sánchez et al. 2018; Ramos-Hernández et al. 2018a). Nevertheless, when HDPAF is mixed with other polymers as WP, the hygroscopicity is reduced significantly (Table 2), since these mixtures can form covalent bonds and hydrogen bonds (López-Rubio and Lagaron 2012) and therefore, can prevent interaction with water in the environment. This behavior has been reported in mixtures of maltodextrin-fructans, decreasing the hygroscopicity from 18.2 ± 0.1 to 16.6 ± 0.1% (Jimenez-Sánchez et al. 2018). Additionally, the values obtained in the capsules (Table 2) are lower than those reported by Souza et al. (2017), which reported mixtures polymerbeing of 11.3 ± 0.1% for maltodextrin:soy protein and 12.7 ± 0.1% for maltodextrin:pectin. with hygroscopicity values of 11.3 ± 0.1% and 12.7 ± 0.1%, respectively; t. Thus, HDPAF–WP capsules have greater hygroscopic stability than other polymers reported. According to the hygroscopicity values, the ratios HDPAF:WP 80:20 was chosen as an adequate formulation to encapsulate the triterpenes extracted from sea grape leaf.
FTIR spectroscopy
The FTIR spectra of HDPAF (Fig. 2a), according to Apolinário et al. (2017) showed the presence of hydroxyl groups (–OH) of carbohydrates (3304 cm−1). The band from 2800 to 3000 cm−1 is similar to the HDPAF capsules spectra reported by Ramos-Hernández et al. (2018a); this band is attributed to C–H stretching. Moreover, the bands in 927–1121 cm−1 have been assigned to C–O and C–C stretching modes and are characteristic of carbohydrates. The WP FTIR spectrum (Fig. 2b) is similar to those of WP reported by Alehosseini et al. (2019). The amide I at 1632 cm−1 and amide II at 1520 cm−1 are the main bands in the spectra.
The capsules spectra showed the presence of HDPAF and WP, since the characteristic bands of both compounds bands were detected (Fig. 2c). The bands in the amide regions (1642 cm−1) were increased, due to the stretching vibrations of the C = O and C-N groups (Kutzli et al. 2019). This behavior is related to the added WP (Alehosseini et al. 2019). Moreover, no changes of characteristic FTIR bands occurred, due to no chemical interactions between HDPAF and WP in the capsules. In the presence of WP, hydrophobic interactions were significantly increased as indicated by the enhanced intensity of the spectra in the range from 2800 to 3100 cm−1 (Fig. 2c), assigned to C–H stretching (Alehosseini et al. 2019). This effect on hydrophobic interactions has been previously reported (Abaee and Madadlou 2016). The aliphatic amino acids exposure and cross-linkages formation (Eissa et al. 2006), can be attributed to an increase of hydrophobicity in –CH2 asymmetrical stretching regions. Moreover, it can be observed a significant increase in the intensity of the band in the range from 3100 to 3700 cm−1 (Fig. 2c), which could be due to a greater number of inter- and intramolecular hydrogen bonds (López-Rubio et al. 2012; Alehosseini et al. 2019). The FTIR spectrum changes of capsules (Fig. 2c) confirm that the mixture of the polymers (HDPAF–WP) significantly impacts the structure and conformation of capsules and improves hygroscopic stability.
Thermal stability of extract and HDPAF–WP capsules
Differential scanning calorimetry (DSC)
Thermograms of HDPAF–WP capsules shown typical transition by the inflection in the curve to determinatewere used to determine the glass transition temperature (Tg) value, which indicates a variation in the specific heat, since the change in heat flow causes differences in heat capacity (Espinosa-Andrews and Urias-Silvas 2012). The values obtained shown showed a significant increase in Tg from 130.05 to 138.08 °C for nead and loaded-capsules without and with extract, respectively (Fig. 3a, b). These results are in concordance with the values reported by Espinosa-Andrews and Urias-Silvas (2012), who obtained a Tg of 125.7 °C for fructans. This Tg displacement and appearance of the peak located at 138 °C (b) indicates that the extract and the HDPAF–WP occurred an interactioninteracted and formed and the formation of a complex, between extract and HDPAF–WP (Souza et al. 2017). The increased Tg of HDPAF–WP capsules (Fig. 3a, b) with respect to fructans, could promote greater thermal stability, and consequently. Besides, this increase in Tg could provide extra protection to the extract (Ramos-Hernández et al. 2018a). Moreover, an improvement in the structural stability of the capsules and their stability during storage has been observed. It is important to mention that a greater Tg can prevent oxygen diffusion through the capsule, reduce hygroscopicity and avoid caking (Espinosa-Andrews and Urias-Silvas 2012). Therefore, adding WP as a stabilizer avoids the presence of these unwanted changes in the HDPAF capsules. The differences in Tg values of the capsules could be explained by the characteristics of the polymers used as wall materials, and to possible differences in their residual water levels (García-Moreno et al. 2018).
Thermogravimetric Analysis (TGA)
Thermogravimetric analysis (TGA) evaluated the thermal decomposition of compounds in HDPAF–WP capsules. The temperature of thermal decomposition according to the mass loss was calculated in thermograms withusing the thermogram derivative. Thermal decomposition of the extract was between 244.45 and 334.08 °C (Fig. 3c). According to Saleem (2009), the decomposition temperateure of lupeol is 215 °C. This difference could be related to the cyclic structure of triterpenes since these compounds normally present a slower thermal decomposition. HDPAF–WP capsules are decomposed between 199.95 and 215.85 °C (Fig. 3d), results that are in concordance with the ones obtained by Ramos-Hernández et al. (2018a) for HDPAF capsules (205.48–257.70 °C), and by Espinosa-Andrews and Urias-Silvas (2012) for agave fructans around 200 °C. However, the temperature range is narrower when WP was added. This behavior could demonstrate that HDPAF–WP capsules can protect HBVC. The encapsulated extract (Fig. 3e) was decomposed between 200.08 and 214.96 °C, this range is similar to the capsules without extract. Since the HBVC decomposition is not visible in the capsules, this could be due to a thermal protective effect (Peinado et al. 2016), whichThis protective effect could be associated with the decomposition of branched chains of fructans by the effect of temperature (Espinosa-Andrews and Urias-Silvas 2012).
In vitro release of extract
The release of the extract from HDPAF–WP capsules is shown in Fig. 4 (a and b respectively) at the simulated stomach and small intestine conditions. A slower extract release was observed during stomach simulation. Under these stomach conditions, a gradual and partial release of the extract was obtained. The maximal release (0.15 mg/mg) occurred at 90 min and the content released was constant up to 180 min. Thus, it can be concluded that under these conditions the wall material constituted by HDPAF–WP was partially hydrolyzed. OtherwiseOn the other hand, during the small intestine simulation, a higher and quicker release was doneoccurred, since because maximal release (0.4 mg/g) was obtained at 60 min. The fact of having a faster release under small intestine conditions suppose could mean that this material is adequate to protect and transport the extract to be bioavailable at small intestine where the absorption of the maximal most of the nutrients occurs. Native β-lactoglobulin is the predominant component of WP and has been shown to be less digestible by pepsin (Peram et al. 2013; Yi et al. 2015). This Iindigestibility can be attributed to the stable conformation of the folded β-sheet- structure of β-lactoglobulin (Yi et al. 2015). However, Tthe amount of extract released from HDPAF–WP capsules treated with pancreatin (Fig. 4b), was greater than the release from pepsin. This result is similar to that reported for WP by Yi et al. (2015). An important point is that the resistances to pepsin digestion, make HDPAF–WP capsules, ideally suited for controlled release delivery.
Conclusion
Mixtures of HDPAF and WP can form capsules by electrospraying. The best morphology and hygroscopicity of the capsules were obtained in concentrations HDPAF 80% and WP 20% of polymers (50%). HDPAF–WP capsules have the capacity to improve the stability of HBVC. Capsules obtained showed a good release in the small intestine of the extract. Thus, capsules could be bioavailable and perform its biological activity. In this work, the incorporation of WP to HDPAF was proposed to increase the hygroscopic stability of HDPAF capsules. The obtained results showed that HDPAF:WP ratios of 80:20 were sufficient to reduce the hygroscopicity of the capsules to 8%. Therefore, this mixture was used to encapsulate the extract of sea grape leaf (Coccoloba uvifera L.) by means of electrospray in order to test the capacity of this polymeric mixture as an encapsulating material. Spherical particles were obtained, with sizes from 655 to 7250 nm. Furthermore, HDPAF–WP capsules were shown to have the ability to improve the thermal stability of sea grape leaf extract. Regarding the release rate of the encapsulated extract, it was observed that the greatest release occurred in the small intestine. Therefore, HDPAF:WP capsules managed to provide stability to the extract and protection until reaching the small intestine. Therefore, the bioavailability of this extract and its biological activity could be expected to increase. These promising results make HDPAF:WP an interesting alternative as a biological encapsulation matrix, suitable for human consumption and able to be used in the food, pharmaceutical and cosmetic industries.
References
Abaee A, Madadlou A (2016) Niosome-loaded cold-set whey protein hydrogels. Food Chem 196:106–113. https://doi.org/10.1016/j.foodchem.2015.09.037
Alehosseini A, Sarabi-Jamab M, Ghorani B, Kadkhodaee R (2019) Electro-encapsulation of Lactobacillus casei in high-resistant capsules of whey protein containing transglutaminase enzyme. LWT 102:150–158. https://doi.org/10.1016/j.lwt.2018.12.022
Al-Kahtani HA, Hassan BH (1990) Spray drying of roselle (Hibiscus sabdariffa L.) extract. J Food Sci 55(4):1073–1076. https://doi.org/10.1111/j.1365-2621.1990.tb01601.x
Apolinário AC, de Carvalho EM, de Lima Damasceno BPG, da Silva PCD, Converti A, JrA Pessoa, da Silva JA (2017) Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind Crops Prod 108:355–362. https://doi.org/10.1016/j.indcrop.2017.06.045
Arrizón J, Urias-Silvas JE, Sandoval G, Mancilla-Margalli NA, Gschaedler AC, Morel S, Monsan P (2014) Production and bioactivity of fructan-type oligosaccharides. Food Oligosacch Prod Anal Bioactivity. https://doi.org/10.1002/9781118817360.ch11
Cervantes VSF, Lincon ED, Soto AS, Roldan HM, González IA (2016) Effect of spray drying temperature and agave fructans concentration as carrier agent on the quality properties of blackberry powder. Int J Food Eng 12(5):451–459. https://doi.org/10.1515/ijfe-2015-0287
Díaz-Ruiz G, Hernández-Vázquez L, Luna H, Wacher-Rodarte M, Navarro-Ocaña A (2012) Growth inhibition of Streptococcus from the oral cavity by α-amyrin esters. Molecules 17(11):12603–12611. https://doi.org/10.3390/molecules171112603
Eissa AS, Puhl C, Kadla JF, Khan SA (2006) Enzymatic cross-linking of β-lactoglobulin: conformational properties using FTIR spectroscopy. Biomacromolecules 7(6):1707–1713. https://doi.org/10.1021/bm050928p
Espinosa-Andrews H, Urias-Silvas JE (2012) Thermal properties of agave fructans (Agave tequilana Weber var. Azul). Carbohydr Polym 87(4):2671–2676. https://doi.org/10.1016/j.carbpol.2011.11.053
Fang Z, Wang R, Bhandari B (2013) Effects of type and concentration of proteins on the recovery of spray-dried sucrose powder. Drying Technol 31(13–14):1643–1652. https://doi.org/10.1080/07373937.2013.770011
Fathi M, Martin A, McClements DJ (2014) Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci Technol 39(1):18–39. https://doi.org/10.1016/j.tifs.2014.06.007
Fox PF, Uniacke-Lowe T, McSweeney PLH, O’Mahony JA (2015) Milk proteins. In: Dairy chemistry and biochemistry, 2 edn. Springer, Switzerland, pp 146–238. https://doi.org/10.1007/978-3-319-14892-2
García-Moreno PJ, Pelayo A, Yu S, Busolo M, Lagaron JM, Chronakis IS, Jacobsen C (2018) Physicochemical characterization and oxidative stability of fish oil-loaded electrosprayed capsules: combined use of whey protein and carbohydrates as wall materials. J Food Eng 231:42–53. https://doi.org/10.1016/j.jfoodeng.2018.03.005
Gómez-Mascaraque LG, Perez-Masiá R, González-Barrio R, Periago MJ, López-Rubio A (2017) Potential of microencapsulation through emulsion-electrospraying to improve the bioaccesibility of β-carotene. Food Hydrocolloids 73:1–12. https://doi.org/10.1016/j.foodhyd.2017.06.019
Guerra A, Etienne-Mesmin L, Livrelli V, Denis S, Blanquet-Diot S, Alric M (2012) Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol 30(11):591–600. https://doi.org/10.1016/j.tibtech.2012.08.001
Härdelin L, Perzon E, Hagström B, Walkenström P, Gatenholm P (2013) Influence of molecular weight and rheological behavior on electrospinning cellulose nanofibers from ionic liquids. J Appl Polym Sci 130(4):2303–2310. https://doi.org/10.1002/app.39449
Jacobsen C, García-Moreno PJ, Mendes AC, Mateiu RV, Chronakis IS (2018) Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu Rev Food Sci Technol 9:525–549. https://doi.org/10.1146/annurev-food-030117-012348
Jimenez-Sánchez DE, Calderon-Santoyo M, Ortiz-Basurto RI, Bautista-Rosales PU, Ragazzo-Sánchez JA (2018) Effect of maltodextrin reduction and native agave fructans addition on the physicochemical properties of spray-dried mango and pineapple juices. Food Sci Technol Int 24(6):519–532. https://doi.org/10.1177/1082013218769168
Jones O, Decker EA, McClements DJ (2010) Thermal analysis of β-lactoglobulin complexes with pectins or carrageenan for production of stable biopolymer particles. Food Hydrocolloids 24(2–3):239–248. https://doi.org/10.1016/j.foodhyd.2009.10.001
Kutzli I, Gibis M, Baier SK, Weiss J (2019) Electrospinning of whey and soy protein mixed with maltodextrin–Influence of protein type and ratio on the production and morphology of fibers. Food Hydrocolloids 93:206–214. https://doi.org/10.1016/j.foodhyd.2019.02.028
Librán CM, Castro S, Lagaron JM (2017) Encapsulation by electrospray coating atomization of probiotic strains. Innov Food Sci Emerg Technol 39:216–222. https://doi.org/10.1016/j.ifset.2016.12.013
Lopez MG, Mancilla-Margalli NA, Mendoza-Diaz G (2003) Molecular structures of fructans from Agave tequilana Weber var. azul. J Agric Food Chem 51(27):7835–7840. https://doi.org/10.1021/jf030383v
López-Rubio A, Lagaron JM (2012) Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov Food Sci Emerg Technol 13:200–206. https://doi.org/10.1016/j.ifset.2011.10.012
López-Rubio A, Sanchez E, Wilkanowicz S, Sanz Y, Lagaron JM (2012) Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids. Food Hydrocolloids 28(1):159–167. https://doi.org/10.1016/j.foodhyd.2011.12.008
Melo CM, Morais TC, Tomé AR, Brito GAC, Chaves MH, Rao VS, Santos FA (2011) Anti-inflammatory effect of α, β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. Inflamm Res 60(7):673–681. https://doi.org/10.1007/s00011-011-0321-x
Peinado I, Mason M, Romano A, Biasioli F, Scampicchio M (2016) Stability of β-carotene in polyethylene oxide electrospun nanofibers. Appl Surf Sci 370:111–116. https://doi.org/10.1016/j.apsusc.2016.02.150
Peram MR, Loveday SM, Ye A, Singh H (2013) In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J Dairy Sci 96(1):63–74. https://doi.org/10.3168/jds.2012-5896
Pinal L, Cornejo E, Arellano M, Herrera E, Nuñez L, Arrizon J, Gschaedler A (2009) Effect of Agave tequilana age, cultivation field location and yeast strain on tequila fermentation process. J Ind Microbiol Biotechnol 36(5):655–661. https://doi.org/10.1007/s10295-009-0534-y
Ramos-Hernández JA, Calderón-Santoyo M, Navarro-Ocaña A, Barros-Castillo JC, Ragazzo-Sánchez JA (2018a) Use of emerging technologies in the extraction of lupeol, α-amyrin and β-amyrin from sea grape (Coccoloba uvifera L.). J Food Sci Technol 55(7):2377–2383. https://doi.org/10.1007/s13197-018-3152-8
Ramos-Hernández JA, Ragazzo-Sánchez JA, Calderón-Santoyo M, Ortiz-Basurto RI, Prieto C, Lagaron J (2018b) Use of electrosprayed agave fructans as nanoencapsulating hydrocolloids for bioactives. Nanomaterials 8(11):868. https://doi.org/10.3390/nano8110868
Saleem M (2009) Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285(2):109–115. https://doi.org/10.1016/j.canlet.2009.04.033
Siddique HR, Saleem M (2011) Beneficial health effects of lupeol triterpene: a review of preclinical studies. Life Sci 88(7–8):285–293. https://doi.org/10.1016/j.lfs.2010.11.020
Souza ACP, Gurak PD, Marczak LDF (2017) Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod Process 102:186–194. https://doi.org/10.1016/j.fbp.2016.12.012
Yi J, Lam TI, Yokoyama W, Cheng LW, Zhong F (2015) Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocolloids 43:31–40. https://doi.org/10.1016/j.foodhyd.2014.04.028
Acknowledgements
This study was supported by the Consejo Nacional de Ciencia y Tecnoligía (CONACyT, Mexico) for the scholarship granted (Number 702624) to Jorge Alberto Ramos-Hernández; CYTED thematic network code 319RT0576 and Spanish Ministry of Science, Innovation and Universities (Project Code RTI2018-097249-B-C21). The author’s thanks Dr. Rosa Isela Ortiz-Basurto for providing the high degree of polymerization Agave fructans (HDPAF) and Claudia Hernández Ambrosio for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos-Hernández, J.A., Lagarón, J.M., Calderón-Santoyo, M. et al. Enhancing hygroscopic stability of agave fructans capsules obtained by electrospraying. J Food Sci Technol 58, 1593–1603 (2021). https://doi.org/10.1007/s13197-020-04672-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04672-3