Abstract
Objective
To evaluate the anti-inflammatory effect of α,β-amyrin, a pentacyclic triterpenoid from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice.
Methods
Acute pancreatitis was induced in Swiss mice by five intraperitoneal injections of cerulein (50 μg/kg), at 1 h intervals. Mice received α,β-amyrin (10, 30 and 100 mg/kg), thalidomide (200 mg/kg), or vehicle (3% Tween 80) orally 1 h before and 12 h after the cerulein challenge. The severity of pancreatitis was evaluated 24 h after cerulein by assessing serum pro-inflammatory cytokines and amylase activity, pancreatic myeloperoxidase (MPO), and thiobarbituric acid-reactive substances (TBARS), as well as by histology.
Results
α,β-Amyrin and thalidomide significantly attenuated the cerulein-induced increase in tumor necrosis factor (TNF)-α, interleukin-6, lipase, amylase, MPO, and TBARS. Moreover, α,β-amyrin greatly suppressed the pancreatic edema, inflammatory cell infiltration, acinar cell necrosis, and expressions of TNFα and inducible nitric oxide synthase.
Conclusions
α,β-Amyrin ameliorates cerulein-induced acute pancreatitis by acting as an anti-inflammatory and antioxidant agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pancreatitis is characterized by activation of digestive proteases, a widespread inflammatory cell infiltration, leukocyte activation, the release of various kinds of inflammatory mediators, and acinar cell necrosis, and is often associated with significant morbidity and mortality [1, 2]. The premature intra-acinar activation of digestive enzymes is a key event in the pathogenesis of acute pancreatitis. The inflammatory response is partly caused by the release of chemokines from acinar cells, which is followed by recruitment of helper T lymphocytes and macrophages, leading to pancreatic edema and accumulation of neutrophils.A role for oxidative stress in the pathogenesis of pancreatic disease has also been described [3, 4]. The local and systemic release of inflammatory mediators, including cytokines, complement and nitric oxide, in excess may lead to the development of systemic inflammatory response syndrome (SIRS) and/or systemic acute respiratory syndrome (ARDS) [5–7]. Repeated attacks of acute pancreatitis have the potential to evolve into chronic disease characterized by fibrosis and loss of pancreatic function [8]. There are no specific therapies for acute pancreatitis. Medical management is aimed at the control of symptoms, the prevention of severe complications, and possibly endoscopic stone removal if common bile duct stones are present or suspected [9]. Agents which combat oxidative stress, inflammation, and acinar cell injury during the early phase of acute pancreatitis may greatly arrest the pathological progression of severe pancreatitis.
Experimental research has shown the efficacy of plants like Hypericum perforatum and Gardenia jasminoides [10, 11], antioxidants like melatonin, ascorbic acid, N-acetylcysteine, and omega-3 fatty acid [12, 13], and the apoptosis inductor, artemisinin [14], in preventing cerulein-induced pancreatitis, an experimental model for human acute pancreatitis characterized by increased serum amylase and lipase activities, release of pro-inflammatory mediators and cytokines, and histological alterations [15].
Protium heptaphyllum (Aubl.) March, commonly known as almécega, is a widely distributed plant in the Amazon and in the northeast of Brazil. The resin collected from its trunk wood is an effective wound healing agent with anti-inflammatory and analgesic properties [16]. The major component of the resin is α,β-amyrin, a pentacyclic triterpene, and our previous studies established its anti-inflammatory, antipruritic, gastroprotective, and hepatoprotective activities [17–20]. A few other studies reported on its suppressive effect on acute visceral and orofacial nociception and bladder inflammation in a mouse model of cystitis [21–23]. Since these studies established the anti-inflammatory, antinociceptive, and antioxidant properties of α,β-amyrin, the present study evaluated its potential to ameliorate pancreatic injury in a cerulein-induced murine model of acute pancreatitis.
Materials and methods
Materials
The extraction and isolation of α,β-amyrin from the crude resin of P. heptaphyllum (March.) was carried out as described earlier [24] and its structural identity was confirmed by 1H- and 13C-NMR spectral analysis, based on the method developed by Gallegos and Roque [25] and comparison to literature data [26]. The ratio of α- and β-amyrin in this mixture was 63:37. Cerulein was purchased from Sigma–Aldrich (St. Louis, MO, USA), Tween 80 from Merck AG (Darmstadt, Germany), and thalidomide from Funed (Brazil). All solvents used were of analytical grade. The α,β-amyrin was dissolved in 3% Tween 80 and diluted just before use in 0.9% saline.
Animals
Male Swiss mice (20–25 g) obtained from the Central Animal House of our University were used. Experimental groups consisted of eight animals per group. They were housed at 24 ± 2°C under a 12 h/12 h light/dark cycle and had free access to standard pellet diet (Purina chow) and tap water. The animals were deprived of food for 15 h before experimentation, but had free access to drinking water. Experimental protocols were approved by the Institutional Committee on Care and Use of Animals for experimentation (No.84/08) in accordance with the guidelines of the National Institutes of Health, Bethesda, USA.
Experimental design
A total of 48 mice were randomly divided into six groups (n = 8 in each group). Group 1 was normal control; group 2 was treated with vehicle (2% Tween 80 in normal saline in a volume of 10 mL/kg, p.o.); groups 3, 4, and 5 were treated with α,β-amyrin (10, 30, and 100 mg/kg, p.o., respectively); and group 6 was treated with thalidomide (200 mg/kg, p.o.). Acute pancreatitis was induced in groups 2–6 by five i.p. injections of cerulein (50 μg/kg) at intervals of 1 h [27]. α,β-Amyrin and thalidomide were administered 1 h before and 12 h after the cerulein administration. The normal control mice were given i.p. saline (0.9%, NaCl) solution instead cerulein injections. Twenty-four hours after the last injection of cerulein or saline, mice were anaesthetized, blood samples were drawn and the animals exsanguinated, and the pancreas was quickly removed and frozen at −70°C until use.
Pancreatic edema and serum amylase
The pancreatic weight/body weight ratio was evaluated to give an estimate of the degree of pancreatic edema [28]. For serum assays, blood samples were centrifuged at 3,000g at 4°C for 10 min. Serum amylase was determined by routine colorimetric method using the commercial kit for amylase (Labtest Diagnostica SA, Lagoa Santa, Brazil) and expressed as U/dL.
Serum cytokines
Serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 were measured using an ELISA kit according to the manufacturer’s instructions (Quantikine®, R&D Systems, Minneapolis, MN, USA). The cytokine levels were calculated from the standard curve and expressed as pg/mL.
Measurement of pancreatic myeloperoxidase activity
The degree of neutrophil infiltration was quantified by the measurement of pancreatic myeloperoxidase (MPO) activity [29]. Pancreatic tissue (50 mg) was minced and homogenized in 0.5 mL of 50 mmol/L phosphate buffer solution (PBS) (pH 6) that contained 0.5% HETAB. The homogenate was subjected to three cycles of freezing (−30°C) and thawing (37°C) and brief periods (15 s) of sonication, after which they were centrifuged at 12,000g for 15 min at 4°C. The supernatant (0.1 mL) was mixed with 2.9 mL of 50 mmol/L PBS, pH 6, which contained 0.167 mg/mL o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 470 nm was then measured for 5 min using a Beckman spectrophotometer (Beckman DU 640B; CA, USA).
Measurement of pancreatic thiobarbituric acid-reactive substances
Thiobarbituric acid-reactive substances (TBARS) level in the pancreatic tissue was determined as an indicator of lipid peroxidation according to a previously described method [30]. Briefly, 500 μL of 10% tissue homogenate in 0.15 mol/L KCl was mixed with 200 μL 8.1% SDS, and then incubated at room temperature for 5 min. The reaction mixture was heated at 95°C for 1 h after the addition of 1.5 mL 20% acetic acid (pH 3.5) and 1.5 mL 0.8% thiobarbituric acid. After the mixture had cooled, 1.0 mL distilled water and 5.0 mL butanol/pyridine (15:1) solution were added under agitation using a vortex. This solution was centrifuged at 1,000g for 15 min, and the resultant colored layer was measured at 532 nm a Beckman spectrophotometer (Beckman DU 640B).
Immunohistochemistry
Immunohistochemical analysis of the expression of TNFα and inducible nitric oxide synthase (iNOS) was performed. Sections of pancreas (4 μm) were transferred to a gelatin-coated slide. The tissue sections were deparaffinized, and endogenous peroxidase activity was blocked by incubation with 3% H2O2 (10 min). Nonspecific protein binding was blocked by incubating the tissue sections with goat serum (1:200 in PBS for 45 min). The slides were then incubated overnight with primary rabbit anti-TNF-α or rabbit anti-iNOS (Sigma, USA) diluted 1:400 in PBS plus bovine serum albumin. The slides were gently washed with Tris-buffered saline (pH = 7.4) and incubated with alkaline phosphatase conjugated secondary antibody (EnVisionTM/AP K1396, DakoCytomation kit). The reaction was developed by applying onto the slides a solution containing levamisole, to block human alkaline phosphatase activity, and Fast Red Substrate (EnVisionTM/AP, DakoCytomation).
Histological examinations
Samples of pancreatic tissue were fixed in 10% buffered formalin solution, embedded in paraffin by standard methods, cut into 5 μm sections, stained with hematoxylin–eosin, and then assessed under light microscopy and examined blind by a morphologist for grading the histological alterations. Pancreatic edema, leukocyte infiltration, acinar vacuolization, and necrosis were described with scores ranging from 0 to 3 as described by Dembiński et al. [31].
Statistical analysis
Statistical analysis was performed by analysis of variance (ANOVA) followed by Kruskal–Wallis or Student Newman–Keuls as post-hoc tests using GraphPadPrisma (GraphPad Software, San Diego, CA, USA). The non-parametric data is expressed as median (with low and high ranges), and parametric data as mean ± s.e.m. P < 0.05 was considered to indicate statistical significance.
Results
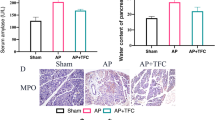
The secretagogue cerulein (5 × 50 μg/kg), administered intraperitoneally, induced acute pancreatitis in mice as evidenced by changes in biochemical and histological parameters. Cerulein caused a significant enhancement in serum levels of amylase enzyme (1,917.0 ± 116.1 U/dL) and the pancreatic weight/body weight ratio (5.944 ± 0.227 mg/g) compared to saline-treated control (1,284.0 ± 15.7 U/dL and 4.624 ± 0.266 mg/g, respectively) (Fig. 1). Pretreatment with α,β-amyrin (10, 30, and 100 mg/kg) showed a dose-related lowering effect on cerulein-induced elevation of serum amylase (801.7 ± 30.6, 536.1 ± 59.3, and 336.5 ± 24.8 U/dL, respectively) (Fig. 1a). At doses of 30 and 100 mg/kg, α,β-amyrin significantly decreased the pancreatic weight/body weight ratio by 14 and 18%, respectively (Fig. 1b). Thalidomide (200 mg/kg), the reference anti-inflammatory drug included in the study, manifested significant reductions in serum amylase activity as well as in pancreatic weight/body weight ratio (Fig. 1).
Effects of α,β-amyrin treatment on serum amylase (a) and pancreatic weight/body weight ratio (b) in cerulein-induced acute pancreatitis in mice. Results are expressed as mean ± SEM from eight mice. *P < 0.05 compared with normal control group; # P < 0.05 compared with vehicle control group (ANOVA followed by Student Newman–Keuls test)
Pancreatic MPO activity and TBARS were significantly elevated in cerulein-induced pancreatitis in mice (Fig. 2). Treatment with α,β-amyrin (10, 30, and 100 mg/kg) significantly reduced the cerulein-evoked increase in pancreatic MPO activity by 58, 62, and 57% and in TBRAS content by 16, 21, and 22%, respectively (Fig. 2a, b). Thalidomide (200 mg/kg) significantly reduced the MPO activity and TBARS content (Fig. 2).
Effects of α,β-amyrin treatment on pancreatic myeloperoxidase (MPO) activity (a) and thiobarbituric acid-reactant substances (TBARS) (b) in cerulein-induced acute pancreatitis in mice. Results are expressed as mean ± SEM from 8 mice. *P < 0.05 compared with normal control group; # P < 0.05 compared with vehicle control group (ANOVA followed by Student Newman–Keuls test)
Serum TNF-α and IL-6 were significantly elevated in cerulein-induced pancreatitis in mice (Fig. 3). Similar to thalidomide, treatment with α,β-amyrin (10, 30, and 100 mg/kg) significantly reduced the cerulein-evoked increase in serum levels of TNF-α and IL-6 (Fig. 3).
Effect of α,β-amyrin treatment on serum TNFα (a) and IL-6 (b) in cerulein-induced acute pancreatitis in mice. Results are expressed as mean ± SEM from eight mice. *P < 0.05 compared with normal control group; # P < 0.05 compared with vehicle control group (ANOVA followed by Student Newman–Keuls test)
Representative TNF-α and iNOS immunostaining of the pancreas for different treatments are shown in Fig. 4 and 5. In control mice, the pattern of TNF-α and iNOS staining was very mild (Fig. 4a, 5a). On the other hand, while there was a high-intensity staining for TNF-α and iNOS in the acinar cells, inflammatory cells, and blood vessels of the pancreas in the cerulein group (Fig. 4b, 5b), in mice pretreated with α,β-amyrin (100 mg/kg) or thalidomide (200 mg/kg) the TNF-α and iNOS immunostaining intensity was much less (Fig. 4, 5).
Effect of α,β-amyrin on TNFα immunoreactivity in cerulein-induced acute pancreatitis in mice. Normal control group showing basal immunostaing (a); vehicle + cerulein showing increased acinar immunostaining (b); α,β-amyrin (100 mg/kg) + cerulein (c); and thalidomide (200 mg/kg) + cerulein (d) showing immunostaining comparable to control group
Effect of α,β-amirin on iNOS immunoreactivity in cerulein-induced acute pancreatitis in mice. Normal control group showing basal immunostaing (a); vehicle + cerulein showing increased acinar immunostaining (b); α,β-amyrin (100 mg/kg) + cerulein (c); and thalidomide (200 mg/kg) + cerulein (d) showing immunostaining comparable to control group
Histological examination of normal controls showed normal architecture and absence of edema, leukocyte infiltration, acinar vacuolization, and necrosis (Fig. 6a and Table 1). In contrast, pancreatic sections from cerulein-administered mice revealed extensive tissue damage characterized by significant disruption of architecture with acinar cell vacuolization, extensive acinar cell necrosis, and inflammatory cell infiltration, and thus gave significantly higher scores (Fig. 6b and Table 1). Pretreatment with α,β-amyrin (100 mg/kg) and thalidomide (200 mg/kg) significantly protected the pancreas from histological damage induced by cerulein, as indicated by lower histological scores (Fig. 6c,d and Table 1).
Representative photomicrographs of haematoxylin–eosin staining of pancreas after 24 h of cerulein-induced pancreatitis in mice. Pancreas in normal control group showing normal architecture (a); pancreas of animals treated with vehicle + cerulein showing disruption of pancreatic architecture with acinar cell vacuolization, extensive acinar cell necrosis, and inflammatory cell infiltration (b); α,β-amyrin (100 mg/kg) + cerulein (c); and thalidomide (200 mg/kg) + cerulein (d)
Discussion
The present study demonstrates that the pentacyclic triterpene, α,β-amyrin, attenuates the severity of cerulein-induced pancreatitis in mice. In particular, we have demonstrated that α,β-amyrin treatment reduces pancreatic inflammation and the associated tissue injury, through suppression of neutrophil infiltration, TNF-α and IL-6 cytokine production, and TNF-α and iNOS expression. TNF-α and IL-6 were found to be enhanced in both experimental pancreatitis as well as in pancreatitis patients [32–34]. TNF-α plays a pivotal role in severe acute pancreatitis, acting early in the disease course [27], and IL-6 constitutes the principal mediator in the synthesis of acute-phase proteins, in addition to transitioning the acute inflammatory response to a chronic response [35]. In this study, α,β-amyrin pretreatment significantly attenuated cerulein-induced TNF-α and IL-6. Furthermore, immunohistochemical staining for TNF-α and iNOS showed that α,β-amyrin could inhibit their expression. Thus, the present study reveal that α,β-amyrin ameliorates acute pancreatitis by suppressing pro-inflammatory cytokines TNF-α and IL-6, and iNOS expression.
Many reports show that cerulein-induced reactive oxygen species (ROS) can activate nuclear factor (NF)-κB, thereby enhancing TNF-α expression [36, 37]. Excessive ROS and nitrogen species (RNS) produced by nitric oxide synthase (NOS) and isoforms of NADPH oxidase, or as by-products of the mitochondrial electron-transport chain, have been implicated in the pathogenesis of acute pancreatitis [4]. Oxidative stress is a deleterious process that can be an important mediator of damage to cell structures, including lipids, membranes, proteins, and DNA. Antioxidants such as N-acetylcysteine, raxofelast, and pyrrolidine dithiocarbamate are efficient inhibitors of NF-κB activation in animal models of pancreatitis [37]. In the present study, α,β-amyrin potently suppressed the neutrophil-mediated MPO enzyme and lipoperoxidation, as evidenced by reduced TBARS formation, events that reflect its antioxidant action. Recent studies have shown that α,β-amyrin and α-amyrin have the capacity to inhibit NF-kB activation [38] and are also effective under a therapeutic regimen in yet another model of l-arginine-induced pancreatitis in which nitric oxide plays a prominent role [39]. Further research is therefore needed to verify that α,β-amyrin would also work under a therapeutic regimen without prophylactic application in this or other models of pancreatitis.
Thalidomide, the positive control used in this study, is a synthetic derivative of glutamic acid that inhibits TNF-α production, modulates adhesiveness in microvascular beds through the modification of surface cell adhesion molecules, and suppresses NF-κB activity [40–42]. Thalidomide manifested amelioration of pancreatic injury almost to a similar degree as α,β-amyrin. This finding with thalidomide is consistent with the observations of Malleo et al. [27] that showed its efficacy in the cerulein model of experimental pancreatitis. Activation of pancreatic primary sensory neurons causes the release of inflammatory neuropeptides, both in the spinal cord to signal pain and in the pancreas itself where they produce plasma extravasation and neutrophil infiltration. Recent studies indicate that primary sensory neurons of the pancreas express transient receptor potential V1 (TRPV1) channels whose activation induces pancreatic inflammation, and blockade of these TRP channels has been suggested to ameliorate pancreatitis inflammation and pain [43, 44]. In this context, our previous studies demonstrated the antinociceptive effects of α,β-amyrin in experimental models of nociception induced by capsaicin (TRPV1 agonist) [20, 45] and in visceral nociception induced by ciclophosphamide, by mechanisms that involve, at least in part, tachykinin NK(1)-receptors and K(+)(ATP) channels [22]. Future studies should examine the efficacy of α,β-amyrin in mitigating pancreatic pain in animal models of acute pancreatitis.
Many plant-derived anti-inflammatory triterpenoids, including oleanone and ursane derivatives, have been previously described to act as competitive and non-competitive inhibitors of the serine proteases trypsin and chymotrypsin, which have a key role in the development of acute pancreatitis [46, 47]. Such an antiprotease effect of α,β-amyrin is quite likely, due to its structural resemblance to ursane and oleanone series of triterpenes.
Conclusions
In conclusion, this study provides the first evidence that α,β-amyrin attenuates the development of cerulein-induced acute pancreatitis by reducing the infiltration of neutrophils and generation of inflammatory cytokines and iNOS. However, the beneficial effect of α,β-amyrin observed in this study is based on prophylactic application. Further research is needed to demonstrate whether it will work also as treatment without prophylactic application in this or other models of pancreatitis. In addition, the protease inhibitory effect of α,β-amyrin on the inflammatory response in experimental acute pancreatitis deserves future study.
References
Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–44.
Regnér S, Manjer J, Appelros S, Hjalmarsson C, Sadic J, Borgström A. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: differences between mild and severe cases and changes over the first three days. Pancreatology. 2008;8:600–7.
Rau B, Poch B, Gansauge F, Bauer A, Nüssler AK, Nevalainen T, et al. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann Surg. 2000;231:352–60.
Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135–66.
Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639–42.
Bhatia M, Wong FL, Fu D, Law HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–5.
Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14:675–84.
Vonlaufen A, Wilson JS, Apte MV. Molecular mechanisms of pancreatitis: current opinion. J Gastroenterol Hepatol. 2008;23:1339–48.
Pandol SJ, Saluja AK, Imrie AK, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–51.
Genovese T, Mazzon E, Di Paola R, Muià C, Crisafulli C, Menegazzi M, et al. Hypericum perforatum attenuates the development of cerulein-induced acute pancreatitis in mice. Shock. 2006;25:161–7.
Jung WS, Chae YS, Kim DY, Seo SW, Park HJ, Bae GS, et al. Gardenia jasminoides protects against cerulein-induced acute pancreatitis. World J Gastroenterol. 2008;14:6188–94.
Foitzik T, Eibl G, Schneider P, Wenger FA, Jacobi CA, Buhr HJ. Omega-3 fatty acid supplementation increases anti-inflammatory cytokines and attenuates systemic disease sequelae in experimental pancreatitis. J Parenter Enteral Nutr. 2002;26:351–6.
Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259–64.
Zhao M, Xue DB, Zheng B, Zhang WH, Pan SH, Sun B. Induction of apoptosis by artemisinin relieving the severity of inflammation in caerulein-induced acute pancreatitis. World J Gastroenterol. 2007;13:5612–7.
van Westerloo DJ, Maris NA, Bruno MJ, van der Poll T. Caerulein induced pancreatitis. Gut. 2003;52:452–3.
Siani AC, Ramos MFS, de Lima MO, Santos R, Ferreira FE, Soares EC, et al. Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium. J Ethnopharmacol. 1999;66:57–69.
Oliveira FA, Lima-Júnior RC, Cordeiro WM, Vieira-Junior GM, Chaves MH, Almeida FRC, et al. Pentacyclic triterpenoids, alpha, beta-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacol Biochem Behav. 2004;8:719–25.
Oliveira FA, Vieira-Júnior GM, Chaves MH, Almeida FRC, Santos KA, Martins FS, et al. Gastroprotective effect of the mixture of alpha- and beta-amyrin from Protium heptaphyllum: role of capsaicin-sensitive primary afferent neurons. Planta Med. 2004;70:780–2.
Oliveira FA, Chaves MH, Almeida FR, Lima RC Jr, Silva RM, Maia JL, et al. Protective effect of alpha-and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. Trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol. 2005;98:103–8.
Holanda Pinto SA, Pinto LM, Cunha GM, Chaves MH, Santos FA, Rao VS, et al. Anti-inflammatory effect of alpha, beta-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacol. 2008;16:48–52.
Lima-Júnior RCP, Oliveira FA, Gurgel LA, Cavalcante IJM, Santos KA, Campos DA, et al. Attenuation of visceral nociception by alpha- and beta- amyrin, a triterpenoid mixture isolated from the resin of Protium heptaphyllum, in mice. Planta Med. 2006;72:34–9.
Lima-Júnior RC, Sousa DI, Brito GA, Cunha GM, Chaves MH, Rao VS, et al. Modulation of acute visceral nociception and bladder inflammation by plant triterpene, alpha, beta-amyrin in a mouse model of cystitis: role of tachykinin NK(1)-receptors, and K(+)(ATP) channels. Inflamm Res. 2007;56:487–94.
Holanda Pinto SA, Pinto LM, Guedes MA, Cunha GM, Chaves MH, Santos FA, et al. Antinoceptive effect of triterpenoid alpha, beta-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine. 2008;15:630–4.
Vieira-Junior GM, Souza CML, Chaves MH. Resina de Protium heptaphyllum: isolamento, caracterização estrutural e avaliação das propriedades térmicas. Química Nova. 2005;28:183–7.
Gallegos RS, Roque NE. Analise de misturas de triterpenos por 13C NMR. Química Nova. 1990;13:278–81.
Mahato SB, Sen S. Advances in triterpenoid research, 1990–1994. Phytochemistry. 1997;44:1165–236.
Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130–40.
Szabolcs A, Varga IS, Varga C, Berkó A, Kaszaki J, Letoha T, et al. Beneficial effect of resveratrol on cholecystokinin-induced experimental pancreatitis. Eur J Pharmacol. 2006;532:187–93.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Dembiński A, Warzecha Z, Ceranowicz P, Warzecha AM, Pawlik WW, Dembiński M, et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur J Pharmacol. 2008;591:284–92.
de Beaux AC, Ross JA, Maingay JP, Fearon KC, Carter DC. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br J Surg. 1996;83:1071–5.
Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–62.
Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, et al. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–8.
Papachristou GI. Prediction of severe acute pancreatitis: Current knowledge and novel insights. World J Gastroenterol. 2008;14:6273–5.
Kim H, Seo JY, Kim KH. NF-kappaB and cytokines in pancreatic acinar cells. J Korean Med Sci. 2000;15:S53–4.
Rakonczay Z Jr, Hegyi P, Takács T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–67.
Medeiros R, Otuki MF, Avellar MC, Calixto JB. Mechanisms underlying the inhibitory actions of the pentacyclic triterpene alpha-amyrin in the mouse skin inflammation induced by phorbol ester 12-O-tetradecanoylphorbol-13-acetate. Eur J Pharmacol. 2007;559:227–35.
Melo CM, Carvalho KM, Neves JC, Morais TC, Rao VS, Santos FA, et al. alpha, beta-amyrin, a natural triterpenoid ameliorates l-arginine-induced acute pancreatitis in rats. World J Gastroenterol. 2010;16:4272–80.
Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703.
Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–80.
Yasui K, Kobayashi N, Yamazaki T, Agematsu K. Thalidomide as an immunotherapeutic agent: the effects on neutrophil-mediated inflammation. Curr Pharm Des. 2005;11:395–401.
Wick EC, Hoge SG, Grahn SW, Kim E, Divino LA, Grady EF, et al. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G959–69.
Liddle RA. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta. 2007;1772:869–78.
Oliveira FA, Costa CL, Chaves MH, Almeida FR, Cavalcante IJ, Lima AF, et al. Attenuation of capsaicin-induced acute and visceral nociceptive pain by alpha- and beta-amyrin, a triterpene mixture isolated from Protium heptaphyllum resin in mice. Life Sci. 2005;77:2942–52.
Rajic A, Akihisa T, Ukiya M, Yasukawa K, Sandeman RM, Chandler DS, et al. Inhibition of trypsin and chymotrypsin by anti-inflammatory triterpenoids from Compositae flowers. Planta Med. 2001;67:599–604.
Granger J, Remick D, et al. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24:45–51.
Acknowledgments
This study was supported by grants from CNPq and CAPES, Brazil and FUNCAP, Ceará. The technical assistance of Francisco Alison Quintito Braga and Anissa Quintino de Miranda are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Melo, C.M., Morais, T.C., Tomé, A.R. et al. Anti-inflammatory effect of α,β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. Inflamm. Res. 60, 673–681 (2011). https://doi.org/10.1007/s00011-011-0321-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0321-x