Abstract
The present work was undertaken with an objective to evaluate the impact of temperature on respiration rate of fresh black carrot using a non-linear enzyme kinetics approach. Two different models viz. Arrhenius equation (temperature effect) and enzyme kinetics (effect of temperature and headspace concentration) have been compared for predicting the respiration rate of black carrot. The respiratory behavior of black carrot was assessed using closed system technique at 5, 10 and 15 °C (± 1 °C) temperature and constant relative humidity (RH) of 85%. The O2 consumption and CO2 evolution rate values were 39.17, 58.88 and 68.08 ml kg−1 h−1; 22.15, 34.63 and 41.86 ml kg−1 h−1 after the attainment of steady-state condition at 5, 10 and 15 °C, respectively. The inhibition by evolved CO2 was found to be predominantly competitive at all temperatures. The average absolute deviation in O2 consumption and CO2 evolution rate for Arrhenius model was 3.5% and 5.3% while for enzyme kinetics model was 8.8% and 6.3%, respectively. Dependency of respiration rate of black carrot on temperature was well defined by Arrhenius model. The outcomes of the study can be further utilized to design the MAP (modified atmosphere packages) for fresh black carrot storage at 5 °C with 85% RH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetables are diverse groups and have been recognized for their human health benefits. Most of the root vegetables have a high content of nutritive and bioactive compounds (Galiana-Belaguer et al. 2018; Guliyev et al. 2018). Black carrot (Dacus carota L.) is one such root vegetable which is also a valuable source of antioxidant property, bioactive compounds and functional components (Kamiloglu et al. 2018). During postharvest storage, black carrot respires to provide energy for its biochemical process and undergoes a complex series of physicochemical reactions along with microbiological changes that were responsible for quality changes i.e. low storage stability (Barbosa et al. 2011). Thus, the self-stability of fresh commodities can be enhanced by controlling O2 and CO2 levels of their storage environment (Rocculi et al. 2006). The quality and storage stability of perishable commodity is usually affected by two important factors i.e. temperature and relative humidity (Tigist et al. 2013). Kirca et al. (2007) also examined and concluded from their results that bioactive and antioxidant compounds of fresh produce degrade at a faster rate if stored at high temperatures. Hence the need was realized for black carrot postharvest management in MAP (modified atmosphere packaging) storage. However, accurate respiration rates and modeling is pivotal to design MAP for agricultural commodity due to their perishability.
Design of an appropriate packaging for extending the shelf life of black carrot depends upon multiple factors include crop (variety, maturity stage, respiration, transpiration, etc.), surrounding environment (storage temperature and relative humidity) and package type (packaging film, permeability, thickness, area exposed and headspace composition) (Rennie and Tavoularis 2009). The incorrectly designed model may be ineffective or even shorten the shelf life of the black carrot. Thereafter, an appropriate handling method, especially storage temperature for post-harvest is required for extending the self-stability of black carrot.
The metabolic process of perishable crop continues even after harvesting as they are directly proportional to the storage temperature because with every 10 °C arise in temperature (stored perishable crop), as it increased by two or three folds (Kirca et al. 2007). The Q10 coefficient can be very useful in predicting a loss in quality of fresh black carrot, with 10 °C augmentation in storage temperature, results in an increase of respiration rate along with depletion of sugars and organic acids from the tissue (Jerry and Jeffrey 2002). At higher temperatures, enzymatic denaturation may occur which are responsible for the quality change of black carrots. Thus, the respiration rate and biochemical reactions can be controlled at a low storage temperature of fresh produce (Iqbal 2009). But even, at too low temperature, physiological injuries may also occur which enhances the respiration rate (Fonseca et al. 2002).
Measuring the respiration rate of fresh produce is important for storage stability but measuring the respiration rate at a particular storage temperature is a time-consuming process. Thus, different mathematical models have been developed in order to correlate the respiration rate with other storage parameters (O2, CO2, time and temperature) (Kaur et al. 2011; Singh et al. 2014). Arrhenius law explains the temperature dependent respiration rate (Fonseca et al. 2002; Benkeblia 2004). Peppelenbos and Leven (1996) proposed the O2 consumption rate which depends on the headspace composition i.e. O2 and CO2 concentration. The fact about the respiratory metabolic activity can be carried out by enzymatic reactions, but the Michaelis–Menten equation has been used by many investigators (Ersan et al. 2010) which assume that evolved CO2 has no inhibition effect to explain the relation of respiration with gas concentration. Moreover, the respiratory quotient has been generally assumed to be unity for predicting the rate of CO2 evolution. Kaur et al. (2011) suggested that including the effect of headspace, temperature and respiration quotient in the same model can help in reducing the deviation of predicted values. Until recently, a few research has been carried out which investigate the combined effect of temperature, headspace concentration, and respiration quotient relation especially with respect to black carrot storage.
Thus, the present study has been designed to evaluate the impact of surrounding temperature, headspace concentration and inhibition by evolved CO2 on the respiratory behavior of fresh black carrot. A mathematical model was developed for predicting respiration behavior of fresh black carrot at different storage temperatures (5, 10 and 15 °C) and comparing the results with respect to the proposed model of Peppelenbos and Leven (1996). Finally, the developed model can be used for selecting optimum packaging material for a fresh black carrot.
Materials and methods
Procurement of raw material
Fresh black carrot (Variety: Punjab Black Beauty) was procured from Vegetable Farm, Punjab Agricultural University (PAU) after harvesting at the maturity stage. It was harvested in the winter season (December-January) and ambient temperature during these days was varied from 8 to 10 °C. The selected crop was pre-cleaned to remove adhering dirt’s and used as raw material for experimental investigation. The black carrot was properly handled to avoid any mechanical injury and bruising.
Physicochemical properties of fresh black carrot
Physicochemical properties of fresh black carrot were carried out in ten replications and the average of these was taken.
Physical dimensions of fresh black carrot
Length of fresh black carrot was measured using measuring scale and diameter of the end (1 cm from tip), mid and top of fresh black carrot were measured using Vernier Caliper (Make: Mitutoyo Corporation, Japan; Model: CD- 6″ R).
Weight of fresh black carrot
Weight of fresh black carrot was measured using a precision electronic weighing balance (Make: A&D Instruments, Japan; Model: GF1200) with the least count 0.0001 g.
Texture Analysis
The market value of black carrot is affected by its firmness (g) which measured using Texture Analyser (Make: Stable MicroSystems, Model: TA XT plus). Test for each sample was performed in triplicate, the stick of black carrot kept horizontal under the Warner/Blazer (HDP/BS) blade of test speed of 5 mm/s and penetration distance of 30 mm (Ayhan et al. 2008). Results were expressed as maximum cutting force (g) which represents the firmness of black carrot.
Color
The color of the freshly harvested black carrot samples was measured by using Color Reader CR-10 (Konica Minolta Sensing Inc.). The instrument was calibrated before color analysis using white and black tiles. For determination of color, the sample was tightly filled in petri dish provided that no light was allowed to pass during the measuring process. The values were recorded as ‘L’, ‘a’ and ‘b’ and the average values were taken for statistical analysis.
Total soluble solids
Total soluble solids of fresh black carrot were measured after extracting juice, using a hand refractometer (Make ERMA) of 0–32°brix range.
Flavonoids content
Flavonoid content of black carrot was estimated using the method given by Chang et al. (2002) with few modifications. One gram of black carrot sample was homogenized with 10 ml of methanol and 0.5 ml of supernatant was diluted with 1.5 ml of methanol. Then add 1 ml of 1% aluminium chloride and 1% potassium acetate to the solution. The resulting solution was diluted with 2.8 ml distilled water and allowed to stand for 30 min. The optical density was measured against the blank without extract at 415 nm. The standard curve was plotted by taking an aliquot of 0.2–1 ml (5 concentrations) from standard solution of quercetin (100 mg in 100 ml of distilled water) and final volume was made up to 1 ml with distilled water. The results were calculated using the obtained standard curve of quercetin and expressed as mg equivalent of quercetin/100 g (fresh weight basis).
Antioxidants activity
Antioxidant activity was determined as a scavenging activity of free radicals using 2, 2-diphenyl-l-picrylhydrazyl (DPPH) radical (HiMedia Laboratories, India) as per the method of De Ancos et al. (2002). 1 g of sample was extracted using 50 ml of 80% methanol for 2 h, clarified with a cold centrifuge (Eltek Limited; MP 400-R; India) (6000 rpm for 5 mm at 4 °C) and repeated extraction again for left behind in centrifuge tube for 1 h more. Aliquots of 0.01 ml of supernatant were mixed with 3.9 ml of methanolic DPPH (0.025 g/l) and 0.09 ml of distilled water. The resulting mixture was incubated at room temperature in dark for 30 min. The absorbance of the mixture was measured against the blank (methanol) at 515 nm. Antioxidant activity was calculated as the ratio of difference between control OD (optical density) and sample OD to control OD and expressed as % inhibition of DPPH scavenging activity.
Anthocyanins content
The anthocyanins content in carrot was determined using the modified method described by Tonutare et al. (2014). Two-gram black carrot sample was ground in pestle and mortar with ethanolic HCL (85:15) until extract becomes colorless. A pinch of sodium sulphate was added to absorb moisture during extraction. After extraction, the final volume was made up to 25 ml with ethanolic HCL and results were taken at 535 nm against ethanolic HCL as blank.
Respiration studies of fresh black carrot
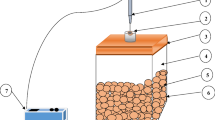
A closed or static system, hermetically sealed glass jar was used to achieve the objective of the present study (Fig. 1) (Lokke et al. 2011). For making airtight glass jar, the screw at the top of the jar was provided to close the lid and was air-sealed using silica gel (Sorbead, India) around its neck (Ghosh and Dash 2018). PTFE Silicone septa, a self-sealing glued to a glass jar for taking the gas sample using a sampling needle of the gas analyzer (Gaspace Advance® electronic analyzer; Make: Systech Instruments; UK). It was injected through the silicone septa glued at the midpoint of a glass jar for measuring O2 and CO2 concentration.
The average weight of black carrot was around 200 ± 5 g of sample in each jar. The true density and weight of black carrot were analyzed using the water displacement method (Ishikawa et al. 1992) and weighing balance, respectively. The total volume of the glass jar (VT) was measured using water which filled up to the brim. For measuring the volume of the black carrot, it was kept in a closed jar and estimated using the Eqs. (1):
The void volume (VV) was estimated using the following relationship in Eqs. (2):
The impermeable glass jars were then kept in a walk-in cooling chamber (Motherson Zanotti, India) with dimensions of 174 × 143 × 216 cm at various temperatures (5, 10, and 15 °C) and relative humidity (RH) of 85% (± 1%). For the respiration experiment, the analysis was carried out in replicates (three) and means values were used for further analysis. The headspace gaseous concentration was continuously measured (%) after every 30 min, at regular intervals using the gas analyzer and converted into partial pressures, using the relation of one atmospheric pressure (101.325 kPa) which is equivalent to 100% gaseous composition. Gas concentrations were measured until the alteration between two consecutive observations become constant indicates the attainment of steady-state.
Respiration rate at each temperature in terms of O2 consumption and CO2 evolution rate was evaluated as outlined below (Singh et al. 2014) in the mathematical form of Eqs. (3) and (4) respectively:
Respiratory quotient (RQ) is the ratio of CO2 evolution rate to O2 consumption rate (Torrieri et al. 2010) as outlined below [Eqs. (5)]:
The values of Q10 were used in calculating the ratio of respiration rates (RR) which measures at two different storage temperatures, explained in Eqs. (6):
Enzyme kinetics model
The effect of headspace concentrations on the respiratory behavior of fresh black carrot was studied on the basis of a model proposed by Peppelenbos and Leven (1996). According to this model, the enzyme kinetics parameters, viz.\({V}_{{m}_{{O}_{2}}}\),\({K}_{{m}_{{O}_{2}}}\),\({K}_{{mc}_{C{O}_{2}}}\) and \({K}_{{mu}_{C{O}_{2}}}\) were estimated using non-linear analyses of respiration data for combined or mixed inhibition (Kaur et al. 2011). Effect of O2 headspace concentration on respiration was evaluated using Michaelis–Menten constant \({K}_{{m}_{{O}_{2}}}\) which is a measure of respiration at saturation point with headspace O2. It epitomizes the oxygen concentration at which half of the maximum respiration rate reached, assuming no inhibition by CO2 but it can be evaluated using inhibition constants \({K}_{{mc}_{C{O}_{2}}}\) and \({K}_{{mu}_{C{O}_{2}}}\). The inhibition constant value is an estimation of level to which respiration inhibited by CO2. The high value of inhibition constants indicates that backward reaction is faster than forwarding reaction thus, CO2 inhibition is not possible. The non-linear analysis shows that inhibition constant represents that black carrot respiration was susceptible to combined inhibition (\({K}_{{mc}_{C{O}_{2}}}\) and \({K}_{{mu}_{C{O}_{2}}}\) are finite and unequal). Further, to incorporate the temperature effect, temperature dependent equations were developed for all enzyme kinetics parameters.
Arrhenius equation
The effect of temperature on respiration rates and enzyme kinetic parameters is calculated using, Arrhenius relationship which is referred for fresh produce. It depends on chemical reactions theory i.e. reactions complexity which has a direct impact on temperature. Respiration rates and enzyme kinetic model parameters were computed by Arrhenius relations as shown in Eqs. (8), (9), (10) and (11), (12), (13), (14) respectively:
where \({R}_{{O}_{2}}\), \({R}_{{CO}_{2}}\) and \(RQ\) are respiration rates and respiratory quotient,\({V}_{{{m}_{O}}_{2}}\), \({K}_{{{m}_{O}}_{2}}\),\({K}_{{{mc}_{CO}}_{2}}\),\({K}_{{{mu}_{CO}}_{2}}\), are enzyme kinetic model parameters \({R}_{{O}_{2,}}\)P, \({R}_{{CO}_{2,}}\)P and \(RQ,\)P are respiration pre-exponential factors,\({V}_{{{m}_{O}}_{2}},\)P,\({K}_{{{m}_{O}}_{2}},\)P, \({K}_{{{mc}_{CO}}_{2}},\)P, \({K}_{{{mu}_{CO}}_{2}},\)P, are enzyme kinetic pre-exponential factors,\({E}_{a1}\), \({E}_{a2}\), \({E}_{a3}\), \({E}_{a4}\), \({E}_{a5}\), \({E}_{a6}\) and \({E}_{a7}\) are activation energies, kJ g−1 mol−1, R is the universal gas constant, 8.314 kJ g−1 mol−1 K−1, T represents storage temperature (K) and Tref represents the reference temperature which is the average of experimental temperatures.
Prediction models of respiration rate
The rates of O2 consumption and CO2 evolution were predicted using two different relationship models viz. The Model I: Based on the Arrhenius equation to study the effect of temperature and Model II: Based on Peppelenbos and Leven’s (1996) equation to study the effect of headspace composition. Model II also incorporated the effect of temperature by introducing temperature dependent enzyme kinetic parameters. RCO2 was determined from RO2 using the RQ relationship.
Statistical analysis
The partial pressures and respiration rates of O2 and CO2 used to examine the various enzyme kinetics parameters using non-linear regression in Graph Pad PRISM® Ver. 5.00.288 software (Graph Pad Software Inc., USA). The average absolute deviation is mostly used to determine the goodness of fit (Mangaraj et al. 2011).
Results and discussion
Physicochemical properties
The physicochemical characteristics of the fresh black carrot were presented in Table 1. The length of black carrot varied from 25.62 to 25.78 cm and the diameter of the end (1 cm from tip), mid-section and top were 0.707–0.758, 2.05–2.1 and 3.3–3.38 cm, respectively. The length and diameter of the black carrot were almost similar to those reported by Rubatzky et al. (1999). The average weight of fresh black carrot was 25.2 g which was in accordance to the results that reported by Dhillon et al. (2016). The color parameters viz. L, a and b values were found to be 41.93, 3.5 and – 2.8, respectively. Total soluble solids (TSS) in fresh black carrot were observed as 8.2°Brix and firmness as 67.28 N. The range of TSS varied from 6.55 to 8.28°Brix for fresh black carrot as reported by Dhillon et al. (2016) and firmness from 80 to 97 N as described in minimally processed fresh carrots by Ahyan et al. (2008). Firmness might also depends on the variety or any other characteristic features. The antioxidant capacity, flavonoids content and anthocyanins content in fresh black carrot were 47.44%, 218.75 mg QUE/100 g and 186.05 mg/100 g, respectively. The obtained results were in accordance with Dhillon et al. 2016 (anthocyanins content) and Kaur (2018) (antioxidant capacity) in black carrot.
Dependence of partial pressures on time and temperature
From the Fig. 2, it has been observed that the partial pressures of oxygen and carbon dioxide decreased and increased, respectively at all the considered temperatures. Partial pressures of oxygen (21.16%) inside the jar decreased to 15.20, 12.20, 10.80% whereas the partial pressures of carbon dioxide increased to 3.40, 5.30, 6.40% from 0.03% with the progress of time and achieved the steady-state condition after 3.5, 4 and 4.5 h corresponding to the temperature 5, 10 and 15 °C, respectively. The temperature has a direct impact on partial pressure which might be due to the higher respiration rates at higher temperatures (Pereira et al. 2017). Similar was reported by Barbosa et al. (2011) in minimally processed carrot. Singh et al. (2014) also reported a comparable trend of partial pressures in fresh baby corn. The rate of decreasing and increasing of \({p}_{{O}_{2}}^{in}\) and \({p}_{{CO}_{2}}^{in}\), respectively fastens as temperature elevates from 5 to 15 °C along with oxygen rate which also have direct impact on respiration rates. Thus not only temperature but also higher O2 concentration fastens the uptake of O2 as respiratory enzymes were exposed more to the reaction substrate. A similar effect of temperature and time on the partial pressures was reported in tomatoes (Castellanos et al. 2016) and yellow bell pepper (Kirandeep et al. 2018).
Dependence of respiration rate on time and temperature
The present study revealed that the respiration rate was significantly influenced by time and temperature. Figure 2 shows the results with respect to the respiration rate that O2 consumption and CO2 evolution rate declines with time. With the increment of time, from 0 to 4.5 h, the respiration rate of O2 and CO2 decreased by 47.44 and 34.31% at 5 °C, respectively. There was a significant difference observed in the respiration rate for all the temperatures. The oxygen consumption rate values were 74.52, 98.18 and 104.09 ml kg−1 h−1 and carbon dioxide evolution rate values were 33.71, 51.46 and 57.37 ml kg−1 h−1 at 5, 10 and15 °C temperature, respectively at the beginning of experiment but later on they stabilized moderately to 39.17, 58.88 and 68.08 ml kg−1 h−1; 22.15, 34.63 and 41.86 ml kg−1 h−1, respectively after attainment of steady-state conditions. Similar was observed by Iqbal et al. (2008) in stored red carrots that the O2 consumption and CO2 evolution rate increased as the storage temperature augmented. In the whole respiration study, partial pressures of O2 and CO2 surrounded by the aerobic respiration limit but fermentation were not observed.
Initially, the change of respiration rate was higher and decreased with the succession of time till the steady-state condition was achieved where the respiration rate attains nearly constant values. This might be due to the decrease in oxygen concentration in a hermetically sealed jar (Ghosh and Dash 2018). Iqbal et al. (2008) also reported that respiration rate augments with storage time in whole carrots. Belay et al. (2017) concluded that time and temperature has a direct impact on the respiration rate of pomegranate arils.
In the present study, it was observed that with a slight increase in temperature, the respiration rate changed drastically. Thus, a higher temperature of stored fresh produce accelerates their metabolic rate The respiration rates at 15 °C were higher as compared to 5 °C. Augmentation in temperatures increased the metabolic activity of a black carrot which results in an enhanced respiration rate. Alike, results in a change of respiration rate along with temperature and time were reported by Singh et al. (2014) in Baby corn. At higher temperatures, moisture condensation may also occur which leads to the spoilage of fresh produce (Kaur et al. 2011). Dependence on the temperature of respiration rates examined using the Arrhenius equation and was calculated by plotting the natural logarithm of respiration rates against the inverse of corresponding temperatures. It was found that the respiration rate immensely dependents on temperature and as expressed by their higher activation energies as RO2 (Ea = 37.39 kJ/mol) and RCO2 (Ea = 42.04 kJ/mol) and temperature dependence relation presented in Table 2. It was found that the nature of dependence of respiration rate was similar. Iqbal et al. (2008) also observed higher activation energy in whole carrots.
Respiratory quotient and Q10
The respiratory quotient (RQ) of fresh black carrot increased gradually as temperature augments as shown in Table 2. The respiration quotient values were 0.56, 0.58 and 0.61 at 5, 10 and 15 °C, respectively, during the steady-state condition. A similar trend of RQ was witnessed in fresh baby corn (Singh et al. 2014). The temperature dependence relationship of RQ was evaluated using the Arrhenius relationship and higher activation energy (Ea = 5.98 kJ/mol) was observed. RQ increased persistently, as the time lapsed which further stabilized as the experiment achieved the steady-state condition. RQ value less than unity revealed that O2 consumption was higher than the CO2 production. This might be due to produce’s skin permeability which does not increase as O2 consumption for a given temperature changed (Beaudry et al. 1992). The Q10 value of freshly harvested black carrot was 1.73 as temperature increased from 5 to 15 °C. A similar trend of RQ and Q10 was reported by Fonseca et al. (2002) in fresh produce that it increased gradually as temperature augmented. Barbosa et al. (2011) also reported in their study that the RQ value of carrot accelerates with temperature.
Dependence of enzyme kinetics on headspace concentrations and temperature
The enzyme kinetics parameters, viz. \({V}_{{m}_{{O}_{2}}}\), \({K}_{{m}_{{O}_{2}}}\), \({K}_{{mc}_{C{O}_{2}}}\) and \({K}_{{mu}_{C{O}_{2}}}\)(Table 2) estimated by non-linear analysis of respiration values for combined or mixed inhibition using the enzyme kinetics equation. The oxygen consumption rate against oxygen concentration (Fig. 3) curve does not shifted down but to the left. As the highest velocity of the enzyme doesn't alter even though enough O2 was supplied, but more O2 was taken to achieve the half-maximal activity. In the present experiment, samples stored at 5, 10 and 15 °C, extra O2 was not supplied, thus O2 concentration level decreased within a few hours, which considerably reduced the half of the maximum velocity of the reaction. The results of the present study revealed that Michaelis–Menten constant for competitive inhibition was less than un-competitive inhibition of O2 consumption by CO2, %. This might be due to the reversible binding of CO2 at the same site as O2 (substrate). Thus, the inhibition of this study was predominantly competitive at all studied storage temperatures and similar was explained by Copeland (2000).
Values of \({K}_{{m}_{{O}_{2}}}\) at all temperatures revealed that fresh black carrot showed a steep incline in respiration with respect to temperature. \({V}_{{m}_{{O}_{2}}}\) For fresh black carrot increased from 93.58 to 115.5 as temperature augmented from 5 to 15 °C. Both \({V}_{{m}_{{O}_{2}}}\) and \({K}_{{mu}_{C{O}_{2}}}\) were not much temperature dependent as expressed by its lower activation energies i.e. 13.98 and 67.56 kJ mol−1 than other parameters. \({K}_{{mc}_{C{O}_{2}}}\) was found to be highly temperature dependent as compared to other inhibition constants (\({K}_{{m}_{{O}_{2}}}\) and \({K}_{{mu}_{C{O}_{2}}}\)). It was found that all the parameters related to black carrot were temperature dependent but the nature of dependence was dissimilar. Many investigators reported that enzyme kinetics model parameters have a dependent relationship with temperature (Benkeblia 2004; Geysen et al. 2005). A similar result of low activation energy for \({V}_{{m}_{{O}_{2}}}\) was reported by Singh et al. (2014) in fresh baby corn.
Model predictions
The respiration rates predicted using Model I and Model II further compared with the experimental results at three different storage temperatures (5, 10 and 15 °C). Experimental results of respiration rates (RO2 and RCO2) were evaluated using a closed system technique. The average absolute deviation for the O2 consumption rate and CO2 evolution rate for Model I was 3.5% and 5.3% while Model II was 8.8% and 6.3%, respectively (Fig. 4). This indicated that the predicted model result fitted experimental results well.
Conclusion
In the present study, respiratory behavior of fresh black carrot was carried out in hermetically sealed glass jar at different temperatures 5, 10 and 15 °C and respiration rates attained steady state after 3.5, 4 and 4.5 h, respectively. Thus, the temperature and time have a direct impact on partial pressure which results in high respiration rates at higher temperatures. The respiration rates at 15 °C were higher as compared to 5 °C because augmentation in temperatures increased the metabolic activity of a black carrot. Respiration rates of O2 and CO2 increased by 50.33% and 56.33%, respectively as temperature amplified from 5 to 10 °C whereas an increase of 73.82% and 89.01% was observed as temperature augmented from 5 to 15 °C. In the enzyme kinetics model, respiration rates follow the predominantly competitive inhibition at all storage temperatures. Arrhenius equation model was found to be better in predicting the respiration rate of fresh black carrot. The application of present results can be helpful in designing the packaging material for the storage of fresh black carrot at 5 °C with 85% RH.
Abbreviations
- \({{p}_{{CO}_{2}}}^{f}\) :
-
Final concentration of CO2, %
- \({{p}_{{CO}_{2}}}^{in}\) :
-
Initial concentration of CO2, %
- \({{p}_{{O}_{2}}}^{f}\) :
-
Final concentration of O2, %
- \({{p}_{{O}_{2}}}^{in}\) :
-
Initial concentration of O2, %
- \({Rco}_{2}\) :
-
Respiration rate (rate of CO2 evolution, ml kg−1 h−1)
- \({Ro}_{2}\) :
-
Respiration rate (rate of O2 consumption, ml kg−1 h−1)
- \({V}_{{m}_{{O}_{2}}}\) :
-
Maximum oxygen consumption rate, ml kg−1 h−1
- \({V}_{s}\) :
-
Volume of black carrot sample, ml
- \({V}_{t}\) :
-
Total volume of jar, ml
- \({V}_{v}\) :
-
Void volume of jar, ml
- \({K}_{{m}_{{o}_{2}}}\) :
-
Michaelis–Menten constant for oxygen, %
- \({K}_{{mc}_{{CO}_{2}}}\) :
-
Michaelis–Menten constant for competitive inhibition of O2 consumption by CO2, %
- \({K}_{{mu}_{{CO}_{2}}}\) :
-
Michaelis–Menten constant for uncompetitive inhibition of O2 consumption by CO2, %
- \({t}^{f}\) :
-
Final time, h
- \({t}^{i}\) :
-
Initial time, h
- \({\rho }_{s} \) :
-
Mean density of fresh black carrot, kg l−1
- E a :
-
Activation energy, kJ mol−1
- RH:
-
Relative humidity, %
- \(W\) :
-
Produce weight, kg
- \({W}_{\mathrm{s}}\) :
-
Weight of fresh black carrot inside the impermeable jar, kg
References
Ancos BD, Sgroppo S, Plaza L, Cano MP (2002) Possible nutritional and health- related value promotion in orange juice preserved by high-pressure treatment. J Sci Food Agric 82:790–796
Ayhan Z, Esturk O, Tas E (2008) Effect of modified atmosphere packaging on the quality and shelf life of minimally processed carrots. Turk J Agric For 32:57–64
Barbosa LDN, Carciofi BAM, Dannenhauer CE, Monteiro AR (2011) Influence of temperature on the respiration rate of minimally processed organic carrots (Daucus Carota L. cv. Brasília). Ciênc Tecnol 31:78–85
Beaudry RM, Cameron AC, Shirazi A, Dostal-Lange AL (1992) Modified atmosphere packaging of blueberry fruit: effect of temperature on package O2 and CO2. J Amer Soc Hort Sci 117:436–441
Belay ZA, Caleb OJ, Opara UL (2017) Enzyme kinetics modeling approach to evaluate the impact of high CO2 and super-atmospheric O2 concentrations on the respiration rate of pomegranate arils. Cyta J Food 15:608–616
Benkeblia N (2004) Effect of maleic hydrazine on respiratory parameters of stored onion bulbs (Allium cepa L.). Braz J Plant Physiol 16:47–52
Castellanos DA, Cerisuelo JP, Hernandez-Munoz P, Herrera AO, Gavara R (2016) Modeling the evolution of O2 and CO2 concentrations in MAP of a fresh product: application to tomato. J Food Eng 168:84–95
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Copeland RA (2000) Enzymes, 2nd edn. Wiley-VCH, New York
Dhillon HS, Dhillon TS, Devi R (2016) Quality characterization in carrot (Daucus carota L.) Germplasm. Indian J Ecol 43:330–332
Ersan S, Gunes G, Zor AO (2010) Respiration rate of pomegranate arils as affected by O2 and CO2, and design of modified atmosphere packaging. Acta Hort 876:189–196
Fonseca SC, Oliveira FAR, Brecht JK (2002) Modeling respiration rate of fresh fruits and vegetables for modified atmosphere packages: a review. J Food Eng 52:99–119
Galiana-Belaguer L, Ibanez G, Cebolla-Cornejo J, Rosello S (2018) Evaluation of germplasm in Solanum section Lycopersicon for tomato taste improvement. Turk J Agric For 42:309–321
Geysen S, Verlinden BE, Conesa A, Nicolai BM (2005) Modeling respiration of strawberry (cv. Elsanta) as a function of temperature, carbon dioxide, low and super atmospheric oxygen concentration. Frutic 5:12–16
Ghosh T, Dash KK (2018) Respiration rate model and modified atmosphere packaging of bhimkol banana. Eng Agric Environ Food 11:186–195
Guliyev N, Sharifova S, Ojaghi J, Abbasov M, Akparov Z (2018) Genetic diversity among melon (Cucumis melo L.) accessions revealed by morphological traits and ISSR markers. Turk J Agric For 42:393–401
Iqbal T, Rodrigues FAS, Mahajan PV, Kerry JP, Gil L, MC Cunha LM (2008) Effect of minimal processing conditions on respiration rate of carrots. J Food Sci 73:396–402
Iqbal T, Rodrigues FAS, Mahajan PV, Kerry JP (2009) Mathematical modeling of the influence of temperature and gas composition on the respiration rate of shredded carrots. J Food Eng 91:325–332
Ishikawa Y, Sato H, Ishitani T, Hirata T (1992) Evaluation of broccoli respiration rate in modified atmosphere packaging. J Pack Sci Technol 1:143–153
Jerry AB, Jeffrey KB (2002) Postharvest physiology and pathology of vegetables. CRC Press, United States
Kamiloglu S, Camp JV, Capanoglu E (2018) Black carrot polyphenols: effect of processing, storage and digestion- an overview. Phytochem Rev 17:379–395
Kaur A (2018) Physicochemical changes in black carrot during growth and development of roots. J Nutr Food Sci 8:72
Kaur P, Rai DR, Paul S (2011) Nonlinear estimation of respiratory dynamics of fresh-cut spinach (Spinacia oleracea) based on enzyme kinetics. J Food Process Eng 53:1459–1473
Kirandeep KP, Singh B, Kumar N (2018) Effect of temperature and headspace O2 and CO2 concentration on the respiratory behavior of fresh yellow bell- pepper (Mirabelli). Int Chem Stud 6:3214–3220
Kirca A, Ozkan M, Cemeroglu B (2007) Effects of temperature, solid content and pH on the stability of black carrot anthocyanin. Food Chem 101:212–218
Lokke MM, Seefedlt HF, Edwards G, Green O (2011) Novel wireless sensor system for monitoring oxygen, temperature and respiration rate of horticultural crops post harvest. Sensors 11:8456–8468
Mangaraj S, Goswami TK (2011) Measurement and modeling of respiration rate of Guava (cv. Baruipur) for modified atmosphere packaging. Int J Food Prop 14:609–628
Peppelenbos HW, Leven JV (1996) Evaluation of four types of inhibition for modeling influence of carbon dioxide on oxygen consumption of fruits and vegetables. Postharvest Biol Technol 7:27–40
Pereira MJ, Amaro AL, Pintado M, Pocas MF (2017) Modelling the effect of oxygen pressure and temperature on respiration rate of ready-to-eat rocket leaves. A probabilistic study of the Michaelis–Menten model. Postharvest Biol Technol 131:1–9
Rennie TJ, Tavoularis S (2009) Perforation–mediated modified atmosphere packaging: part I. Development of a mathematical model. Postharvest Biol Technol 51:1–9
Rocculia P, Nobile MAD, Romani S, Baiano A, Rosa MD (2006) Use of a simple mathematical model to evaluate dipping and MAP effects on aerobic respiration of minimally processed apples. J Food Eng 76:334–340
Rubatzky VE, Quiros CF, Simon PW (1999) Carrots and related vegetable umbelliferae. CABI, New York
Singh M, Kumar A, Kaur P (2014) Respiratory dynamics of fresh baby corn (Zea mays L.) under modified atmospheres based on enzyme kinetics. J Food Sci Technol 51:1911–1919
Tigist M, Workneh TS, Woldetsadik K (2013) Effects of variety on the quality of tomato stored under ambient conditions. J Food Sci Technol 50:477–486
Tonutare T, Moor U, Szajdak L (2014) Strawberry anthocyanin determination by ph. differential spectroscopic method—how to get true results? Acta Sci Pol-Hortoru 13:35–47
Torrieri E, Perone N, Cavella S, Masi P (2010) Modeling the respiration rate of minimally processed broccoli (Brassica rapa var. sylvestris) for modified atmosphere package design. Int J Food Sci Technol 45:2186–2193
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singla, M., Kumar, A., Kaur, P. et al. Respiratory properties of fresh black carrot (Dacus Carota L.) based upon non-linear enzyme kinetics approach. J Food Sci Technol 57, 3903–3912 (2020). https://doi.org/10.1007/s13197-020-04422-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04422-5