The second author “Abhaya Kumar Srivastava” (Associate Professor) lost his life while battling a deadly cancer. He stood brave till the last breath of his life. Even after he was diagnosed with cancer, he wholeheartedly carried out his research and other academic activities. The Department of Post-Harvest Engineering and Technology, Aligarh Muslim University, deeply mourns the sad demise of this great personality and prays for the departed soul.
Abstract
Application of hydrocolloid based edible coatings is widely investigated as a promising means to retain quality and to extend the shelf life of food products. Present investigation was aimed to analyze influence of treatments, with different concentrations of lemon extract (0, 5, 10 and 15)% and coating with (0 and 5)% soy protein isolate (SPI), on fresh-cut melons. After the treatments, the samples were packed in polypropylene containers and kept at 4 °C for quality and shelf life analyses. The study involved 8 combinations of melon samples which were monitored in triplicate on specific days for different quality parameters including headspace gases, physicochemical, sensory and microbiological changes over the storage period. Lowest weight loss was indicated by samples treated with both lemon extract and soy protein isolate. When compared to control, coated samples indicated 4.36 log CFU/g lesser total plate count, and 2.39 log CFU/g lesser yeast and mold count at the completion of storage. Treatments showed effectiveness to retain vitamin C of melon samples. Total soluble solids, pH and titratable acidity varied remarkably through the storage life. Significant differences were observed in sensory attributes of control and coated samples. Chroma and color change (ΔE) values also reflected the potential of soy protein isolate coating to protect foods. Overall, the results suggested that lemon extract and soy protein isolate can help in retaining quality and extending the shelf life of fresh-cut melon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fruits and vegetables constitute a necessary portion of diet for humans. For convenience of consumers, fresh-cut and other minimally processed horticultural products have secured a large part of global market. Many fresh-cut agricultural products are nowadays available in major supermarkets across the world. Commonly available fruits include fresh-cut pineapple, fresh-cut musk melon, fresh-cut water melon, fresh-cut dragon fruit, fresh-cut papaya. Different varieties of melon (Cucumis melo) are consumed throughout the world. Kajari variety of melon, considered to be originated from India, is found in country’s markets but only for a short seasonal time. This variety of melon has smooth orange tinted outer covering. There are alternate white and green portions on the skin surface. This type of melon has a large seed cavity and the color of its flesh is usually green.
Though there are many merits of fresh-cut products, but most of the minimal processing steps result in the reduction of shelf life and hence pose a challenge to the food processors. Different strategies have been worked up on by the researchers including the application of various emerging technologies to counter this challenge. In recent past, edible coatings have been widely used as an interesting and effective strategy for preservation of different kinds of food stuff including fresh-cut fruits and vegetables. Edible coatings/films prepared by utilizing food-grade components can serve as primary packaging substances and are being reported to help in prolonging the shelf life by causing a reduction in weight loss, decline in respiration and oxidation reactions (Quirós-Sauceda et al. 2014). An array of biological substances such as hydrocolloids, lipids and their derivatives have been used in the edible coating formulations for fresh-cut fruits and vegetables (Yousuf et al. 2018a).
Protein based coatings have received a great focus in the recent past. Despite their low moisture barrier characteristics, protein based coatings have been largely employed as packaging materials for different types of food stuffs (Yousuf and Srivastava 2019). Proteins offer various functional characteristics, owned to their higher intermolecular-binding capability via different kinds of bonds (Calva-Estrada et al. 2019). Among the proteins, soy protein isolate (SPI) is one of the potential candidates having excellent film/coating forming properties. Some recent studies have shown the coating or film forming ability of SPI and also discussed the possibilities for improving its properties (Kang, et al. 2016; Xia et al. 2016; Galus 2018; Han et al. 2018; Martelli-Tosi et al. 2018; Nassar et al. 2018). Soy protein isolate is abundantly available and cheap soybean oil by-product containing a high amount of proteins.
A range of naturally occurring extracts obtained from many plant sources had been investigated and employed for prolonging the shelf life of food products (Barbosa-Pereira et al. 2014). Recent times have witnessed an increasing interest in natural food preservatives. Compounds such as polyphenols having some functional properties can be used as preservatives for foods and are usually preferred by the consumers (Randazzo et al. 2018). Natural antioxidant and antimicrobial agents are widely used for food preservation. Extracts derived from herbs or any other plant sources may contain many natural compounds having beneficial effects such as antimicrobial activity. In this context, these extracts have been used to serve as antibrowning agents, or antimicrobial agents against a broad range of microorganisms. For instance, green tea extract, a polyphenol rich extract has demonstrated strong antioxidant, anticarcinogenic, anti-inflammatory and antimicrobial (bactericidal and virucidal) activities against a range of foodborne pathogens (Falcó et al. 2019). Recently, Supapvanich et al. (2012) investigated konjac glucomannan coating along with pineapple fruit extract to inhibit brown discoloration in fresh-cut rose apple fruit. Many other examples include extract from fenugreek (Trigonella foenumgraecum) for ground beef patties (Hettiarachchy et al. 1996), pineapple juice for effective prevention of browning discoloration of banana (Chaisakdanugull et al. 2007). Antibacterial effect of lemon extracts has been investigated (Conte et al. 2007; Bansode and Chavan 2012; Kumar et al. 2012), but to our knowledge no study has been conducted to evaluate its influence on quality and shelf life of any fresh-cut fruits.
In the present study, treatments with lemon extract and SPI coating were performed on fresh-cut melon. The effect of lemon extract treatment and SPI coating on different quality attributes and overall shelf life was investigated over a specified period of time.
Material and methods
Melon and other materials
Melons (Kajari variety) were purchased from local market of Aligarh, India. The melons were of regular shape, size, maturity and did not had any bruises. The fruits were washed immediately after arrival in the Laboratory and equilibrated at 5 °C. The hard covering or rind on the outer side of the melons was peeled-off by the help of sharp edged knife. Then the melons were divided in 2 halves. Seeds were removed by scraping them from the seed cavity. Thereafter, they were cut into uniform trapezoidal pieces with the help of sharp knife. Food-grade poly-ethylene hand gloves were used while preparing the samples. These gloves were changed after every step in the process in order to avoid any contamination.
The other material used in the study include Polypropylene (PP) trays and transparent polyvinyl chloride cling films for packaging of melon pieces, soy protein isolate (SPI), and lemons. All the chemicals were of analytical grade.
Preparation of coating solutions and plan of experiments
Preparation of coatings was carried out by cautiously dispersing the coating components in deionized water. Different combinations of treatments were carried out with the help of soy protein isolate (SPI) and lemon extract (LE) as elaborated ahead. Prepared trapezoidal pieces of melon were grouped in eight combinations of samples. The first 4 samples were subjected to just LE treatment at concentration of 0%, 5%, 10% and 15%. Next 4 samples were subjected to LE treatment at same concentration as 0%, 5%, 10% and 15% followed by coating with 5% SPI in all the 4 samples. The eight combinations of samples thus include, 0% LE and 0% SPI, 5% LE and 0% SPI, 10% LE and 0% SPI, 15% LE and 0% SPI, 0% LE and 5% SPI, 5% LE and 5% SPI, 10% LE and 5% SPI, and 15% LE and 5% SPI. The samples which were treated with both lemon extract and SPI coating, were initially dipped in the desired LE concentration solutions for 2 min. Thereafter, the excess solution was permitted to drip-off by placing the melon samples on paper towel for 3 min. Subsequently, these samples were then subjected to a dip in SPI coating solution for 2 min. Here again, the excess coating solution was permitted to drip-off the melon samples. The sample treated with 0% LE and 0% SPI was labeled as control sample. On completing the treatments, samples were packed in polypropylene containers which were then covered with transparent PVC cling sheets. These containers were then kept at 4 °C for conducting quality and shelf life investigations.
Monitoring changes in headspace gas composition
Package headspace gas concentrations were estimated with the help of an automatic gas analyzer (Gaspace Advance GS3/P, Systech Instruments, Thame Oxfordshire). The suction end of the gas analyzer was pierced, across a rubber septum kept on the PVC cling cover, into the package. The device was auto-calibrated with gas composition of atmosphere prior to analyzing the fruit samples. Percentage of different gases in the headspace was noted from display screen of this machine.
Weight loss
Lab. scale digital weighing machine having accuracy standard of 0.001 g was engaged to check the weight of samples. Weight of entire package was measured on every sampling day and the final weight loss values were calculated. The results are presented as percent weight loss.
Total soluble solids (TSS), pH, titratable acidity and vitamin C
Different chemical parameters were analyzed during the storage on specific days of analysis.
The total soluble solids of the juice extracted from the samples were measured with the help of a bench refractometer (Metzer Optical Instruments, Mathura, India). The results of TSS are given as degree brix (°B).
pH of the melon samples was measured by electronic pH meter (Biogen Scientific, Meerut, India) on specified days of evaluation. The results are given as unit of pH.
For determination of titratable acidity, AOAC method was followed and the results are presented as percentage with respect to citric acid.
Vitamin C of melon samples was measured by 2,6-dichloro-indophenol titration method and the values were presented as mg per100 g of tissue. Briefly, 10 g melon was weighed and ground. The sample was then mixed with 3% metaphosphoric acid (HPO3) solution. Titration of this mixture was carried out against the dye (2,6-dichlorophenol-indophenol) until the appearance of pink color which represents end point. Prior to this, the dye solution was initially standardized with respect to standard ascorbic acid solution. The values of titre were noted and calculations were made to determine amount of vitamin C by using the formulae given below.
Color measurements
The color of samples was measured throughout the shelf life by using Hunter Lab Colorimeter (ColorFlex® EZ, Hunter Associates Laboratory, Reston, USA). Before, measuring color of the samples, the machine was standardized against black and white plates. The machine had a display screen on which the results were displayed as color coordinates L* (lightness), a* (green–red) and b* (yellow–blue). Color change (ΔE) was obtained by putting the values of color coordinates in this equation
where, L, a, b represents color readings at a specified time and L0, a0, b0 are starting color values.
The values obtained for each color coordinates (L*, a* and b*) were average of three readings.
In addition to this, to calculate chroma (C*), the below given formulae as reported in Raybaudi-Massilia et al. (2008) was used.
Sensory evaluation
Sensory analysis was conducted according to nine-point Hedonic scale in which, 1 = extremely dislike or unusable; 3 = limit of usability; 5 = neither like nor dislike; 7 = very good; 9 = extremely like. The sensory panel consisted of 30 members, who assigned scores to quality attributes of coated and uncoated samples. Effects of various coating treatments on acceptability of attributes including visual appearance, texture, aroma, juiciness and overall quality were observed.
Microbiological analysis
Total plate count and yeast and mold growth determinations were conducted using standard methods of microbiological evaluations. The samples were checked for microorganism populations on all the specified days of sample evaluation. Discussing in brief, in case of every treatment 10 g of sample was subjected to homogenization in 90 ml of sterilized saline mixture. Thereafter serial dilutions were performed and 1 ml sample was transferred to pre-sterilized petri-dishes. This was followed by adding nutrient agar for total plate count and potato dextrose agar for yeast and mold count evaluations. Incubation conditions to obtain total plate count were 48 h at 37 °C, and to obtain yeast and mold count it was 25 °C for 3 days. Microbiological counts were represented as log colony-forming units per gram of the melon sample (log CFU g−1).
Statistical analysis
Statistical analysis was performed with the help of analysis of variance (ANOVA) technique and the data was represented as mean ± standard deviation. The data was evaluated using ANOVA by subjecting it to Statistical software, SPSS 19.0 software (Chicago, IL, USA). The statistical analysis of variance was carried out by virtue of one-way analysis of variance (ANOVA) test. Duncan's multiple range test was employed to check significant differences between the means (at the level of P < 0.05).
Results and discussion
Changes in headspace gas composition
The effect of various protein-based edible coating combinations on respiratory activity was investigated (Fig. 1). The stress caused due to fresh-cut operations such as cutting and slicing, is expected to accelerate the respiration process. In this study, the in-package oxygen concentration dropped rapidly from initial 21% to the range of 9.8–11.7% for every samples on first day of storage. The drop in oxygen concentration continued throughout storage reaching the range of 3.7–5.6% on the twelfth day. However, no significant differences were observed among treatments with respect to oxygen concentration at any sampling time. It may be concluded that treatments/coatings were not effective in reducing oxygen consumption to a large extent. Furthermore, no specific trend differentiating uncoated and coated fruit was seen.
Changes in headspace gas a Oxygen concentration and b Carbon dioxide concentration (mean of three replicates ± standard deviation) of fresh-cut melon treated with lemon extract (LE) and coated with soy protein isolate (SPI), and stored at 4 °C. ( ) 0% LE + 0% SPI, (
) 0% LE + 0% SPI, ( ) 5% LE + 0% SPI, (
) 5% LE + 0% SPI, ( ) 10% LE + 0% SPI, (
) 10% LE + 0% SPI, ( ) 15% LE + 0% SPI, (
) 15% LE + 0% SPI, ( ) 0% LE + 5% SPI, (
) 0% LE + 5% SPI, ( ) 5% LE + 5% SPI, (
) 5% LE + 5% SPI, ( ) 10% LE + 5% SPI, (
) 10% LE + 5% SPI, ( ) 15% LE + 5% SPI
) 15% LE + 5% SPI
On the other hand, carbon dioxide concentration exhibited an elevation over the shelf life. From an initial 0.3%, the carbon dioxide concentration on day 1 had reached in the range of 1.3–3.0% in all the samples with highest concentrations corresponding to control. Carbon-dioxide levels inside the packages increased slowly during the first 4 days, and indicated a more rapid increase in the follow-up days. Significant difference in carbon dioxide concentration was seen between the uncoated and coated samples at each stage during the storage. Highest headspace carbon dioxide concentration was observed in the package containing uncoated control melon sample while coated samples maintained comparatively lower concentrations throughout the storage. Elevated carbon-dioxide generation could be as a result of micro-organism growth and a general degradation of the cut-fruit tissue (Villanueva et al. 2004). Lower oxygen conditions usually hinder the growth of aerobic micro-organisms (Bai et al. 2001) but, extremely little oxygen levels could also result into an-aerobic fermentation.
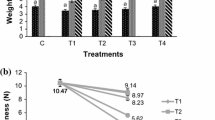
Weight loss
In the light of fact that value of fresh fruits and vegetables is greatly influenced by the mass, mass loss due of water loss has an adverse impact which may result in huge economic losses. Moreover, physiological modifications as a consequence of water loss could cause postharvest losses in terms of nutritional, functional and sensory quality. Edible films/coatings may be used to retard these undesired influences due to water loss in fruits and vegetables. In this investigation, every sample was seen to undergo weight loss with time (Fig. 2). There was a slight increase in weight loss in case of all the samples from initial time up to day 4. From day 4 comparatively higher weight loss in the treated/coated samples and a remarkably greater weight loss was observed in the control melon. At completion of storage period significant differences occurred in weight loss of uncoated and coated fresh-cut melon. Highest weight loss was seen in control melon (with no treatments). In addition, it was analyzed that both storage and treatments significantly affected the degree of weight loss. Treatments with lemon extract reduced weight loss. However, SPI was not found to be capable of further reducing the weight loss. That means no additional effect was observed on weight loss of fresh-cut melon while using combination of lemon extract treatment and soy protein isolate coating. Generally, protein or polysaccharide based coatings exhibit poor water vapor barrier properties in comparison to lipid based coatings. However, these hydrocolloid-based edible coatings form a semipermeable barrier which could be improved by adding lipid components (Maqbool 2011). Thus, the present study favors the need for formulation of composite coatings for efficient water vapor barrier properties. In an apparent observation, weight loss largely occurs by loss of moisture and leakage of fluid part from the fruit tissue. Respiratory processes and other senescence related metabolic pathways during storage may also cause weight loss, but this contribution is believed to be negligible.
Effect on weight loss (mean of three replicates ± standard deviation) of fresh-cut melon treated with lemon extract (LE) and coated with soy protein isolate (SPI), and stored at 4 °C. ( ) 0% LE + 0% SPI, (
) 0% LE + 0% SPI, ( ) 5% LE + 0% SPI, (
) 5% LE + 0% SPI, ( ) 10% LE + 0% SPI, (
) 10% LE + 0% SPI, ( ) 15% LE + 0% SPI, (
) 15% LE + 0% SPI, ( ) 0% LE + 5% SPI, (
) 0% LE + 5% SPI, ( ) 5% LE + 5% SPI, (
) 5% LE + 5% SPI, ( ) 10% LE + 5% SPI, (
) 10% LE + 5% SPI, ( )15% LE + 5% SPI
)15% LE + 5% SPI
TSS, pH, titratable acidity and vitamin C
The chemical quality attributes for example, total soluble solids, pH, titratable acidity and vitamin C of fresh-cut melon were evaluated on pre-specified dates with a 4-days gap between 2 sampling times (Table 1).
The TSS of melon tissue ranged from 6.5 to 7.0°Brix, varying with respect to the physiological state at the time of processing. TSS of all samples decreased along the time, with minor and random variations (the decrease in TSS didn’t follow any particular trend) between samples. A resembling pattern was also reported by Iglesias et al. (2018) in case of fresh-cut pears. Statistically, no significant changes were found in relation to TSS among samples at any particular time of storage.
The pH of fresh-cut melon was determined initially and on every sampling day. Highest pH was observed in control sample while as 15% LE + 0% SPI and 15% LE + 5% SPI had lowest pH at the initial stage. This might be due to these samples being treated with lemon extract which might have contributed to the pH. Up to day 8 of storage the pH followed a consistently decreasing trend in case of all the samples but, after day 8 the control sample showed a rapid decline. However, this rapid decline behavior was not observed in the coated samples. Effect of lemon extract and storage time was significant (P < 0.5).
TSS and titratable acidity are important properties that reflect the sweetness and tartness respectively in harvested fruit. Titratable acidity increased over the storage time for all the treatments. There were significant differences among the titratable acidity of different samples during the initial storage period but at completion of storage, insignificant difference was seen. Like on pH, the lemon extract treatments had influence on the titratable acidity as samples treated with 0% LE + 0% SPI and 0% LE + 5% SPI indicated lowest titratable acidity at day 1. This is due to zero concentration of lemon extract in these treatments.
The advantageous influence of fruit and vegetables have been attributed to different phytochemical components, vitamins and antioxidants present in them. Melon is having value because of occurrence of bio-active components such as phenolic substances, vitamin C and β-carotene, although occurring in little amounts but are having essential impact on health of humans (Lester and Hodges 2008). Melon quality in terms of its nutritional value, such as ascorbic acid is rapidly degraded while they are stored after harvest, and even at a much rapid rate if subjected to processing (Kalt 2005). Vitamin C content of fresh-cut melon was determined and remarkable difference in the different samples was observed right from the starting day of storage. These higher differences at the initial stage among the samples may be due to the fact that treatments with lemon extract might have contributed towards vitamin C measurements as the treatments involving zero concentration of lemon extract were found to have lowest vitamin C content. The vitamin C content at day 1 ranged between 84–104 mg/100 g which slowly and constantly kept on decreasing irrespective of the treatments/coating combinations applied. However, the treatments/coatings were found to maintain higher concentration of ascorbic acid through the storage. The ascorbic acid content of uncoated control sample reached to 30 mg/100 g sample at the end of storage period, which was lowest among all the samples. Moreover, significant difference in vitamin C content of uncoated and coated melon occurred at every point during the storage.
Color measurement
The L* values increased slightly with storage. There were no significant differences in L* color coordinates of different melon samples at any particular sampling time. Insignificant change in fresh-cut melon was also documented by Oms-Oliu et al. (2007a). The a* color coordinate had more negative values on the starting day, later on elevated towards zero as time passed. Negative values for a* color coordinate indicate green color. Therefore, melon color degraded with the passage of time as negative values kept on increasing towards zero representing loss of original green color of melon flesh. Nonetheless, a* color coordinate indicated no significant differences between uncoated and most of the coated melon samples at completion of storage.
b* values exhibited a decline with time. The extent of decrease in values of b* color coordinate in 12-day storage period was less in coated samples than the uncoated/control and the sample coated with SPI but not treated with lemon extract. At the initial sampling day there were significant differences among the samples, though thereafter decrease in b* value occurred in every samples but, towards completion of storage they did not differed significantly.
Color change and chroma are represented in Fig. 3. The color change increased along with storage time irrespective of the treatments, indicating degradation of original desired color of fresh-cut melon with time. Aguayo et al. (2004) related the color degradation with translucence injury, a result of physiological disease featured by dark and glassy tissue. Translucency may be a result of an advanced stage of ripeness. Appearance of translucency is considered one of the primary visual changes representing degradation of fresh-cut melon packed in modified atmosphere (Aguayo et al. 2004; Bai et al. 2001; Connor-Shaw 1994). Though at starting day there were significant differences among the color change of different samples, but at completion of storage time insignificant differences occurred. Nonetheless, highest color change values were recorded against the control sample at the final sampling day, despite at the starting time the control samples showed least color change compared to coated melon samples which may be due to the reason that the treatments/coatings might have affected the surface color of the melon samples.
Effect on a color change (mean of three replicates ± standard deviation), b chroma (mean of three replicates ± standard deviation) of fresh-cut melon treated with lemon extract (LE) and coated with soy protein isolate (SPI), and stored at 4 °C. ( )0% LE + 0% SPI, (
)0% LE + 0% SPI, ( )5% LE + 0% SPI, (
)5% LE + 0% SPI, ( )10% LE + 0% SPI, (
)10% LE + 0% SPI, ( ) 15% LE + 0% SPI, (
) 15% LE + 0% SPI, ( )0% LE + 5% SPI, (
)0% LE + 5% SPI, ( ) 5% LE + 5% SPI, (
) 5% LE + 5% SPI, ( ) 10% LE + 5% SPI, (
) 10% LE + 5% SPI, ( ) 15% LE + 5% SPI
) 15% LE + 5% SPI
Chroma of fresh-cut melons decreased with the passage of time both in uncoated and coated samples. Again like for color change, initially chroma varied significantly between the samples, however, towards the end of storage insignificant differences were seen. Control sample maintained higher chroma values up to day 8 of storage and thereafter, a comparatively steep decrease was observed. Decrease in chroma of fresh-cut melon during storage was also documented by Moreira et al. (2014) and Zambrano-Zaragoza et al. (2017).
Sensory evaluation
Generation of essential bio-active components and volatile organic compounds may be negatively affected by low storage temperatures. Therefore, a balance of sensory and nutritional characteristics is a challenging task (Amaro et al. 2018). Due to its appealing sensory properties, melon is a greatly desired and appreciated fruit. But at the same time, fresh-cut melon is readily prone to softening up on extended storage. Moreover, minimal processing damages the flesh which accelerates the rate of respiratory processes (Raybaudi-Massilia 2008), and consequently leads to damage of sensory attributes. For present study, changes in sensory attributes such as color, taste, texture, aroma, and overall acceptability of coated and un-coated melon for eight storage days are represented in Fig. 4. The sensory quality decreased throughout storage. On day one color had no major impact but the use of higher concentration of lemon extract was found to influence the original aroma, taste and subsequently the overall acceptability of melon samples. The treatments with higher concentration of lemon extract provided a little acidic taste to fresh-cut melon, it was not regarded to be a negative quality impact by the panel as long as 10% level. However, at 5% concentration LE had negligible influence on these attributes. On fourth day of storage, the samples had good taste, texture and overall quality in all treatments but remarkably different in comparison to initial scores at day 1 for most of the quality attributes. The overall degree of acceptance reflected no significant variations between treatments at day 4.
By day 8, significant differences developed between samples for all measured quality attributes. The control sample was assigned lowest scores for all the attributes. Also, taste was affected in samples that were treated with 15% LE. Color was least affected in coated melon samples. The texture of control sample was worst affected. The development of translucent appearance occurred which adversely affected appeal and over-all acceptability. Other samples also showed signs of developing translucency but on comparison with control it can be affirmed that coating/treatments were effective in delaying such damage. The overall acceptability of control sample reached close to score of 5 on eighth day. Sensory testing was not performed beyond the 8th day of storage considering microbiological concerns, because earlier investigations reported the stable storage time for fresh-cut melon was limited from 1 week to10 days (Oms-Oliu et al. 2007a).
Based on our own observations while carrying out this study, we found that the quality of melons was influenced by the treatments, though the magnitude of effect varied with the particular combination of treatments. The compatibility of plant extracts should be checked keeping in consideration the effect on sensory properties. In case of our study the lemon extract did not affect the sensory properties of melon until the concentration was kept below 10%. The higher concentrations depicted an adverse effect on quality of melons.
Microbiological analysis
The processing or fresh-cut preparation of fruits and vegetables means removal or damage of most of the protective barriers of the fruit tissue. The processing of fresh materials not only impacts on physicochemical quality but also raises the risk for contamination with spoilage microorganisms and pathogens due to the disruption of cell barriers and subsequent damage as a result of leakage of cell components (Collazo et al. 2018). Therefore, whole fruit is assumed to be safer in microbiological terms, but fresh-cut fruits are considered to be carriers of transmitting human pathogens and are often believed to result in foodborne out-breaks. Amongst many strategies, application of edible coatings has been used as a means to retard the microbial contamination (Yousuf et al. 2018b). Microorganism growth can lead to heavy degradation and loss of quality attributes during storage. Microorganism growth as total plate count, and yeast and mold count for melon samples is presented in Fig. 5. At initial sampling day, 2.4 log CFU/g and 1.70 log CFU/g microbial load in terms of total plate and yeast and mold counts were found in fresh-cut melon. However, the microbiological counts increased with storage for every treatment. The growth of microorganisms (both bacteria and, yeast and mold) in uncoated melon was highest, reaching the counts of 11.06 and 8.74 log CFU/g for total plate, and yeast and mold respectively, at the end of storage. On contrary, the counts of coated samples did not exceed 6.7 log CFU/g at completion of shelf life.
Yeasts and molds were lesser in number compared to bacteria on fresh-cut melon during entire storage, the observation also seen in Bai et al. (2001) for fresh-cut Cantaloupe and in Oms-Oliu et al. (2007b) for fresh-cut ‘Piel de Sapo’ melon. Yeasts and molds have also been found in less number compared to aerobic mesophiles, in fresh-cut “Cantaloupe” (Ayhan et al. 1998) and “Barattiere” melons (Conte et al. 2009). The US and most countries in Europe have laws for fresh-cut products, under which aerobic micro-organisms counts are limited to to 6 log cfu/g on expiring date (Martin-Belloso et al. 2006). Though this limit was exceeded in control sample within eight days, the treatments/coatings were able to cause a delay of few days to reach this limit.
Conclusion
Treatment of fresh-cut melon using various concentrations of lemon extract and coating with soy protein isolate showed some promising results. The present study also revealed that soy protein isolate coating on fruits could retard the respiration. The coatings were also found to limit the water loss, thereby protecting the cut tissue from wilting, which is otherwise prone to it. There were no drastic effects of coating on the soluble solids of the melon. Vitamin C and color of melons were preserved by the SPI coating and LE treatments. Microorganism growth was also retarded. The effect of LE on fresh-cut melon was not perceived to be a negative quality as long as treatment was given at 10% level. Overall the sensory attributes were preserved to a remarkable extent. Further studies can be conducted on use of lemon extract and other plant extracts for maintaining quality of fruits and vegetables. However, such studies also would need to optimize the concentration of extracts to be used.
References
Aguayo E, Escalona VH, Artes F (2004) Metabolic behavior and quality changes of whole and fresh processed melon. J Food Sci 69:148–155
Amaro AL, Spadafora ND, Pereira MJ, Dhorajiwala R, Herbert RJ, Muller CT, Rogers HJ, Pintado M (2018) Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem 241:222–231
Ayhan Z, Chism GW, Richter AR (1998) The shelf-life of minimally processed fresh cut melons. J Food Quality 21:29–40
Bai J, Saftner RA, Watada AE, Lee YS (2001) Modified atmosphere maintains quality of fresh-cut cantaloupe (Cucumis melo L.). J Food Sci 66:1207–1211
Bansode DS, Chavan MD (2012) Studies on antimicrobial activity and phytochemical analysis of citrus fruit juices against selected enteric pathogens. Int Res J Pharmacy 3:122–126
Barbosa-Pereira L, Aurrekoetxea GP, Angulo I, Paseiro-Losada P, Cruz JM (2014) Development of new active packaging films coated with natural phenolic compounds to improve the oxidative stability of beef. Meat Sci 97:249–254
Calva-Estrada SJ, Jiménez-Fernández M, Lugo-Cervantes E (2019) Protein-based films: advances in the development of biomaterials applicable to food packaging. Food Eng Rev 11:78–92
Chaisakdanugull C, Theerakulkait C, Wrolstad RD (2007) Pineapple juice and its fractions in enzymatic browning inhibition of banana [Musa (AAA group) Gros Michel]. J Agricul Food Chem 55:4252–4257
Collazo C, Gine-Bordonaba J, Aguilo-Aguayo I, Povedano I, Bademunt A, Vinas I (2018) Pseudomonas graminis strain CPA-7 differentially modulates the oxidative response in fresh-cut ‘Golden delicious’ apple depending on the storage conditions. Postharvest Biol Technol 138:46–55
Connor-Shaw RE, Roberts R, Ford AL, Nottingham SM (1994) Shelf-life of minimally processed Honeydew, kiwifruit, papaya, pineapple, and Cantaloupe. J Food Sci 59:1202–1206
Conte A, Scrocco C, Brescia I, Del-Nobile MA (2009) Different packaging strategies for fresh-cut “baratiere” melon cultivar (Cucumis melo L.). Int J Food Sci Technol 44:1422–1428
Conte A, Speranza B, Sinigaglia M, Del-Nobile MA (2007) Effect of lemon extract on foodborne microorganisms. J Food Protec 70:1896–1900
Falcó I, Flores-Meraz PL, Randazzo W, Sánchez G, López-Rubio A, Fabra MJ (2019) Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocol 87:611–618
Galus S (2018) Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocol 85:233–241
Han Y, Yu M, Wang L (2018) Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocol 75:13–21
Hettiarachchy NS, Glenn KC, Gnanasambandam R, Johnson MG (1996) Natural antioxidant extract from fenugreek (Trigonella foenumgraecum) for ground beef patties. J Food Sci 61:516–519
Iglesias MB, Echeverria G, Vinas I, Lopez ML, Abadias M (2018) Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT- Food Sci Technol 87:581–588
Kalt W (2005) Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci 70:11–19
Kang H, Wang Z, Zhang W, Li J, Zhang S (2016) Physico-chemical properties improvement of soy protein isolate films through caffeic acid incorporation and tri-functional aziridine hybridization. Food Hydrocol 61:923–932
Kumar S, Nancy SD, Kumar V (2012) Evaluating the antibacterial activity of plant extracts against bacterial pathogens. J of Drug Del Therapeu 2:182–185
Lester GE, Hodges DM (2008) Antioxidants associated with fruit senescence and human health: novel orange-fleshed non-netted honey dew melon genotype comparisons following different seasonal productions and cold storage durations. Postharvest Biol Technol 48:347–354
Maqbool M, Ali A, Alderson PG, Mohamed MTM, Siddiqui Y, Zahid N (2011) Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol Technol 62:71–76
Martelli-Tosi M, Masson MM, Silva NC, Esposto BS, Barros TT, Assis OB, Tapia-Blácido DR (2018) Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr Polym 198:61–68
Martin-Belloso O, Soliva-Fortuny R, Oms-Oliu G (2006) Fresh-cut fruits. In: Hui YH (ed) Handbook of fruits and fruit processing. Blackwell Publishing, Ames, pp 129–144
Moreira SP, Carvalho WM, Alexandrino AC, Paula HCB, Rodrigues MCP, Figueiredo RW, Maia GA, Figueiredo EMAT, Brasil IM (2014) Freshness retention of minimally processed melon using different packages and multilayered edible coating containing microencapsulated essential oil. Int J of Food Sci Technol 49:1–9
Nassar SF, Dombre C, Gastaldi E, Touchaleaume F, Chalier P (2018) Soy protein isolate nanocomposite film enriched with eugenol, an antimicrobial agent: Interactions and properties. J Appl Polym Sci 135:45941
Oms-Oliu G, Raybaudi-Massilia MRM, Soliva-Fortuny R, Martin-Belloso O (2007a) Effect of superatmospheric and low oxygen modified atmospheres on shelf-life extension of fresh-cut melon. Food Control 19:191–199
Oms-Oliu G, Soliva-Fortuny R, Martiın-Belloso O (2007b) Effect of ripeness on the shelf-life of fresh-cut melon preserved by modified atmosphere packaging. Euro Food Res Technol 225:301–311
Quirós-Sauceda AE, Ayala-Zavala JF, Olivas GI, González-Aguilar GA (2014) Edible coatings as encapsulating matrices for bioactive compounds: a review. J Food Sci Technol 51:1674–1685
Randazzo W, Fabra MJ, Falco I, López-Rubio A, Sánchez G (2018) Polymers and biopolymers with antiviral activity: potential applications for improving food safety. Compr Rev Food Sci Food Saf 17:754–768
Raybaudi-Massilia RM, Mosqueda-Melgar J, Martín-Belloso O (2008) Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int J Food Microbiol 121:313–327
Supapvanich S, Prathaan P, Tepsorn R (2012) Browning inhibition in fresh-cut rose apple fruit cv. Taaptimjaan using konjac glucomannan coating incorporated with pineapple fruit extract. Postharvest Biol Technol 73:46–49
Villanueva MJ, Tenorio MD, Esteban MA, Mendoza MC (2004) Compositional changes during ripening of two cultivars of muskmelon fruits. Food Chem 87:179–185
Xia C, Zhang S, Shi SQ, CaiL GAC, Rizvi HR, D'Souza NA (2016) Property enhancement of soy protein isolate-based films by introducing POSS. Int J Biol Macromol 82:168–173
Yousuf B, Qadri OS, Srivastava AK (2018a) Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: a review. LWT - Food Sci Technol 89:198–209
Yousuf B, Qadri OS, Srivastava AK (2018b) Antimicrobial packaging: basic concepts and applications in fresh and fresh-cut fruits and vegetables. In: Siddiqui MW, Rahman MS, Wani AA (eds) Innovative packaging of fruits and vegetables: strategies for safety and quality maintenance. Apple Academic Press Inc., Oakville, pp 136–155
Yousuf B, Srivastava AK (2019) Impact of honey treatments and soy protein isolate-based coating on fresh-cut pineapple during storage at 4 °C. Food Pack Shelf Life 21:100361
Zambrano-Zaragoza ML, Quintanar-Guerrero D, Real AD, Pinon-Segundo E, Zambrano-Zaragoza JF (2017) The release kinetics of carotene nanocapsules/xanthan gum coating and quality changes in fresh-cut melon (cantaloupe). Carbohy Polym 157:1874–1882
Acknowledgements
The first author acknowledges the award of CSIR-Senior Research Fellowship from the Council of Scientific and Industrial Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Ethical approval
Moreover, the authors have read this paper and agreed to submit it to the Journal of Food Science and Technology.
Additional information
Abhaya Kumar Srivastava: Deceased.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousuf, B., Srivastava, A.K. & Ahmad, S. Application of natural fruit extract and hydrocolloid-based coating to retain quality of fresh-cut melon. J Food Sci Technol 57, 3647–3658 (2020). https://doi.org/10.1007/s13197-020-04397-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04397-3