Abstract
Fresh-cut ‘Piel de Sapo’ melon was processed at different ripeness stages and stored under modified atmosphere packaging for 35 days at 5 °C. Raw material firmness and soluble solids content ranged from 6.5 to 3.9 N and 11.1 to 14.3 °Brix, respectively. The effects of a 2.5 kPa O2+7 kPa CO2 packaging atmosphere and a dip of 1% ascorbic acid and 0.5% calcium chloride on physiology, microbiological stability as well as color and firmness were evaluated. An intermediate stage of ripeness at processing was the most suitable to extend the shelf-life of fresh-cut ‘Piel de Sapo’ melon. Green-mature fresh-cut melon reduced CO2 accumulation and ethanol production. In addition, a treatment with ascorbic acid and calcium chloride in combination with modified atmosphere packaging, contributed to a greater extension of the shelf-life of fresh-cut melon than that reported for fruits stored under non-modified atmosphere, slowing down the growth of microbial populations, maintaining the original color and reducing softness. Thus, the shelf-life of green-mature fresh-cut ‘Piel de Sapo’ melon dipped in an ascorbic acid and calcium chloride solution and packaged under modified atmosphere was about 10 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
‘Piel de Sapo’ melon (Cucumis melo L.) is a major commercial cultivar in Spain. The fruits are oval shaped with reticular greenish color skin and white sweet flesh. Like other members of the Cucurbitaceae, ‘Piel de Sapo’ is chilling injury sensitive. This physiological disorder appears when melons are stored at low temperature. Changes in membrane structure in response to chilling temperatures are considered as the primary events of chilling injury and lead to a loss of permeability and metabolic dysfunction. Secondary reactions include ethylene production, increased respiration or accumulation of toxic compounds such as ethanol and acetaldehyde [1].
Processing of fresh-cut fruits involves wounding stress as a result of mechanical injury when peeling or cutting, leading to an increase in the respiration rates of fresh-cut commodities in comparison to those of the corresponding whole fruits [2]. Minimal processing damages the tissue integrity, leading to several biochemical deteriorations such as browning, off-flavors and texture breakdown [3]. Many factors may affect the physiological response of the fruit, including the cultivar, maturity stage, temperatures or packaging atmospheres.
The stage of ripeness appears to be essential to keep quality of fresh-cut produce and to extend their shelf-life. The more mature the fruit, the shorter the shelf-life of the fresh-cut commodity. Moreover, if the fruit is not sufficiently mature at processing the product will not reach the optimal sensorial attributes [4]. To our knowledge, there are no data available comparing the physiological and microbiological response of fresh-cut melon processed at different stages of maturity.
It has been reported that the modification of package atmospheres, in concomitance with chemical treatments, effectively retards the detrimental phenomena that occur during fresh-cut processing. Low O2 and/or high CO2 atmospheres have been shown to reduce respiration, decrease ethylene production and inhibit or delay enzymatic reactions. A storage atmosphere of 2–5 kPa O2 plus 10–15 kPa CO2 at 0–5 °C has been proved to maintain the quality of fresh-cut ‘Cantaloupe’ or ‘Honeydew’ melons [5, 6]. Fresh-cut ‘Amarillo’ melon maintained its fresh quality attributes under a passive modified atmosphere for 10 days at 5 °C [7]. Temperatures below 7–10 °C are associated with the occurrence of chilling injury in intact melons. However, fresh-cut produce is recommended to be held at temperatures below 5 °C because of a significant reduction in the respiratory metabolic rates at low temperatures. In addition, lower microbial population and longer shelf-life of fresh-cut produce stored at 5 °C has been reported rather than at 10 °C [6].
The addition of chemical agents is the most common way to control browning and softening phenomena. Some blends of additives have been proved to extend the storage life of fresh-cut produce. Dips containing ascorbic acid have been generally used to delay browning on cut fruit surfaces [8, 9]. Ascorbic acid (AA) content is regarded as a quality indicator for fresh-cut fruits and vegetables. Because of its antioxidant activity it plays an important role in many metabolic pathways that have a direct impact on the oxidative stability of fruits [10]. On the other hand, treatments with calcium salts have been shown to be effective in preserving fresh-cut melon from softening [11, 12]. The action of calcium chloride (CaCl2) treatments in fruits and vegetables may be generally attributed to its diffusion into the tissue and its complexion to polygalacturonic acid residues, present in the cell wall and middle lamella, to provide stabilization of membrane systems [13].
In this work, we aimed to determine the effect of ripeness at processing of fresh-cut ‘Piel de Sapo’ melon preserved by modified atmosphere packaging and dipped in a solution containing ascorbic acid and calcium chloride on respiration rate, ethylene production, anaerobic metabolites, microbiological stability and quality attributes.
Materials and methods
Evaluation of fruit ripeness
‘Piel de Sapo’ melons, grown in Castilla-La Mancha (Spain), were harvested in August at a slightly under-ripe maturity stage. The fruits were grouped in three lots of 20 each, stored in a ventilated room at 10 °C and periodically exposed to temperatures of 20 °C for periods of 48–72 h until the desired ripeness was achieved.
At each storage interval, three melons in optimal conditions were randomly removed from each group. The fruits were peeled and cut into eight sections parallel to the longitudinal axis. The rind and seeds were removed, and the pulp was sliced into small portions. Melon pieces were combined and three samples removed for raw fruit characterization through determination of soluble solids content (Atajo RX-100 refractometer; Atago Company Ltd., Japan), total acidity (AOAC 2000), pH (Crison 2001 pH-meter; Crison Instruments SA, Alella, Barcelona, Spain), pulp color (Minolta CR-400 chroma meter; Konica Minolta Sensing, Inc., Osaka, Japan) and firmness (TA-XT2 Texture Analyzer; Stable Micro Systems Ltd., Surrey, UK). Thus, three physiological stages of the fruit were selected in order to study the effect of the stage of ripeness at processing on the shelf-life of fresh-cut melon.
Sample preparation
Melons were sanitized in a 200 ppm NaClO solution for 2 min, rinsed with tap water, and dried by hand. The fruits were sliced and cut to obtain trapezoidal sections. The fruit pieces were dipped for 1 min in a solution of 1% w/v l-ascorbic acid and 0.5% w/v calcium chloride at a product:solution ratio of 1:2. Control pieces were dipped into distilled water. The excess of water was completely drained and then, 100 g of melon pieces were packaged in polypropylene trays. The O2 and CO2 permeability of the film were 110 cm3 m−2 day−1 bar−1 and 500 g m−2 day−1 bar−1 at 23 °C and 0% RH, respectively (ILPRA Systems España, S.L. Mataró, Spain). The modification of the package atmosphere was carried out by flushing a mixture of 2.5 kPa O2+7 kPa CO2 (N2 balanced) in a ratio melon:gas mixture of 1:2 (v/v) and thermosealing with a vacuum packing machine ILPRA Food Pack Basic V/6 (ILPRA Systems. CP, Vigevono, Italy). Trays were stored at 4±1 °C in darkness and analyzed throughout 35 days of storage in duplicate.
Headspace gases analysis
The gas composition of the package headspace was determined by a gas analyzer (Micro-GC CP 2002, Chrompack International, Middelburg, The Netherlands) equipped with a thermal conductivity detector. A sample of 1.7 ml was automatically withdrawn from the headspace atmosphere with a pin-needle connected to the injection system. The determination of the oxygen concentration was carried out by injecting a sample of 0.25 μl to a CP-Molsieve 5 Å column (4 m×0.35 mm, df=10 μm) at 60 °C and 100 kPa whereas a 0.33 μl portion was injected to a Pora-PLOT Q column (10 m×0.32 mm, df=10 μm) at 75 °C and 200 kPa for carbon dioxide, ethylene and ethanol analysis.
Color and firmness evaluation
The color of fresh-cut melon was determined with a Minolta CR-400 chromameter (Konica Minolta Sensing, Inc., Osaka, Japan). The equipment was set up for a D75 illuminant and 10 ° observer angle. Ten replicates from two packages were evaluated for each treatment. Three readings were obtained for each replicate by changing the sample position to get uniform color measurements. CIE L * (lightness), a * (red-green) and b * (yellow-blue) color parameters were obtained through reflectance values. Color changes of processed melon were expressed as chroma (C *) and whiteness index (WI).
Firmness evaluation was performed using a TA-XT2 Texture Analyzer (Stable Micro Systems Ltd., Surrey, UK) by measuring the maximum penetration force. Cylindrical samples of 2.0 cm high were obtained from the trapezoidal melon pieces and were positioned to be penetrated by a 4 mm diameter rod through their geometric center. The downward distance was set at 10 mm at a rate of 5 mm s−1 and automatic return. Ten samples from two trays of fresh-cut melon were randomly withdrawn from each treatment to obtain representative readings.
Microbiological stability
The microbiological stability of fresh-cut ‘Piel de Sapo’ melon was evaluated through the determination of total mesophilic aerobic bacteria and yeast and mold populations. Two replicate counts were obtained each time from two packages at the same experimental condition. The analyses were carried out weekly for 35 days. A sample of 10 g melon was homogenized for 2 min with 90 ml of 0.1% sterile peptone solution with a Stomacher Lab Blender 400 (Seward medical, London, UK). Serial dilutions of fruit homogenates were plated on plate count agar (PCA) at 30 °C±1 °C for 72 h±3 h for mesophilic aerobic bacteria counts [14] and chloramphenicol glucose agar (GCA) at 25 °C±1 °C for 5 days for yeast and mold counts [15].
Statistical analysis and mathematical modeling
Statistical analysis was performed using the Statgraphics plus v.5.1 software (Manugistics, Inc., Rockville, MA, USA). Data were analyzed by multifactor analysis of variance. The analysis of covariance was used to decide whether significant differences (p<0.05) existed among treatments. The Duncan's multiple-range test was applied to determine differences among levels of each factor.
The growth of microorganisms as affected by the studied conditions was modeled according to a modification of the Gompertz equation [Eq. (3)], which has been proposed to predict the shelf-life of fresh-cut apple slices [16].
where k, initial count estimated by the model [log (cfu g−1)]; A, maximum growth attained at the stationary phase [log (cfu g−1)]; μ max, maximal growth rate [Δlog (cfu g−1) day−1]; λ, lag time (days); t, storage time (days).
Results and discussion
Physicochemical characteristics of ‘Piel de Sapo’ melon
Melon maturity was determined on the basis of the pulp sugar content, total acidity (TA), pH, color and firmness. The soluble solids content (SSC) in melon flesh ranged from 11.1 to 14.3 °Brix, depending on their physiological stage at processing. The sugar content followed an upward trend through ripening (Table 1). Villanueva et al. [17] reported SSC of 11.0–15.5 °Brix in moderately ripe or ripe ‘Piel de Sapo’ melons. These authors suggested minimum sugar content for this fruit of 8 °Brix, since the melons are not usually fit for consumption below that level.
The ripening process from green to green-mature melon involved a decrease in TA and an increase of pH (Table 1). The pH values in green-mature or mature stages were about 6, in agreement with those reported by Villanueva et al. [17] for moderately ripe and fully ripe ‘Piel de Sapo’ melon. Aguayo et al. [18] reported lower initial TA in ‘Galia’ and ‘Cantaloupe’ than in ‘Amarillo’ or ‘Piel de Sapo’ fresh-cut melon.
Table 1 shows that color changes on melon flesh through ripening were due to a decrease in a * and b * values whereas no significant differences in lightness (L *) were detected. The more advanced the fruit maturity at processing, the greater decrease experienced by C * [Eq. (1)] and WI [Eq. (2)] parameters. This depletion was also reported by Aguayo et al. [18] as a consequence of translucency injury on fresh-cut melon, which has been found to be related to a physiological disorder exhibited as symptom of senescence. Translucency may be a consequence of an advanced stage of ripeness, treatments that accelerate ripening or high storage temperatures.
The firmness significantly depleted from 6.5 to 3.9 for green and green-mature melons (Table 1). Fruit ripening is characterized by tissue softening, that is associated with the enzymatic degradation of cell wall compounds like pectic substances and neutral detergent fiber (NDF) such as cellulose, hemicelluloses and lignin. A slight decrease of NDF content as well as degradation of pectic substances at final stage of ripening of ‘Piel de Sapo’ melon has been reported by Villanueva et al. [17].
Package atmosphere composition
Oxygen and carbon dioxide partial pressures in trays headspace throughout storage of fresh-cut melon are shown in Figs. 1 and 2. The oxygen consumption of fresh-cut melon was influenced neither by the fruit ripeness condition at processing nor by dips of 1% AA and 0.5% CaCl2. On the other hand, the composition of packaging atmosphere significantly affected O2 concentration inside packages through storage (p<0.001). The O2 concentration in packages that were initially flushed with 2.5 kPa O2+7 kPa CO2 depleted to levels below 1 kPa O2 beyond 14 days storage in all the assayed conditions and treatments (Fig. 1A and B). On the other hand, O2 levels progressively decreased under non-modified atmosphere but concentrations above 1 kPa were maintained throughout storage (Fig. 1C and D).
Evolution of O2 concentration in the package headspace throughout storage of fresh-cut melon processed in different ripeness stage. (a, b) Melon packaged under 2.5 kPa O2+7 kPa CO2 conditions; (c, d) melon packaged under non-modified atmosphere. (a, c) Melon dipped in a solution of 1% ascorbic acid and 0.5% CaCl2 ((•) green, (▪) green-mature, (▴) mature); (b, d) control melon ((○) green, (□) green-mature, (▵) mature)
Evolution of CO2 concentration in the package headspace throughout storage of fresh-cut melon processed in different ripeness stage. (a, b) Melon packaged under 2.5 kPa O2+7 kPa CO2 conditions; (c, d) melon packaged under non-modified atmosphere. (a, c) Melon dipped in a solution of 1% ascorbic acid and 0.5% CaCl2 ((•) green, (▪) green-mature, (▴) mature); (b, d) control melon ((○) green, (□) green-mature, (▵) mature)
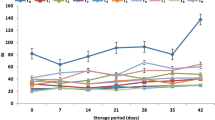
The stage of ripeness at processing had a significant effect on CO2 accumulation inside the packages throughout storage (p<0.001). Thus, the more advanced the state of ripeness at processing, the higher the CO2 production (Fig. 2). Mature melon evolved more CO2 than green and green-mature melons, especially beyond 17 days of storage. Mature melon packaged under non-MA and without dipping treatment exhibited a maximum CO2 accumulation of 45.5 kPa at the end of storage (Fig. 2D). Enhanced CO2 production may be due to microbial growth and a general deterioration of the tissue of fresh-processed melon [17]. A dipping treatment of 1% AA and 0.5% CaCl2 was found to slow down the CO2 accumulation under non-MA packaging (p<0.001). According to these results, a low temperature calcium treatment during processing of fresh-cut ‘Cantaloupe’ melon has been shown to effectively retard CO2 production [11, 19].
Low O2 levels may cause a risk of anaerobic fermentation and thus, minimum 1 kPa O2 and maximum 15 kPa CO2 have been suggested as safe levels to prevent toxicity or anoxia inside packages of fresh-cut melon [6]. An atmosphere composition of 4 kPa O2 and 12–13 kPa CO2 was reached in packages of fresh-processed ‘Amarillo’ melon stored under non-MA at 5 °C for 14 days. This packaging condition was effective to keep sensorial quality and microbial safety and to avoid loss of weight and translucency [18]. Similar O2 and CO2 concentrations were reached in packages of fresh-cut ‘Piel de Sapo’ melon stored under non-MA after 14 days of storage at 5 °C (Fig. 1C and D).
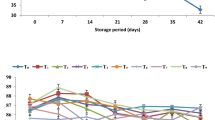
‘Piel de Sapo’ melon evolved small amounts of ethylene thus indicating a low physiological activity. Neither the stage of ripeness at processing nor the dipping treatment affected significantly ethylene levels inside the packages (p>0.05). On the other hand, ethylene production inside packages stored under non-MA was higher than under 2.5 kPa O2+7 kPa CO2 conditions (p<0.05). Ethylene levels reached values of 0.5 and 0.8 ppm in packages stored under a 2.5 kPa O2+7 kPa CO2 atmosphere and non-MA, respectively (Fig. 3). Melon cultivars such as ‘Cantaloupe’ have exhibited a climacteric pattern of ethylene production during ripening [20]. However, ‘Piel de Sapo’ melon shows a low physiologic activity and has been reported to exhibit a non-climacteric ripening pattern [21].
Evolution of ethylene concentration in the package headspace throughout storage of fresh-cut melon processed in different ripeness stage. (a, b) Melon packaged under 2.5 kPa O2+7 kPa CO2 conditions; (c, d) melon packaged under non-modified atmosphere. (a, c) Melon dipped in a solution of 1% ascorbic acid and 0.5% CaCl2 ((•) green, (▪) green-mature, (▴) mature); (b, d) control melon ((○) green, (□) green-mature, (▵) mature)
Ethanol content in packages of green fresh-cut ‘Piel de Sapo’ melon increased throughout time, reaching maximum concentrations ranging from 80 to 125 ppm (Fig. 4). Irrespective of packaging conditions or dipping treatment, fermentative pathways were triggered with higher intensity in green melons, which suggests that slightly under-ripe fruits are more susceptible to undergoing anaerobic metabolism. The enhanced increase of ethanol content in green ripeness stage beyond the 21st day of storage could be due to a stress response that may lead to a loss of membrane integrity. In fact, physiological disorders and membrane damage in fruits are attributed to the effects of stressful levels of too-low oxygen or too-high carbon dioxide [22]. Green-mature fresh-cut melon exhibited the least accumulation of ethanol at the end of storage. These results agreed with those reported by Soliva-Fortuny et al. [9] in fresh-cut ‘Conference’ pears. Moreover, ethanol accumulation further increased under 2.5 kPa O2+7 kPa CO2 atmospheres in comparison to non-MA, regardless of the dipping treatment (p<0.001). Ethanol production was avoided under a 2.5 kPa O2+7 kPa CO2 atmosphere during the first 10 days of storage. On the other hand, packages of fresh-cut melon stored under initial non-MA were kept 14 days without ethanol accumulation. Anaerobic metabolism may be promoted by a low availability of O2 and high accumulation of CO2 in packages preserved under initial 2.5 kPa O2+7 kPa CO2 atmospheres. Lakakul et al. [23] pointed out the importance of maintaining O2 level just above the fermentation threshold and keeping CO2 concentration below the range that causes injury to fruits.
Evolution of ethanol concentration in the package headspace throughout storage of fresh-cut melon processed in different ripeness stage. (a, b) Melon packaged under 2.5 kPa O2+7 kPa CO2 conditions; (c, d) melon packaged under non-modified atmosphere. (a, c) Melon dipped in a solution of 1% ascorbic acid and 0.5% CaCl2 ((•) green, (▪) green-mature, (▴) mature); (b, d) control melon ((○) green, (□) green-mature, (▵) mature)
Color and firmness evolution
Color changes on fresh-cut ‘Piel de Sapo’ throughout storage was mostly due to L * and WI depletion (Table 2). Aguayo et al. [18] reported that WI decreased when translucency injury increased on fresh-processed melon, as a consequence of a physiological disorder characterized by dark and glassy flesh. A dip containing AA and CaCl2 was effective (p<0.001) to reduce translucency in packages of green-mature and mature melon. On the other hand, translucency was not observed on cut surfaces of green melons throughout storage. It seems that translucency is a symptom of senescence attributed to advanced stages of ripeness [5]. In addition, greater percentage of translucency was observed on fresh-cut ‘Piel de Sapo’ melon stored under 2.5 kPa O2+7 kPa CO2 atmospheres than under non-modified atmosphere conditions. The deterioration of fresh-cut ‘Honeydew’ or ‘Cantaloupe’ melon packaged under MA has been found to be primarily due to the development of translucency [5–7, 18, 24].
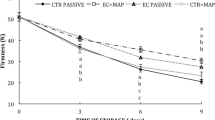
Softening of fresh-cut melon differed significantly among ripeness conditions (p<0.001). Green fresh-cut melon underwent a dramatic decrease in firmness (Table 3) as a consequence of physiological stress. Such loss of firmness could be correlated with an enhanced accumulation of ethanol in green melon packages (Fig. 4). Packaging with 2.5 kPa O2+7 kPa CO2 atmospheres in concomitance with dipping treatment reduced softening of green and green-mature melons (p<0.05) but could not further delay senescence in mature melon. Recent studies have reported the effectiveness of a dip of 1% AA and 0.5% CaCl2 combined with packaging under a low O2 atmosphere to maintain firmness of fresh-cut apples and pears during several weeks [25, 26]. Restricted oxygen concentrations appear to inhibit many biochemical reactions which may lead to a loss of cellular integrity. In addition, calcium salts interact with pectin to form a cross-linked polymer network that increases mechanical strength, thus delaying senescence and controlling physiological disorders in fruits and vegetables [26]. Calcium chloride (1–5%) dips for 1–5 min provided a firming effect on fresh-cut ‘Cantaloupe’ melon stored at 5 °C [11].
Microbial stability and shelf-life modeling
Initial counts of aerobic mesophilic microorganisms on just processed green or green-mature ‘Piel de Sapo’ melons were approximately 3 log (cfu g−1) whereas mature melon loads were about 6 log (cfu g−1). Too-soft initial texture of raw material may be responsible for the appearance of exudates on fruit cut surface at processing, which contain some compounds that may be used to native or exogenous microorganisms to grow on the product surface rapidly [27]. On the other hand, populations of yeasts and molds for all ripeness conditions ranged from 1 to 2.5 log (cfu g−1) at the beginning of storage. Our results for green-mature fresh-cut melon were similar to those found by Bai et al. [5] for fresh-cut ‘Cantaloupe’ melons at a similar stage of ripeness. These authors reported initial bacteria counts of 3.2 log (cfu g−1) under 4 kPa O2+10 kPa CO2 atmospheres and non-MA. Under these packaging conditions, yeast and molds counts were less than 1.9 log (cfu g−1).
Packaging atmosphere and ripeness state at processing affected significantly to microbiological counts during refrigerated storage (p<0.05). The more advanced the stage of ripeness, the higher the microbiological counts (Fig. 5). Tables 4 and 5 show the kinetic constants estimated by a modification of the Gompertz model [28] (Eq. 3) to describe the growth of aerobic mesophilic microorganisms and fungi on fresh-cut melon. The maximal growth rate of mesophilic aerobic microorganisms (μ max) was higher than that of yeasts and molds except for mature fresh-cut melon (Figs. 4 and 5). Bai et al. [5] reported that bacterial populations in fresh-cut ‘Cantaloupe’ stored under a 4 kPa O2+10 kPa CO2 atmosphere or non-MA exhibited faster growth than yeasts and molds.
Evolution of microbial growth throughout storage of fresh-cut melon processed in different ripeness stage: aerobic mesophilic microorganisms (•) green, (▪) mature-green, (▴) mature); yeast and molds ((○) green, (□) mature-green, (▵) mature). (a, b) Melon packaged under 2.5 kPa O2+7 kPa CO2 conditions; (c, d) melon packaged under non-modified atmosphere. (a, c) Melon dipped in a solution of 1% ascorbic acid and 0.5% CaCl2 ((•) green, (▪) green-mature, (▴) mature); (b, d) control melon ((○) green, (□) green-mature, (▵) mature)
Low oxygen atmospheres generally inhibit the growth of aerobic microorganisms [5]. A low O2 atmosphere combined with a dipping treatment reduced the proliferation of aerobic mesophilic microorganisms on green or green-mature fresh-cut ‘Piel de Sapo’ melon (p<0.05). Counts were particularly low on green fresh-cut melon for 14 days (Fig. 5A). On the other hand, melon processed at the most advanced ripeness exhibited the highest counts under all conditions, exceeding counts of 6 log (cfu g−1) at the beginning of storage (Fig. 5). The United States and most European countries have regulations relative to fresh-cut produce, which limit the counts of aerobic microorganisms to 6 log (cfu g−1) at expiration date [29]. Table 4 shows that a dipping treatment combined with a 2.5 kPa O2+7 kPa CO2 atmosphere on green or green-mature melons reduced the maximal growth rate (μ max) and the counts attained at the stationary phase (A). The lag phase period (λ) of aerobic mesophilic microorganisms on green and green-mature fresh-cut melon was around 7–10 days.
Yeasts and molds were less numerous than bacteria on fresh-cut ‘Piel de Sapo’ melon throughout storage (Fig. 5), which was also reported on fresh-cut ‘Cantaloupe’ [5] and on other fresh-cut produce [30]. Initial counts grew slightly on green melons compared to fruits processed at more advanced ripeness (p<0.05) (Fig. 5). Low O2 atmospheres combined with AA and calcium treatment led to a significant reduction of maximal growth rate of fungi in comparison with the other studied conditions (Table 5). However, the counts attained at the stationary phase were not significantly affected by the different packaging conditions. The lag phase of fresh-cut melon dipped in a solution of AA and calcium chloride was lengthened to a minimum of 5 days whereas that of untreated samples was less than 5 days, regardless of the atmosphere conditions (Table 5). Without adding calcium, the decrease of membrane strength and integrity would cause a decompartmentalization of enzymes and substrates leading to a rise in fluids and solute exchanges and thus, promoting the proliferation of microorganisms.
Conclusions
Ripeness stage at processing is a limiting factor for the shelf-life of fresh-cut ‘Piel de Sapo’ melon. Green-mature fresh-cut melon exhibited lower rates of CO2 and ethanol accumulation than mature melon. Low O2 atmospheres in combination with AA and CaCl2 treatments significantly reduced CO2 accumulation and ethylene production as well as the maximal growth rate of bacteria, yeasts and molds, except for fully ripe melon which exhibited the highest initial counts. The dipping treatment combined with packaging under 2.5 kPa O2+7 kPa CO2 atmospheres was also effective to prevent the development of translucency and softening of green or green-mature fresh-cut melon. In addition, low O2 atmospheres prevented the production of fermentative metabolites like ethanol for 10 days. Therefore, for commercial purposes, a shelf-life of 10 days is suggested for green-mature fresh-cut ‘Piel de Sapo’ melon dipped in 1% AA and 0.5% CaCl2 solution and packaged under a 2.5 kPa O2+7 kPa CO2 atmosphere.
References
Valdenegro M, Flores FB, Romojaro F, Ramírez M, Martínez-Madrid MC (2004) Susceptibility of Spanish melon fruits to chilling injury during cold storage. In: Mencarelli F, Tonutti P (eds) Proceedings of the Fifth International Postharvest Symposium, 6–11 June 2004, Acta Horticulturae 682. International Society for Horticultural Science, Belgium, vol 2, pp 1219–1225
Watada AE, Ko NP, Minott DA (1996) Postharv Biol Technol 9:115–125
Varoquaux P (1991) Rev Gén Froid 81:33
Beaulieu JC, Ingram DA, Lea JM, Bett-Garber KL (2004) J Food Sci 69:250–254
Bai JH, Saftner RA, Watada AE, Lee YS (2001) J Food Sci 66:1207–1211
Bai J, Saftner RA, Watada AE (2003) Postharv Biol Technol 28:349–359
Aguayo E, Allende A, Artés F (2003) Eur Food Res Technol 216:494–499
Soliva-Fortuny RC, Oms-Oliu G, Martín-Belloso O (2002) J Food Sci 67:1958–1963
Soliva-Fortuny RC, Alós-Saiz N, Espachs-Barroso A, Martín-Belloso O (2004) J Food Sci 69:244–248
Lurie S (2003) Antioxidants. In: Hodges DM (ed) Postharvest oxidative stress in horticultural crops. Food Products Press, New York, pp 131–150
Luna-Guzmán I, Cantwell M, Barreto D (1999) Postharv Biol Technol 17:201–213
Luna-Guzmán I, Barrett DM (2000) Postharv Biol Technol 19:61–72
Poovaiah BW (1986) Food Technol 40:86–89
ISO (1991) Methods for microbiological examination of food and animal feeding stuffs. ISO, Geneva
ISO (1988) General guidance for enumeration of yeasts and moulds. ISO, Geneva
Lanciotti R, Corbo MR, Gardini F, Sinigaglia M, Guerzoni ME (1999) J Agric Food Chem 47:4769–4776
Villanueva MJ, Tenorio MD, Esteban MA, Mendoza MC (2004) Food Chem 87:179–185
Aguayo E, Escalona VH, Artés F (2004) J Food Sci 69:148–155
Lamikanra O, Watson MA (2004) J Food Sci 69:468–472
Hadfield KA, Rose JKC, Bennett AB (1995) J Exp Bot 46:1923–1925
Valdenegro M, Ramírez M, Flores FB, Romojaro F (2004) Influencia de 1-MCP y etileno sobre los daños por frío en melón español durante el almacenamiento. In: Instituto Nacional de Investigação Agrária (ed) Maturação e Pós-colheita 2004—Frutos e Hortícolas, 6–9 Octubro 2004. Instituto Nacional de Investigação Agrária, Oeiras, Portugal, pp 519–521
Lester GE (2003) Oxidative stress affecting fruit senescence. In: Hodges DM (ed) Postharvest oxidative stress in horticultural crops. Food Products Press, New York, USA, pp 113–129
Lakakul R, Beaudry R, Hernandez RJ (1999) J Food Sci 64:105–110
O’Connor-Shaw RE, Roberts R, Ford AL, Nottingham SM (1994) J Food Sci 59:1202–1206
Soliva-Fortuny RC, Grigelmo-Miguel N, Hernando I, Lluch MA, Martín-Belloso O (2002) J Sci Food Agric 82:1682–1688
Soliva-Fortuny RC, Lluch MA, Quiles A, Grigelmo-Miguel N, Martín-Belloso O (2003) J Food Sci 68:312–317
Soliva-Fortuny RC, Martín-Belloso O (2003) Eur Food Res Technol 217:4–9
Zwietering MH, Jongenburger I, Rombouts FM, Riet K van’t (1990) Appl Environ Microbiol 56:1875–1881
Martín-Belloso O, Soliva-Fortuny R, Oms-Oliu G (2006) Fresh-cut fruits. In: Hui YH (ed) Handbook of fruits and fruit processing. Blackwell Publishing, Ames, Iowa, USA, pp 129–144
Nguyen-The C, Cartin F (1994) Crit Rev Food Sci Nutr 34:371–401
Acknowledgments
This research was supported by the Ministerio de Ciencia y Tecnología (Spain) through the AGL 2003-09208-C01 project and the Departament d’Universitats Recerca i Societat de la Informació of the Generalitat de Catalunya (Spain), which also awarded author G. Oms-Oliu a pre-doctoral grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oms-Oliu, G., Soliva-Fortuny, R. & Martín-Belloso, O. Effect of ripeness on the shelf-life of fresh-cut melon preserved by modified atmosphere packaging. Eur Food Res Technol 225, 301–311 (2007). https://doi.org/10.1007/s00217-006-0415-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0415-9