Abstract
Bioactivity of “espinheira-santa” leaves can be evaluated through solvent purification processes. Species of the same genus may contain different compounds, which may be separated by conventional chromatography using different solvents. The extraction process also influences the contents of these compounds. In order to optimize the above process research is required on variables such as solvents, temperature and extraction time. The objective of this work was to evaluate the antioxidant activity (ABTS, DPPH and FRAP) of extracts and purified fractions from leaves of Maytenus ilicifolia and M. aquafolium. Percolation at 28 °C for 7 days, and ultrasound at 50, 60 and 70 °C for 15 min were the extraction methods tested. Open column chromatography (silica gel) was used in the fractionation of extracts, and hexane, chloroform and methanol were the elution solvents used. The evaluation methods of the antioxidant activity were DPPH, FRAP and ABTS. Plant species showed no significant differences between methods for DPPH, but the fractions obtained with chromatography had significant differences, the crude ethanolic extract (CEE) and the methanolic been in general superior. “espinheira-santa” is a plant with antioxidant potential, but the extraction methods are not optimal. Still, it can be said that the separation by chromatography provided a better visualization of the nature of the components of that plant that best stood out in relation to the antioxidant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maytenus ilicifolia and M. aquafolium are plant species commonly refered as “espinheira-santa” native to Brazil. Leaves of both species have proven pharmaceutical properties against stomach diseases such as gastric ulcers and gastritis (Negri et al. 2009; de Souza et al. 2008) that contain alkaloids, polyphenols, diterpenes and friedelan triterpenes among others (Cunico et al. 2002). Phenolic compounds are the main plant antioxidant agents that, together with other nutritional reducing agents such as vitamins C and E and carotenoids, protect cells from oxidative damage, preventing the body from the risk of various degenerative diseases associated with oxidative stress. In folk medicine, the use of the holy “espinheira-santa” is made from dried leaves in the form of tea (Mariot and Barbieri 2007). Its therapeutic effects are most likely related to its content and composition of phenolic compounds.
The extraction or purification of phenolic compounds from plants is influenced by several factors such as the method used, particle size, procedure time and the presence of interfering substances. The phenolic compounds may have different hydroxyl groups which may be conjugated to sugars, acids or alkyl groups. Accordingly, the polarities of phenolic compounds vary greatly and it is difficult to develop a single and efficient method for all phenolic compounds. Therefore, the optimization of the extraction process is fundamental for an accurate evaluation (Mokrani and Madani 2016).
The search for plants with medicinal effects is increasing, and there is a need for methodological standards of extraction that optimize the recovery of compounds. For this reason, it is necessary to take into account variables such as time and temperature of extraction and type of solvent (Tiwari et al. 2011; Atl 2016) which may interfere in the composition and bioactivity of extracts of the leaves of “espinheira-santa”. Since plant species of the same genus may contain different compounds, it is possible that these compounds are better evaluated and compared by conventional column chromatography separation and purification processes with different elution solvents. The objective of this work was to evaluate (1) the effect of temperature and time of extraction, and (2) chromatographic separation solvent in the antioxidant activity from leaves of M. ilicifolia and M. aquafolium.
Materials and methods
Plant material
Leaf samples of M. ilicifolia (Mart. Ex Reissek) and M. aquafolium (Mart.) were collected at Marechal Cândido Rondon, Paraná, Brazil (24°33′29.7″S54°02′44.0″W) from June to September 2017. The leaves were collected manually and stored in plastic bags for transportation to the laboratory, where they were sanitized with 2% hypochlorite solution and dried at room temperature. Leaves were dehydrated under air circulation at 55 °C for 3 days and milled in a knife mill (TE-680, Wiley) and the powder was placed in a plastic bag and refrigerated.
Extraction
The crude ethanolic extract (CEE) was prepared by two solid–liquid extraction methods: (1) percolation (7 days) at room temperature and (2) ultrasound (15 min) at different temperatures (50, 60 and 70 °C). The solid–liquid ratio was 10% (w/v) with 80% hydroethanolic solvent. When extraction was completed, the extractive solvent was evaporated under reduced pressure (50 °C, 55 rpm). The dry crude extracts were then weighed, diluted in hexane (2.0 mg mL−1) and stored under refrigeration. All extracts were prepared in triplicate.
Separation and purification in open column chromatography
A 20 cm long, 2.0 cm diameter glass chromatographic column packed with 70–230 mesh silica gel adsorbent was used. At the top of the column were placed sand and cotton. The solvents from the mobile phase were, in this elution sequence of hexane, chloroform and methanol. Fractions were collected by staining. Each fraction collected was evaporated under reduced pressure (50 °C, 55 rpm) and the dry weights recorded. Afterwards, each dry fraction was diluted in 80% ethanol and stored under refrigeration for analyzes of antioxidant activity, ABTS·+ [2,2′-azino-bis- (3-ethylbenz-thiazoline-6-sulfonic acid)], DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (Ferric Reducing Antioxidant Power).
Capture of free radical ABTS·+
The ABTS·+ was obtained according to methodology described by Re et al. (1999) with some modifications, in which the method starts from the reaction of 7 mM ABTS with 140 mM potassium persulfate, with incubation at room temperature in the absence of light for 16 h. The solution was then diluted in 100% ethanol together with the spectrophotometer to achieve absorbance of 0.700 ± 0.200 measured at 734 nm. In the test tubes, 30 μL of samples and 3 mL of the solution containing the radical were added. After 6 min of reaction the absorbance was determined in a spectrophotometer. For the construction of the standard curve the synthetic antioxidant Trolox was used in the concentrations of 100–1500 µmol L−1 with results in µmol Eq. Trolox g−1.
Capture of free radical DPPH
The working solution is obtained according to methodology described by Brand-Willian et al. (1995), with the addition of 0.5 mL of the previously diluted standards or samples, followed by 3.0 mL of 50% (v/v) ethanol, 0.3 mL of DPPH in solution of ethanol (0.5 mmol L−1). The reaction took 45 min at room temperature and sheltered from light. After the reaction time the reading was performed in a spectrophotometer at 517 nm. For the negative control, the extract volume was replaced by solvent extraction (ethanol 80%). A blank sample was prepared by substituting the volume of the DPPH solution for an equal volume of ethanol. The curve was prepared with the synthetic antioxidant Trolox at concentrations ranging from 20 to 140 µmol Trolox with results in mg Eq. Trolox g−1.
Iron reduction method (FRAP)
According to methodology described by Benzie and Strain (1996) with some modifications, FRAP reagent was prepared in which 25 mL of an acetate buffer solution (300 mM, pH 3.6), 2.5 mL of the TPTZ solution (10 mM TPTZ in 40 mM HCl) and 2.5 ml of FeCl3 (20 mM) in aqueous solution were mixed. An aliquot of 100 μL of the sample previously diluted to 3 mL of the FRAP reagent was added to the test tube and allowed to react at a temperature of 37 °C in a water bath for 30 min. After the reaction time, the absorbance was measured with a spectrophotometer, being zeroed with the FRAP solution. The standard curve was constructed with ferrous sulfate at concentrations of 2–12 μmol L−1 with results in μmol FeCl3 g−1.
Statistical analysis
The experimental design was a completely randomized. The results were submitted to analysis of variance, and Tukey’s test was applied for means whose effects were significant. Pearson’s linear correlation analysis was also used with level of significance at p < 0.05 on Sisvar (Ferreira 2000) and Excel.
Results and discussion
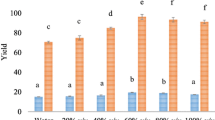
The analysis of the results indicated that in relation to the antioxidant DPPH activity (Fig. 1a, b) there was no significant influence (p > 0.05) among the studied plant species. The percolation extraction method at 28 °C showed antioxidant activity statistically similar to the extraction with ultrasound, regardless of temperature, except for the fraction purified with hexane which showed lower antioxidant activity for all extraction and ultrasonic temperatures. This result indicates that the percolation method may be indicated for simplicity and practicality, although it is more time consuming.
DPPH antioxidant activity of fractions extracted from leaves of M. ilicifolia (a) and M. aquafolium (b) species by different methods. Means followed by the same letter, lowercase, within each extraction method do not differ from each other. Means followed by the same letter, upper case, between methods and even solvent do not differ at p < 0.05 (Own authorship)
The fraction purified with methanol showed the highest antioxidant activity DPPH, followed by fractions obtained with chloroform and hexane (Fig. 1a, b). Since methanol is the solvent with the highest polarity among the solvents tested. The results suggest that the antioxidant profile of the raw extract of the “espinheira-santa” leaves is represented mostly by polar compounds. In addition, when the fraction with methanol was compared to its respective crude ethanol extract (CEE) there was a statistical similarity of the antioxidant activity only for the percolation extractions, by ultrasound at 50 °C and 60 °C independent of the plant species, while for the ultrasonic extraction at 70 °C the methanolic fraction was higher than the CEE. The former suggests a negative effect of temperature, which may be indicative of compound degradation with increasing temperature. This effect was observed on leaves from both species.
The antioxidant capacity measured by the DPPH method showed higher levels when the extraction used lower temperatures (28 °C and 50 °C). Total gross extract (TGE) and the extract obtained with methanol were superior and differed from the others for leaves of both species (Fig. 1). Visually, it was observed that both the CEE and the methanolic fraction obtained in the extraction methods at lower temperatures (28 °C and 50 °C) showed darker coloration than hexane and chloroform fractions indicating that the compounds of greater antioxidant activity of the leaves of the studied “espinheira-santa” have dark pigmentation. In agreement with the results found, Negri et al. (2009) studied the effect of temperature on the preparation of samples of “espinheira-santa” leaves and reported that temperatures of 40 °C and 50 °C showed higher antioxidant DPPH activity.
The data obtained were similar to those found in the literature, conferring reliability on the data found. Haida et al. (2012) evaluated different concentrations of extracts from “espinheira-santa” with values between 69.69 and 83.08 μg mL−1, data lower than those found in this work for the raw ethanoic extract. Gomes et al. (2008) and Vellosa et al. (2007) also find values similar to those described by Haida et al. (2012). According to the above information, the extraction values presented in this work are efficient, although with possibility of optimization from changes in extraction method, such as the increase in ultrasound time or ultrasound application in the percolation method.
Regarding the results of ABTS antioxidant activity (Fig. 2a, b), analysis of variance (results not shown) showed that there was a significant interaction between all variables (extraction method, extraction temperature and plant species). The CEE obtained from the M. ilicifolia leaves differed (p < 0.05) from the other extracts for all treatments, being the extract with the highest antioxidant activity, followed by the extract obtained with methanol. The same was verified for M. aquafolium leaves.
ABTS antioxidant activity of fractions extracted from leaves of the species M. ilicifolia (a) and M. aquafolium (b) by different methods. Means followed by the same letter, lowercase, within each extraction method do not differ from each other. Means followed by the same letter, upper case, between methods and even solvent do not differ at p < 0.05 (Own authorship)
For both species, the behavior of the graph in relation to antioxidant activity as a function of the extracts and the extraction forms are similar, suggesting that the compounds of both species have similar characteristics. For the analysis of the ABTS antioxidant activity the percolation was superior, followed by the method with ultrasound at 50 °C. The TGE and the methanolic fractions had mainly superior results. The extract of lower antioxidant activity was obtained with hexane, conferring a polar character to the compounds. For samples from both species, the temperature influence was quite significant in the extraction, with the lowest temperatures being the most efficient. Haida et al. (2012) demonstrated that aqueous and alcoholic extracts from M. ilicifolia are rich in phenolic compounds. The same authors also found that M. ilicifolia has the highest content of phenolic compounds, in accordance with our data.
There were significant differences (Fig. 3) between extraction methods and extracts analyzed. Similarities were found in the data obtained in the TGE and the extract obtained with methanol (p < 0.05). The extraction method at lower temperature (28 °C) for FRAP method was highlighted in the extraction of compounds with antioxidant activity, a fact that was observed for all methods of evaluation of the antioxidant activity described in this study. Interpretation of the ANOVA indicated that results from the two species was not different (p < 0.05). It is possible to observe similarity graphs from results of the two species of antioxidant activity with similar concentrations with same influence of temperature.
FRAP antioxidant activity of fractions extracted from leaves of the species M. ilicifolia (a) and M. aquafolium (b) by different methods. Means followed by the same letter, lowercase, within each extraction method do not differ from each other. Means followed by the same letter, upper case, between methods and even solvent do not differ at p < 0.05 (Own authorship)
Regarding antioxidant activity evaluated by FRAP, the extraction methods showed influence, being the lower temperatures more effective in the extraction of compounds. The compounds evaluated for both methods are mostly found in crude extracts and extracts obtained with methanol, except ABTS, which had the extracts obtained with similar methanol and chloroform. It can mean that the antioxidant compounds for the ABTS radical are diverse and may be of different molecular weights.
Considering the diversity of antioxidant substances present in food and vegetables, several methods have been developed to estimate the in vitro antioxidant capacity of these substances. However, the lack of standardization of these methods makes it difficult to compare data published by different research groups, mainly due to the use of different solvents and ways of expressing the results. Likewise, variations in the antioxidant complex of each food can provide different responses in each method. Therefore, the literature recommends the combination of at least three of these methods to provide a more complete and representative result of antioxidant capacity (Perez-Jiménez and Saura-Calixto 2006).
Fractions extracted from the leaves of M. ilicifolia showed higher antioxidant activity in all the evaluated methods (DPPH, ABTS and FRAP). With the exception of the ABTS method, samples from both species showed similar results for the methods used and this may explain the fact that both species are widely used as drug products. The use of both species for medicinal purposes is common because both have proven activity on stomach problems. One possible explanation for the medicinal properties of “espinheira-santa” is its antioxidants, which belong to a class of secondary metabolites widely distributed in plants, which are usually tannins related to healing processes (Silva and Da Silva 1999).
Bressani (1993) evaluated polyphenols in beans under cooking and showed that the extraction processes influence the levels of antioxidant activity. According to those authors, high temperature promotes greater release of polyphenols from beans in cooking water. Radomski and Scheffer (2004) suggested care with external interferences for the contents of antioxidant activity affirming that plants that grow in full sun, compared to those that in the shade have higher levels of polyphenols and that the contents of polyphenols vary according to the luminosity. Therefore, factors such as climate, region, management and post-harvest techniques also influence and should be taken into account with regard to antioxidant levels.
Conclusion
Leaves of M. ilicifolia and M. aquafolium have similar antioxidant activity. The crude extracts and extracts obtained with methanol had superior results for all the methods of evaluation of the antioxidant activity. The extraction methods with lower temperatures gave higher antioxidant activity (percolation 28 °C and ultrasound 50 °C).
References
Atl M (2016) Effect of different extraction techniques on the yield, antioxidant activity, total dosages, and profile by hplc-dad of Dicksonia sellowiana (Presl.). Hook, dicksoniaceae. Rev Bras Plantas Med 18(1983–084x):230–239. https://doi.org/10.1590/1983-084X/15_106
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Brand-Willian W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Bressani R (1993) Grain quality of common beans. Food Rev Int 9(2):237–297. https://doi.org/10.1080/87559129309540960
Cunico MM, Cirio GM, Miguel OG, Miguel MD, Montrucchio DP, Auer CG, Grigoletti Júnior A (2002) Study of antifungal activity of Maytenus ilicifolia Mart. ex Reiss., Celastraceae. Rev Bras Farmacogn 12(2):69–73. https://doi.org/10.1590/S0102-695X2002000200003
de Souza LM, Cipriani TR, Serrato RV, da Costa DE, Lacomini M, Gorin PAJ, Sassaki GL (2008) Analysis of flavonol glycoside isomers from leaves of Maytenus ilicifolia by offline and online high performance liquid chromatography-electrospray mass spectrometry. J Chromatogr 1207(1–2):101–109. https://doi.org/10.1016/j.chroma.2008.08.032
Ferreira DF (2000) Análise estatística por meio do SISVAR (Sistema para Análise de Variância) para Windows versão 4.0. In: REUNIÃO ANUAL DA REGIÃO BRASILEIRA DA SOCIEDADE INTERNACIONAL DE BIOMETRIA, vol 45. UFSCar, Anais, São Carlos, pp 255–258
Gomes MF, Silva VTB, Laverde A Jr, Takemura OS (2008) Avaliação da atividade antioxidante de extratos das folhas de Bixa orellana (Bixaceae) e Maytenus ilicifolia (Celastraceae). Arq Ciênc Saúde Unipar 12(3):169–173
Haida KS, Haas J, de Lima DS, Haida KY, da Silva FJ, Limana S, Rodrigues RT (2012) Anti-oxidant activity and phenolic compounds of Maytenus ilicifolia and Maytenus aquifolium. Rev Saúde Pesqui 5(2):360–368
Mariot MP, Barbieri RL (2007) O Conhecimento Popular Associado ao Uso da Espinheira-santa (Maytenus ilicifolia e M aquifolium). Revis Bras Biociênc 5(1):666–668
Mokrani A, Madani K (2016) Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol 162(January):68–76. https://doi.org/10.1016/j.seppur.2016.01.043
Negri MLS, Possamai JC, Nakashima T (2009) Antioxidant activity of “espinheira-santa” - Maytenus ilicifolia Mart. ex Reiss., leaves dried in different temperatures. Braz J Pharmacogn 19(2B):553–556. https://doi.org/10.1590/S0102-695X2009000400007
Perez-Jiménez J, Saura-Calixto F (2006) Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res Int 39:791–800. https://doi.org/10.1016/j.foodres.2006.02.003
Radomski MI, Scheffer MC (2004) Características Fenotípicas de 44 Progênies de Maytenus ilicifolia Mart. Cultivadas no Município de Ponta Grossa, PR. Colombo: Embrapa Florestas, 2004. 6p (Embrapa Florestas. Circular técnica, 86)
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Riceevans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Silva MR, Da Silva MAAP (1999) Aspectos nutricionais de fitatos e taninos. Rev Nutr 12(1):21–32. https://doi.org/10.1590/S1415-52731999000100002
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011) Phytochemical screening and extraction - a review. Int Pharm Sci 1(1):98–106
Vellosa JCR, Barbosa VF, Khalil NM, Santos VAFFM, Furlan M, Lourenço I, Oliveira OMMF (2007) Profile of Maytenus aquifolium action over free radicals ande reactive oxygen species. Braz J Pharm Sci São Paulo 43(3)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiapetti, T.P., Malavasi, U.C., Braga, G.C. et al. Effects of the extraction method and chromatographic separation solvent in the antioxidant activity of different species of “espinheira-santa”. J Food Sci Technol 56, 5056–5062 (2019). https://doi.org/10.1007/s13197-019-03978-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03978-1