Abstract
In the present study, the extraction techniques like maceration, ultrasound and soxhlet extraction were employed to obtain different extracts from the powdered roots of Nepeta leucophylla using the solvents of different polarity and the percentage yield of the different extracts was compared. These extracts were screened for their total polyphenolic content (TPC), total flavonoids content (TFC) and for their antioxidant potential using different assays like 2,2-diphenyl-1-picrylhydrazyl radical scavenging (DPPH), nitric oxide scavenging, ferric reducing antioxidant power (FRAP) and total antioxidant capacity (TAC). The results of percentage yield of different extracts ranged from 0.9 to 8.07 %. The methanol extract obtained by Soxhlet method showed a higher value of TPC [94.02 mg GAE/g of dry plant extract (DPE)], FRAP (130.75 mg Fe(II) E/g of DPE), percentage inhibition of DPPH (91.4 %) and NO scavenging (51.6 %), whereas the same extract obtained by ultrasound assisted extraction method showed higher values of TFC (228.75 mg RE/g of DPE) and TAC (40.8 mg AAE/g of DPE) as compared to all other extracts. Reverse phase high-performance liquid chromatography-photodiode array detector analysis showed the presence of myricetin, caffeic acid, catechin hydrate and vanillic acid as key polyphenolic in the different methanol extracts, whereas gas chromatography mass spectrometry analysis showed the presence of long chain hydrocarbons, steroids, fatty acids and benzoid classes of natural products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The research on antioxidants has increased rapidly during the past few decades due to their potential to prevent the generation of free radicals—the causative agent of various degenerative diseases like diabetes, cancer, cardiovascular, neurological disorders, etc. [1]. The recent reports have shown that the dietary intake of antioxidant rich foods has lowered the risk of various degenerative human diseases [2]. The synthetic antioxidants, like butylated hydroxyanisole, butylated hydroxytoluene, tertiary butyl hydroquinone and propyl gallate etc. have been extensively used, but now-a-days their use has been limited due to their wide range of side effects [3]. The plants, a rich repository of diverse secondary metabolites (SMs) like polyphenols, flavonoids, carotenoids, ascorbic acid, tocopherols, etc. have shown pronounced antioxidant potential that reduces in vitro free radical generation and oxidation of low-density lipoprotein, controls blood pressure, inhibits platelet aggregation, etc. Further, these compounds have also been well known for their antiallergic, antibacterial, antimutagenic and antiviral activities [4, 5]. Thus, nowadays the interest in exploring new ecofriendly natural antioxidant has increased significantly.
Nepeta leucophylla, a member of the genus Nepeta belongs to the family Lamiaceae. Out of the 300 species of this genus found all over the world, 41 are found in Western Himalayan region of India [6]. The terpenoids (iridoids nepetalactones and their derivatives) along with steroids, flavonoids and polyphenols are the principal classes of SMs present in this genus [7, 8]. The genus is well known for its therapeutic potential since ancient times as its different species have been used as antispasmodics, antiasthmatics, antitussives, diaphoretics, diuretics, anti-inflammatory, antimicrobial, antiviral agents, fungicides, sedatives [9–11] antioxidants, disinfectants, [12, 13]. Further, these species have also been effective against angina pectoris, cardiac thrombosis, stomach, liver, kidney and tooth ailments [7, 8].
Nepeta leucophylla is found on the open slopes of mountains in the Himalayan region of Himachal Pradesh, Uttarakhand, J & K and Nepal. The phytochemical analysis of essential oil and various extracts obtained from different parts of this plant has shown the presence of iridodial dienol diacetate, iridodial β-monoenol acetate, dihydroiridodial diacetate, coleon U 12-methyl ether and ursolic acid, etc. [14–16]. Traditionally, the paste prepared from leaves of N. leucophylla has been used to cure malarial fever [17]. The leaves have been also used to prepare tea by the local peoples. Further, the essential oil and various extracts of N. leucophylla have been also known to show antifungal and antibacterial activity [18].
But, recent knowledge on the polyphenolic composition of N. leucophylla and antioxidant potential is limited. Therefore, in the present investigation, we focused on the comparison of the percentage yield, qualitative phytochemical composition, antioxidant potential, total polyphenolic and flavonoids content of various extracts obtained from air dried roots of N. leucophylla using different extraction methods [maceration (MM), ultrasound assisted extraction method (UAEM), Soxhlet extraction method (SEM)] and solvents (hexane, chloroform, methanol). Reverse phase high-performance liquid chromatography-photodiode array detector (RP-HPLC-DAD) and gas chromatography mass spectrometry (GC–MS) analysis of bioactive extracts was also carried out to enlighten the chemical compositions of the various extracts.
Materials and methods
Plant material
Nepeta leucophylla plants were collected from Manimahesh hills (at an elevation of >2000 m) near to Hadsar (latitude 32°15′–32.26°N and longitude 76°19′–76.32°E) in Chamba district of Himachal Pradesh on October, 2013. The plant material was identified by Dr. M.I.S. Saggoo (Professor, Department of Botany) of Punjabi University, Patiala and the specimen (voucher no. PUN58876) was deposited in the Herbarium of Punjabi University, Patiala. The plant material was air dried (30 days) and pulverized with the help of electrical grinder and stored in air tight polyethylene bags at low temperature (<4 °C) for further use.
Chemicals
The various standards (HPLC) used in the present study were purchased from Loba Chemie, Otto Chemie, Sigma Aldrich and Thermo Fisher Scientific (Acros organics), India Pvt. Ltd, Mumbai. HPLC grade methanol and acetonitrile were purchased from Merck, India Pvt. Ltd, Mumbai. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and HPLC grade glacial acetic acid were procured from Otto Chemie, India Pvt. Ltd, Mumbai. All other chemicals, different reagents and solvents used in the study were of analytical grade and obtained from Fluka, Loba Chemie, Otto Chemie, Sd-Fine Chemicals and Thermo Fisher Scientific (Acros organics), India Pvt. Ltd. The Millipore water (obtained from Millipore Direct Q 3 with pump, instrument) was used throughout the experimental work.
Extraction of plant material
The various extracts of powdered roots of N. leucophylla were obtained by following methods [19].
Maceration method (MM)
The same plant material (20 g) was extracted three times in a row with 3 × 100 mL of solvent and extraction time was 3 × 32 h. The extraction was carried out in the dark at room temperature (30 ± 3 °C). The liquid extract isolated from the solid residue was filtered twice with whatman filter paper no. 1. Further, the filtrate was evaporated using a rotary vacuum evaporator (at 45 °C) to dryness and the percentage yield was noted. Finally, the dry plant extracts were stored at temperature <4 °C for further studies.
Ultrasound assisted extraction method (UAEM)
In this method, the same plant material (20 g) was extracted three times and each time 120, 90 and 90 mL of solvent were used, whereas the time of extraction was 30, 15 and 15 min, respectively. The extraction was done using temperature controlled sensitive prove at 55 °C (±1 °C). Further processing of different extracts was carried out using the same procedure as described in the above method.
Soxhlet extraction method (SEM)
The powdered roots (40 g) were extracted for 8 h with each solvent (at boiling point of solvent used). After the completion of extraction, the processing of different extracts was carried out in the same manner as illustrated in the previous methods.
Total polyphenolic content (TPC)
The method described by Stoilova et al. [20] was followed for the quantitative assessment of the total polyphenolic content of various extracts using the Folin–Ciocalteu reagent. 10 mL deionized water was mixed with 1 mL of different extracts (1 mg/mL) or standard solution (0–150 mg/L). To this solution, 1 mL of Folin–Ciocalteu phenol reagent was added and the mixture was incubated for 5 min. Then, 2 mL of sodium carbonate solution (20 %) was added to the mixture. Finally, the content was again incubated in the dark for 60 min and the absorbance of the samples was noted at 750 nm on UV–Vis spectrophotometer 1800 (Shimadzu). The gallic acid was used as a standard (Y = 9.788x + 0.0137, R2 = 0.9993). The results were expressed as mg of gallic acid equivalents (GAE)/1 g of dry plant extract (DPE) as mean of three replicates.
Total flavonoids content (TFC)
The aluminum chloride method described by Zhishen et al. [21] was used for the quantification of TFC. 4 mL deionized water was mixed with 1 mL of the different extracts (1 mg/mL) or standard solutions (0–300 mg/L). Then, 0.3 mL of sodium nitrite solution (5 %, w/v) was added to the above mixture. After 5 min incubation, 0.3 mL of aluminum chloride (10 %, w/v) followed by 2 mL of sodium hydroxide (1 M) was added. Further, the deionized water was used to make the total volume 10 mL. Finally, the absorbance was read at 510 nm. The rutin trihydrate was used as standard (Y = 1.1643x + 0.0018, R2 = 0.9984) and the results of TFC were expressed as mg of rutin trihydrate equivalents (RE)/1 g of DPE. Each sample was analyzed in triplicate.
Qualitative analysis of phytochemical
The different root extracts were screened for the presence of alkaloids, carbohydrates, coumarins, diterpenoids, flavonoids, glycosides, lignins, polyphenols, proteins, resins, saponins, steroids and triterpenoids using standard qualitative tests in order to establish their chemical profiles [22, 23]. The results of qualitative analysis of phytochemical were represented as + or − which indicates the presence or absence of a particular SM in the extract.
Antioxidant potential
The following four assays were used for the evaluation of antioxidant potential of different root extracts. The quercetin dihydrate and ascorbic acid were used as standard control in each assay.
DPPH free radical scavenging assay
The DPPH free radical scavenging potential was determined according to the procedure used by Uddin et al. [24]. 0.2 mL of different extracts and standard (1 mg/mL) was mixed with 3 mL of methanolic solution of DPPH (0.004 %). Then, the incubation (30 min) of different samples was done in the dark at room temperature. Finally, the result of absorbance was read at 517 nm. The following equation was used to calculate the results of DPPH free radical percentage inhibition (I %):
where AC and AS are the absorbance of the control and samples or standard. The each sample was analyzed in triplicate.
Nitric oxide scavenging assay
The method demonstrated by Hazra et al. [25] with some modification was used to evaluate the nitric oxide scavenging potential of different extracts. To 0.2 mL of different extracts (1 mg/mL), 0.8 mL of sodium nitroprusside solution (10 mM) prepared in phosphate buffered saline (pH 7.4) was added and different samples were incubated at room temperature for 150 min. Then, 1 mL sulfanilamide (0.33 %, w/v in 20 % glacial acetic acid) was added to each sample and again samples were incubated for 5 min. After this, 1 mL of napthyl ethylenediamine dihydrochloride solution (0.1 %, w/v) was mixed with different sample solutions. Finally, after the incubation of 30 min at room temperature, the absorbance of the solution was read at 540 nm on UV–Vis spectrophotometer. The following equation was used to evaluate the percentage inhibition (I %) of NO free radical:
where AC and AS are the absorbance of the control and samples or standard. The value of percentage inhibition was evaluated in triplicate for each sample.
FRAP assay
The FRAP assay was carried out according to the method of Benzie and Strain [26]. 0.2 mL of different extracts (0.5 mg/mL) and standards (0–300 mg/L) was added to 2.8 mL of FRAP solution [25 mL acetate buffer (300 mM), 2.5 mL of TPTZ (2,4,6-tripyridyl-s-triazine) (10 mM in 40 mM HCl) and 2.5 mL FeCl3∙6H2O (20 mM)]. The samples were incubated for 30 min in the dark at room temperature. Finally, the absorbance was noted at 593 nm. Ferrous sulphate was used as standard (Y = 4.5682x − 0.0347, R2 = 0.9993). Each experiment was carried out in triplicate and the results were expressed as mg of ferrous II equivalent (Fe(II) E)/1 g of DPE.
TAC assay
The phosphomolybdenum reducing method stated by Prieto et al. [27] was used to assess the total antioxidant capacity of the different extracts. 0.3 mL of different extract (1 mg/mL) or standard (0–300 mg/L) was added to 3 mL of the reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). All the samples were incubated at 95 °C for 90 min. After cooling to room temperature, the absorbance of each sample was measured at 695 nm on UV–Vis spectrophotometer. Ascorbic acid was used as standard (Y = 3.5665x − 0.0503, R2 = 0.9992) and the results were represented as mg of ascorbic acid equivalent (AAE)/1 g of DPE. Each sample was evaluated in triplicate.
RP-HPLC-DAD analysis
The composition of polyphenolic compounds in the methanolic extracts obtained by different extraction methods from roots of N. leucophylla were analyzed by a modified method of Wu et al. [28]. The study was carried out in detail using a Waters (USA) 1525 binary pump, a 2998 photo diode array detector (in range 210–400 nm), a 2707 auto-sampler and inline degasser AF. The Sunfire C-18 reversed phase column (125 × 4.6 mm length, 5 µm particle size, Waters (USA) was used for the separations. A gradient mode was used with a solvent A—acetic acid:water (2:98, v/v) and solvent B—1 % acetic acid:acetonitrile (1:1, v/v) to elute the different samples and standards at room temperature. The different samples and standards were dissolved in HPLC grade methanol and were filtered through 0.22 µm Millipore Millex GV (hydrophilic PVDF) syringe filter, whereas the different solvents used for elution were filtered with Millipore solvent filtration assembly having durapore GVWP 0.22 µm, 47 mm filter prior to HPLC injection. 20 µL of sample or standards were injected at the flow rate of 1 mL/min. The elution gradient applied was: B:A (15:85) from 0 to 1 min, B:A (35:65) from 1 to 30 min, B:A (75:25) from 30 to 40 min, B:A (90:10) from 40 to 45 min, B:A (100:0) from 45 to 52 min, B:A (20:80) from 52 to 56 min and B:A (5:95) from 56 to 60 min, respectively. The polyphenolic compounds like benzoic acid, caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, gallic acid, p-hydroxybenzoic acid, syringic acid, vanillic acid, trans-cinnamic acid, catechin hydrate, myricetin, quercetin dihydrate and rutin trihydrate were used as standards. Each sample was examined in triplicate. The polyphenolic compounds were identified by comparing their retention times and UV–Vis chromatogram to the commercial standards and their quantification were carried out on the basis of peak areas and by using calibration curves drawn for each standard (Linear equations used and their respective R2 are provided in supplementary data). The method proved to be very sensitive as displayed from the values of average recovery (75–86 %), limits of detection (LOD) (0.15–0.69 µg/g) and limits of quantification (LOQ) (0.79–2.1 µg/g). The results of polyphenolic composition were represented as ug/g of DPE.
GC–MS analysis
There are various reports where GC–MS technique was used to analyze the phytochemical composition of various bioactive extracts obtained from plants [29]. In the present study, the different extracts were (1 µL) subjected to GC–MS analysis in Shimadzu (GC-2010 Plus) GC system coupled with Mass spectroscopy (GC–MS-QP 2010 Ultra). RTx-5Sil MS capillary column (30 m × 0.25 mm × 0.25 mm, Restesk USA) was used during the study. Helium was used as carrier gas at a flow rate of 1 mL/min and split ration was 1:5. The injection temperature was 280 °C and the oven temperature was programmed as: Initial temperature was 100 °C for 1 min and then increased to 250 °C at the rate of 4 °C/min after hold of 5 min temperature further increased to 280 at the rate of 5 °C/min. Finally temperature was hold at 280 °C for 30 min. Mass spectra were obtained at 70 eV using a spectral range of 40–700 m/z with electron ionization (EI) mode. Ion source and interface temperature was maintained at 200 and 280 °C. The various components were identified on the basis of comparison of their mass spectral data with mass spectral database of Wiley8 and NIST11 library. The method proved to be very sensitive as displayed from the values of average recovery (85–96 %), limits of detection (LOD) (0.002–0.14 µg/g) and limits of quantification (LOQ) (0.03–0.30 µg/g).
Statistical analysis
The results have been expressed as the mean ± SD. The results were analyzed by one way analysis of variance (p < 0.05) followed by Duncan’s multiple range test. The marked correlations (among the different assay) were significant at p < 0.05. The results were obtained by the computer programmes: MS excel and Statistica 7 software.
Results and discussion
Extractive yield
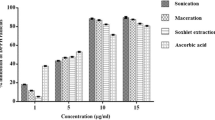
The extraction methods and solvent used largely affect the percentage yield of different extracts which varied from 0.9 to 8.07 % (Fig. 1). Among the solvents used, methanol showed the best extraction efficiency, which may be ascribed to its polar nature because the polar solvents facilitates the solubility of SMs through deep penetration in plant cells [30]. The similar trend of percentage yield (methanol > chloroform > hexane) was also reported in case of Torilis leptophylla whole plant [31]. The order of percentage yield of chloroform and hexane extracts obtained by different extraction techniques was UAEM > SEM > MM. The higher percentage yield in the extracts obtained by UAEM in lesser time is due to the phenomenon of acoustic cavitation generated by ultrasound waves in the solvent, which disrupts the cell wall and helps the solvent to penetrate deep into the plant cell. Owing to this, there is an increase in the diffusion of solutes from the plant cell to the solvent [32]. On the other hand, in case of methanol extract the order was SEM > MM > UAEM which may be attributed to the fact that in case of SEM the exhaustive extraction of plant material was carried out. Similar results, where the SEM attained the highest percentage yield in polar extracts as compared to other extraction methods were reported in the case of aerial parts of Potentilla atrosanguinea [30], leaves, fruit, pulp and seed of Hippophae rhamnoides [33], the bark of Pinus radiate [34].
Total polyphenolic content
The polyphenolic compounds act as efficient antioxidants such as metal chelators and radical scavengers due to this the antioxidant potential of various plant extracts is assigned to the content of polyphenolic compounds in these extracts [35]. The TPC in the studied extracts ranged from 2.25 to 94.02 mg GAE/g DPE (Table 1). The TPC of different solvents used was in the order of methanol > chloroform > hexane. The higher TPC of methanol extract may be attributed to the polar nature of most of the polyphenolic compounds, which increases their solubility in methanol. A similar trend of TPC was also found previously in the case of whole plant of T. leptophylla [31] and the bark of Terminalia arjuna [36]. Among the extraction methods, the TPC of methanol and chloroform extract followed the order SEM > MM > UAEM. But, the hexane extracts did not show the presence of any polyphenolic compound, which may be attributed to its non polar nature. Milic et al. [37] also reported similar results for TPC in case of aerial parts of Galium mollugo. Further, the lower polyphenolic content in different extracts obtained by UAEM may be due to some kind of oxidation and degradation of the some SMs under ultrasonication conditions [32].
Total flavonoids content
The TFC of various extracts varied from 5.73 to 228.75 mg RE/g DPE (Table 1). The TFC in different extracts followed the order methanol > chloroform > hexane with respect to solvents used. These results were in good correlation with the findings of Shahriar et al. [36] where methanol extract of T. arjuna bark had higher TFC followed by chloroform and hexane extract, respectively. The TFC of the methanol extract obtained by different extraction techniques was in the order of UAEM > SEM > MM. A similar result, where TFC of methanol extract increases with sonication was reported previously by Karabegovic et al. [38] in cherry laurel leaves and fruit. Further, the order of TFC of chloroform and hexane extract followed the order SEM > MM > UAEM and SEM > UAEM > MM.
Qualitative analysis of phytochemicals
The results of phytochemical analysis revealed the presence of carbohydrates, coumarins, diterpenoids, flavonoids, glycosides, polyphenols, steroids and triterpenoids in the different extracts. According to qualitative analysis of phytochemicals, the phytochemical composition of different extracts followed the order Methanol > chloroform > hexane with respect to the solvents used, which revealed the superiority of methanol among the solvents used in the extraction of secondary metabolites. On the other hand, the different extraction techniques had no significant effect on the composition of SMs in the different extracts. The present results were in good agreement with the previous reports of the other research groups in case of Nepeta cataria, Nepeta praetervisa and Nepeta nepetella [13, 39, 40] (Table 2).
Antioxidant potential
The antioxidant potential of natural antioxidants has been attributed to various mechanisms like binding to transition-metal ion catalysts, decomposition of peroxides, radical scavenging, reductive capacity, prevention of chain initiation and continued hydrogen abstraction [41]. Due to this, a single antioxidant assay does not give a correct profile of antioxidant potential of plant extracts. Therefore, it is always better to evaluate the antioxidant potential by a number of different methods in order to draw a complete antioxidant profile of the plant extracts. The results of antioxidant potential of various antioxidant assays are shown in Table 1.
DPPH free radical scavenging assay
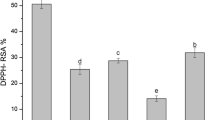
The hydrogen donating power of the natural antioxidant affects the stability of dark violet colored DPPH free radical solution. Larger the hydrogen donating power more rapidly the violet color of DPPH solution gets discharged [42]. The DPPH free radical scavenging potential of the different extracts ranged between 9.3 and 91.4 %. The order of percentage inhibition of DPPH with respect to the solvents used was methanol > chloroform > hexane. This order was in good agreement with the order of TPC and TFC indicating that the polyphenolics were the principal compounds responsible for the antioxidant potential of tested extracts. Previously, Prasad et al. [43] and Shahriar et al. [36] reported similar results with same order in different extracts obtained from leaves of Ipomoea aquatic and bark of T. arjuna. The results of DPPH percentage inhibition of methanol extract followed the order SEM > MM > UAEM, whereas in case of chloroform and hexane extract the order was SEM > UAEM > MM. The ascorbic acid and quercetin used as standard and showed the percentage inhibition of 58.42–89.52 %. The results of percentage inhibition of methanol extracts were comparable to that of the standard used. The present results were in good agreement with previous finding, where the extracts isolated by conventional extraction methods (SEM and MM) using polar solvents had high potential to scavenge DPPH free radical in the solution [44, 45].
Nitric oxide scavenging assay
In this assay, sodium nitroprusside spontaneously produces nitric oxide at physiological pH in the aqueous solution, which reacts with oxygen and causes the production of nitrite ions which can be measured using Greiss reagent. The natural antioxidants scavenge nitric oxide, compete with oxygen, resulting in reduced generation of nitrite ions [46].
The results of NO radical percentage inhibition varied from 2.58 to 51.6 %. However, with respect to the solvent used, the NO percentage inhibition results followed the order methanol > chloroform > hexane. This trend was similar to that of DPPH percentage inhibition results, which directly correlated to the polyphenolic contents of these extracts. Shahriar et al. [36] found similar results in various extracts obtained from the bark of T. arjuna. With respect to extraction techniques, the NO percentage inhibition of methanol extracts followed the order SEM > UAEM > MM, whereas for chloroform and hexane extracts the order was UAEM > SEM > MM. The ascorbic acid and quercetin were used as standard and showed the percentage inhibition of 48.85–75.48 %, which was comparable to the methanol extracts obtained by various extraction methods. The similar results were also reported previously by Aly et al. [47] where the different polar extracts obtained by SEM from aerial parts of N. cataria had higher NO percentage inhibition potential as compared to medium polar and non-polar extracts.
FRAP assay
In the FRAP assay the antioxidant potential of different extracts was estimated based upon their power to reduce 2,4,6-tripyridyl-s-triazine–Fe(III) [TPTZ–Fe(III)] complex to TPTZ–Fe(II) complex. The production of Fe(II) can be estimated from the formation of colored complex during the reaction. The results of FRAP values of the different extracts varied from 2.54 to 130.75 mg Fe(II) E/g DPE. The results of FRAP values for different extracts were ranked as methanol > chloroform > hexane with respect to the solvent used. Further, the order for methanol and chloroform extracts with respect to different extraction techniques was SEM > MM > UAEM, whereas the hexane extracts followed the order SEM > UAEM > MM. The ascorbic acid was used as a positive control and showed the FRAP value of 385.3 mg Fe(II) E/g of ascorbic acid, which was much higher than the FRAP values of all the tested extracts. The results of FRAP values were in good correlation with total polyphenolic and total flavonoids content of different extracts.
Total antioxidant capacity
In this assay, Mo(VI) was reduced to Mo(V) with the formation of a green phosphate/Mo(V) complex at acid pH, which was read at 695 nm. The results of TAC of the different extracts ranged between 2.81 and 40.8 mg AAE/g DPE. The order of TAC results for various extracts was methanol > chloroform > hexane based on the different solvents used. Shahriar et al. [36] also reported alike result, previously in case of different extracts obtained from the bark of T. arjuna. The results revealed that the methanol extract obtained by UAEM had the highest value of TAC followed by MM and SEM, respectively. Whereas, the order of TAC values for chloroform and hexane extract was SEM > MM > UAEM and SEM > UAEM > MM. The quercetin was used as a positive control and showed a TAC value of 488.95 mg AAE/g of quercetin, which was much higher that the different extracts examined in the present study.
The present results revealed that the antioxidant potential, total polyphenolic and flavonoid content of different extracts obtained by different extraction methods using solvents of different polarity varied significantly. It was also confirmed from the one way ANOVA (p < 0.05) of the all results followed by Duncan’s multiple range test (Table 1).
The highest antioxidant potential of methanol extracts over chloroform and hexane extracts may be attributed to the presence of high content of the polyphenolic and flavonoids. Further, it was observed that the most of extracts obtained by SEM had higher antioxidant potential as compared to the corresponding extracts isolated with UAEM and MM, which may be due to the higher TPC and TFC in these extracts. Similar results were also observed by Kalia et al. [30] in case of different extracts obtained by SEM from aerial parts of Potentilla atrosanguinea.
The present results showed that the polyphenolic and flavonoids were the key compounds present in the different extracts, which directly relates to their antioxidant potential. These results were also supported by previously published data, which revealed that the higher polyphenolic content of different extracts was the main reason for their higher antioxidant potential [48]. This activity of polyphenolic compounds is supposed to be primarily due to their ability to quench different free radical reactions and to decompose peroxides present in the living systems [35].
RP-HPLC-DAD analysis of polyphenolic composition
The polyphenolic composition of different bioactive extracts (methanol extract) obtained from root of N. leucophylla were analyzed using RP-HPLC-DAD (Fig. 2). Out of the fourteen polyphenolic standards analyzed, five were recognized in each methanol extract. The overall concentration of identified polyphenolic compounds was higher in the case of methanol extract isolated using SEM followed by MM and UAEM, respectively. Out of 14 analyzed standards, no polyphenolic compound was observed in chloroform and hexane extracts isolated by various extraction methods. The concentration of various polyphenolics in the different methanol extracts follows the order myricetin > caffeic acid >catechin hydrate > vanillic acid > gallic acid (Table 3). Caffeic acid, ρ-coumaric acid, gallic acid and ferulic acid were also identified previously in other species of genus Nepeta [7]. To the best of our knowledge, this is the first study where the polyphenolic profiles of N. leucophylla root extracts were determined.
RP-HPLC chromatogram of methanol extract obtained by a Soxhlet extraction method, b Ultrasound assisted extraction method, c Maceration method. (a-1, b-1 and c-1 was the zoom of a, b and c chromatogram.). Targeted compounds are labeled with numbers: gallic acid (1), catechin hydrate (2), caffeic acid (3),vanillic acid (4), myricetin (5)
GC–MS analysis
GC–MS is the one of the most sensitive techniques used for the detection of bioactive constituents of various plant extracts. The GC–MS study of various plant extracts was also carried out previously for the determination of different biological properties viz. antimicrobial, antiproliferative, anticancer and chemopreventive properties, etc. [29].
The GC–MS analysis of most bioactive hexane, chloroform and methanol extracts (among different hexane, chloroform and methanol extracts) obtained by the Soxhlet method showed the presence of 23, 30 and 16 constituents, respectively. The hexane extract resulted in major long chain hydrocarbons and steroids classes of non polar compounds. On the other hand, chloroform and methanol extracts showed the presence of phenolic and benzoid classes of compounds along with steroids, fatty acid and long chain hydrocarbons classes of non polar compounds. Table 4 shows the list of major constituents detected in the analyzed extracts while Fig. 3 represents the GC–MS chromatograms of hexane, chloroform and methanol extracts. So, the antioxidant potential of these extracts probably attributed to the presence of these phenolic, benzoid, steroids and fatty acid classes of non polar compounds.
The GC–MS analysis of methanol extract obtained from leaf of Cinnamomum iners [29] ethanol and acetone extracts of Aloe vera leaves [49] also corresponded to major fatty acid and steroids classes of non-polar compounds. These constituents showed a broad range of applications in various pharmaceutical, nutraceutical and food industries [29]. Thus, the various bioactive root extracts of N. leucophylla can also be used as a potent source for the isolation of these bioactive constituents.
Correlation
The correlations among the results of the DPPH scavenging activity, NO scavenging activity, FRAP, TAC, TPC and TFC for various extracts obtained by different extraction methods from dried roots of N. leucophylla are given in Table 5. There was good correlation between the results of different antioxidant assays, total polyphenols and flavonoids content as shown by correlation coefficients (r). The r-values were in the range of 0.91–1. The values of r for the correlation of TPC results with DPPH, NOS, FRAP and TAC were equal to 1, 0.97, 1, 0.98, respectively. On the other hand, the values of r for the correlation of TFC results with DPPH, NOS, FRAP and TAC were equal to 0.99, 0.95, 0.99, 0.98, respectively, which revealed that there existed a direct relationship between various antioxidant assays and polyphenolic and flavonoids content. Further, the results of various antioxidant assays showed good correlation among themselves also. These results confirmed that the antioxidant potential of different root extracts of N leucophylla is due to the presence of various kinds of polyphenolic compounds. These results are in accordance to the previously published data, where the highest antioxidant potential of different plant extracts is attributed to their higher total polyphenolic content [50].
Conclusion
To the best of our knowledge, this is the first report in which antioxidant potential, polyphenolic and phytochemical composition of N. leucophylla roots were evaluated. The highest polyphenolic composition and antioxidant potential was reported in the methanol extracts. Among the various extraction methods, the Soxhlet extraction method provided the best results of percentage yield, TPC, FRAP, percentage inhibition of DPPH and NO, whereas UAEM gave best results in case of TFC and TAC. The extract obtained by SEM and UAEM showed comparable antioxidant potential, but the time of extraction in case UAEM was very less, which makes it cheap and economical in contrast to the traditional extraction methods used. The results of DPPH scavenging, NOS, FRAP and TAC assay showed good correlation with that of TPC and TFC. The GC–MS analysis of hexane, chloroform and methanol extracts showed the presence of long chain hydrocarbons, fatty acid, steroids and benzoid classes of non-polar compounds, whereas the RP-HPLC-DAD analysis of methanol extracts revealed the presence of five polyphenolic compounds out of which Myricetin, caffic acid and catachin were the major ones. The high antioxidant potential and polyphenolic content of N. leucophylla roots clearly highlight its potential as the potent source of natural antioxidants that may promote good health, reduce the risk of degenerative diseases and replace synthetic antioxidants for their use in food and pharmaceutical industries.
References
D. Huang, B.R.L. Ou, J. Agr. Food Chem. 53, 1841–1856 (2005)
H. Sies, Redox Biol. 4, 180–183 (2015)
S. Santanu, K.M. Upal, K.P. Dilip, P. Sambit, J. Sourabh, Iran J. Pharm. Res. 9, 75–82 (2010)
R. Blomhoff, M.H. Carlsen, L.F. Andersen, D.R. Jacobs, Br. J. Nutr. 96, 52–60 (2006)
E. Choe, D.B. Min, Compr. Rev. Food Sci. Food Saf. 8, 345–358 (2009)
J. Hussain, G. Abbas, S. Ahmed, Afr. J. Biotechnol. 8, 935–940 (2009)
C. Formisano, D. Rigano, F. Senatore, Chem. Biodivers. 8, 1783–1818 (2011)
A. Sharma, D.S. Cannoo, Pharmacophore 4, 181–201 (2013)
N. Micelia, M.F. Tavianoa, D. Giuffridab, A. Trovatoa, O. Tzakouc, E.M. Galati, N. Kahkeshani, J. Ethnopharmacol. 97, 261–266 (2005)
U. Suschkea, F. Sporera, J. Schneelea, H.K. Geissb, J. Reichlinga, Nat. Prod. Commun. 2, 1277–1286 (2007)
A. Shafaghat, K. Oji, Nat. Prod. Commun. 5, 625–628 (2010)
P. Salehi, A. Sonboli, L. Allahyari, J. Essent. Oil Bear. Plants 10, 324–331 (2007)
T.I. Edewor, L.A. Usman, Afr. J. Pure Appl. Chem. 5, 503–506 (2011)
C.S. Mathela, A. Gupta, P. Upreti, A.K. Pant, M.M. Olmstead, H. Hope, J. Nat. Prod. 54, 910–912 (1991)
A.T. Bottini, V. Dev, G.C. Shah, C.S. Mathela, A.B. Melkani, A.T. Nerlo, N.S. Strum, Phytochemistry 35, 1653–1657 (1992)
J. Saxena, C.S. Mathela, Appl. Environ. Microbiol. 62, 702–704 (1996)
V.K. Bisht, C.S. Rana, J.S. Negi, A.K. Bhandari, V. Purohit, C.P. Kuniyal, J. Med. Plants Res. 6, 4281–4291 (2012)
D.S. Bisht, C.S. Mathela, P. Lal, L. Singh, V. Pande, R.C. Padalia, J. Serb. Chem. Soc. 75, 739–747 (2010)
H.V. Annegowda, M.N. Mordi, S. Ramanathan, M.R. Hamdan, S.M. Mansor, Food Anal. Methods 5, 226–233 (2012)
I. Stoilova, A. Krastanov, A. Stoyanova, P. Denev, S. Gargova, Food Chem. 102, 764–770 (2007)
J. Zhishen, T. Mengcheng, W. Jianming, Food Chem. 64, 555–559 (1999)
J.B. Harborne, Phytochemical Methods (Springer Pvt. Ltd, New Delhi, 2005), p. 17
C.K. Kokate, A.P. Purohit, S.B. Gokhale, Pharmacognosy (Nirali Prakashan, Pune, 2009), pp. 6.16–6.17
S.N. Uddin, M.E. Ali, M.N. Yesmin, Amer. J. Plant Physiol. 3, 96–100 (2008)
B. Hazra, S. Biswas, N. Mandal, B.M.C. Complement. Altern. Med. 8, 63–73 (2008)
I.F.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70–76 (1996)
P. Prieto, M. Pineda, M. Aguilar, Anal. Biochem. 269, 337–341 (1999)
J.W. Wu, C.L. Hsieh, H.Y. Wang, Food Chem. 113, 78–84 (2009)
N.K. Udayaprakash, M. Ranjithkumar, S. Deepa, N. Sripriya, A.A. Al-Arfaj, S. Bhuvaneswari, Ind. Crop Prod. 69, 175–179 (2015)
K. Kalia, K. Sharma, H.P. Singh, B. Singh, J. Agric. Food Chem. 56, 10129–10134 (2008)
N. Saeed, M.R. Khan, M. Shabbir, B.M.C. Complement. Altern. Med. 12, 221–232 (2012)
M. Vinatoru, Ultrason. Sonochem. 8, 303–313 (2001)
U.K. Sharma, K. Sharma, N. Sharma, A. Sharma, H.P. Singh, A.K. Sinha, J. Agric. Food Chem. 56, 374–379 (2008)
E. Aspe, K. Fernández, Ind. Crop Prod. 34, 838–844 (2011)
V. Louli, N. Ragoussis, K. Magoulas, Bioresour. Technol. 92, 201–208 (2004)
M. Shahriar, S. Akhter, M.I. Hossain, M.A. Haque, M.A. Bhuiyan, J. Med. Plants Res. 6, 5286–5298 (2012)
P.S. Milic, L.P. Stanojevic, K.M. Rajkovic, S.M. Milic, V.D. Nikolic, L.B. Nikolic, V.B. Veljkovic, Hem. Ind. 67, 89–94 (2013)
I.T. Karabegovic, S.S. Stojicevic, D.T. Velickovic, Z.B. Todorovic, N.C. Nikolic, M.L. Lazic, Ind. Crop Prod. 54, 142–148 (2014)
G. Fareed, N. Afza, A. Malik, U.R. Mughal, R.B. Tareen, M.A. Varsiani, M. Fareed, M. Lateef, J. Chem. Soc. Pak. 35, 1366–1370 (2013)
M. Seladji, C. Bekhechi, F. Beddou, H. Dib, N. Bendimerad, J. Appl. Pharm. Sci. 4, 12–16 (2014)
A. Yildirim, A. Mavi, M. Oktay, A.A. Kara, O.F. Algur, V. Bilaloglu, J. Agr. Food Chem. 48, 5030–5034 (2000)
G. Miliauskas, P.R. Venskutonis, T.A. Van Beek, Food Chem. 85, 231–237 (2004)
K.N. Prasad, S. Divakar, G.R. Shivamurthy, S.M. Aradhya, J. Sci. Food Agric. 85, 1461–1468 (2005)
V. Povilaityte, P.R. Venskutonis, J. Am. Oil Chem. Soc. 77, 951–956 (2000)
A. Dapkevicius, T.A. van Beek, G.P. Lelyveld, A. Van Veldhuizen, A. de Groot, J.P.H. Linssen, P.R. Venskutonis, J. Nat. Prod. 65, 892–896 (2002)
A. Kumaran, R.J. Karunakaran, LWT Food Sci. Technol. 40, 344–352 (2007)
H.F. Aly, M.E. Ebrahim, H.M. Metawaa, E.A.A. Hosni, F.M. Ebrahim, J. Am. Sci. 6, 364–386 (2010)
A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouma, P. Stocker, N. Vidal, Food Chem. 97, 654–660 (2006)
S. Arunkumar, M. Muthuselvam, World J. Agri. Sci. 5, 572–576 (2009)
M. Holasova, V. Fiedlerova, H. Smrcinova, M. Orsak, J. Lachman, S. Vavreinova, Food Res. Int. 35, 207–211 (2002)
Acknowledgments
The authors are grateful to DST (Department of Science and Technology), Govt. of India, New Delhi for financial assistance under INSPIRE program (INSPIRE Code IF120715). Further, the authors are also grateful to Prof. M.I.S. Saggoo (Department of Botany, Punjabi University, Patiala) for his help in the identification of plant material and Sant Longowal Institute of Engineering and Technology (SLIET), Longowal, Sangrur (PB) for providing necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We certify that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, A., Cannoo, D.S. Comparative evaluation of extraction solvents/techniques for antioxidant potential and phytochemical composition from roots of Nepeta leucophylla and quantification of polyphenolic constituents by RP-HPLC-DAD. Food Measure 10, 658–669 (2016). https://doi.org/10.1007/s11694-016-9349-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9349-5