Abstract
Selected moth beans (Vigna aconitifolia) were subjected to different processes such as sprouting and cooking. Their respective flours were prepared and evaluated for their physicochemical and functional characteristics. From our study, it was observed that the ash content of raw moth bean flour was considerably higher in comparison to the sprouted and cooked moth bean flour. On the other hand, the crude lipid and fiber content of sprouted moth bean flour were remarkably higher compared to raw and cooked moth bean flour, respectively. The raw moth bean flour exhibited considerably better emulsifying activity compared to the sprouted moth bean flour. Sprouted bean flour was showing higher emulsion stability than the raw bean flours and the cooked bean flour reported zero emulsion stability. The value of foaming stability was not significantly different among raw and sprouted moth bean, but it was significantly low in cooked moth bean flour. Raw moth bean flour was found to exhibit higher gelation concentration than the sprouted and cooked flours. This study highlights the variations observed in the physicochemical and pasting characteristics of moth bean seeds (raw, sprouted and cooked) and their respective flours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulses, being rich in protein are of great importance in the diet of low-income people. They are being considered as the second most crucial staple food after cereals. The pulses also contain ample amount of dietary fibers, complex carbohydrates, minerals and vitamins that possess high-energy values (Tharanathan and Mahadevamma 2003). Moth bean (Vigna aconitifolia), an underutilized legume is known to be a possible source of protein and different nutrients. Globally, the mature moth bean seeds are cultivated and consumed by humans, mainly from underdeveloped countries (Siddhuraju and Becker 2005). However, there is confined use of leguminous products such as the moth bean because of the existence of anti-nutritional elements such as saponins, phytic acid, trypsin inhibitors (Reyes-Bastidas et al. 2010). The pulse seeds thus require further processing so as to enhance their dietary constituents and to reduce their anti-nutritional elements such as phytic acid, trypsin inhibitor prior to consumption (Martín-Cabrejas et al. 2009). Sprouting has been reported as a potent process to eliminate anti-nutrient factors and catalyzing secondary metabolites like phytates and α-galactosides. Anti-nutrients like trypsin inhibitors and flatulence-causing oligosaccharide levels can be reduced or inactivated by the cooking process (Hamid et al. 2016). Within the cultivars of moth bean, the antitryptic activity is known to vary considerably from 190 to 272 unit g−1 (Gupta et al. 2016). Moth bean contains comparatively low amounts of saponin than chickpea and soybeans and minor quantity of tannins are found in the moth bean seed (Deshpande and Campbell 1992). Also, considerably lesser amount of anti-nutritional factors has been indicated in moth bean in contrast with various other common legume grains (Jain et al. 2009). It must replicate on the overall higher nutrient content of the seeds of this nourishment legume.
Soaking, roasting, sprouting, and cooking are the conventional processing methods used before consumption of moth bean seeds. Cooked and fried moth bean dal and whole seeds are consumed, and sprouted seeds are taken as breakfast food which is an excellent habit of the rural Indian population. However, high heat cooking is the commonly used processing technique for pulses which results in a considerable depletion of oligosaccharides, phenolic content, minerals, tannins and phytic acid (Wang et al. 2009). Hence, the sprouting of pulses is considered as a powerful method for maximizing their antioxidant activity (Świeca et al. 2012).
Utilization of pulses flour is an emerging trend to develop functional foods, depending on nutritional compositions. The utilization of bean flours as additives for value addition of making food products such as breads, biscuits, pasta, cakes has been investigated previously (Han et al. 2010). As per our information, not one of the reports has yet been published on the physiochemical attributes of raw, sprouted and cooked moth bean flours. This study focused on assessing the consequences of sprouting and cooking on the physical and functional attributes of moth bean (V. aconitifolia).
Materials and methods
Materials
Fresh seeds of moth bean (V. aconitifolia) were procured from a local market in Pune, India. The seeds were washed and cleaned to eliminate any extraneous materials and were then subjected to drying at 40 °C for 8 h (Memmert GmbH+Co. KG, D-91126 Schwabach, Germany). For further analysis, the airtight containers were used to store the seeds at room temperature.
Processing methods
Raw seed powder
Moth bean seeds (200 g) were powdered with a blender (Philips HP 2100/01Thailand) and sieved to get 300 µm particle size (USA Standard Testing Sieve No. 50). The airtight glass jars were then used to store the seed flours at 25 °C till further use.
Sprouting
Moth bean seeds (200 g) were rinsed and subjected to soaking for 6 h in 600 mL distilled water (DW) at 25 °C. Sprouting was done in dark conditions for 48 h at 25 °C. The sprouted seeds were then oven dried for 10 h at 50 °C, ground with a blender (Philips HP 2100/01 Thailand) and sieved through a 300 µm sieve (USA Standard Testing Sieve No. 50). The powder was then stored at room temperature in airtight glass jars till further use.
Cooking
DW (500 mL) was used to rinse and soak the moth bean seeds (200 g) for 4 h. This was followed by cooking at 100 °C. Hardness of the seeds was evaluated by crushing them between two glass slides (Seena and Sridhar 2005). The complete absence of internal white core of crushed seeds indicated that they were fully cooked. Cooked moth seeds were then oven dried at 50 °C till they dried completely. Dried cooked seeds were then ground using a grinder (Philips HP 2100/01) and sieved through a 300 µm sieve (USA Standard Testing sieve No. 50). The powder was stored in airtight glass jars at 25 °C till further use.
Proximate analysis
The moisture, fat, ash, protein and crude fiber content of the raw, sprouted and cooked moth bean flour samples were determined and calculated using the AOAC official methods (AOAC 2000). Carbohydrate content was calculated by the difference.
Physical properties
Seed weight
Moth bean grains (100 kernels) were selected randomly, counted and weighed. The results were then expressed in grams (Kaur et al. 2009).
Seed volume
DW (25 mL) was quantified using a 50 mL measuring cylinder that was used to transfer the seeds (n = 50) for measuring seed volume. Instantly, the differences in the volume were noted and the volume per seed was determined after dividing by 50.
Bulk density
Bulk density of beans was quantified following a slightly modified method of Kaur et al. (2009).
Husk content
10 g of seeds were submerged in DW (50 mL) for 12 h at 25 °C. An oven was used to dry the husks and cotyledons separately for 12 h at 70 °C, which were then cooled at 25 °C for an hour. The calculation of husk content was then done after weighing the dried materials.
Minimum cooking time
Minimum cooking time was measured with slight modifications according the method of Kaur et al. (2009).
Hydration capacity
A measuring jar with 100 mL of DW was used to keep the enumerated seeds or cotyledons (100 g) at 25 °C. After 24 h, the water was exuded out; seeds/cotyledons were blotted so as to get rid of the adhered surface water and their weight was measured (Hamid et al. 2016). The hydration capacity was then calculated as follows:
Swelling capacity
The enumerated seeds or cotyledons (100 g) were used to determine their volume prior to soaking and were then kept for soaking overnight in distilled water. A graduated cylinder was used to measure the soaked seeds and cotyledon volume (Hamid et al. 2016). The swelling capacity of the seeds/cotyledons was evaluated as follows:
Color parameters of whole beans and bean flours
The determination of color from seeds and flours was done following the method of Siddiq et al. (2010) with a Hunter Colorimeter model D25 L optical sensor (Hunter Associates Lab, Virginia, USA). A thick layer of beans or flour was kept in the measurement cup, and measured for color following the L*, a*, and b* system.
The values of Croma and hue angle were quantified as follows:
Functional properties of moth bean flour
Least gelation concentration (LGC)
LGC of bean flour was determined following a slightly modified method of Ajibola et al. (2016). The flour at different concentrations (2–30% w/v) was dispersed in test tubes containing DW and kept for heating in a water bath for an hour at 95 °C. The samples were then allowed to cool to 10 °C for 14 h in inverted tubes. Visual observation was done to check for any drops slipping out from the emulsion and was recorded for LGC determination. The results of LGC were illustrated in terms of no (−), complete (+), or partial (±) gelation.
Foaming properties
The foaming properties of bean flours were assessed following a slightly modified method of Siddiq et al. (2010). DW (100 mL) was utilized to disperse the flour (2 g) which was then homogenized at 20,000 rpm for 2 min with IKA stirrer (RW20, Germany). By using the following equation, the foaming capacity was determined:
The remaining foam volume following incubation at 25 °C for 8 h was used to determine the foam stability which was illustrated in terms of percentage of the primary foam volume.
Emulsifying properties
The emulsifying activity (EA) and stability (ES) of bean flour was determined as stated by Neto et al. (2001) with few changes. The flour suspension (50 mg of flour dispersed in 5 mL DW) was subjected to homogenization with soybean oil (5 mL) to form emulsions. The emulsions obtained were then subjected to centrifugation (1100 × g, 5 min) (Hsiang Tai, CN 2060 made in Taiwan) and the total height and height of the serum layer were documented. The EA was then evaluated as follows: emulsifying activity (%) = (Height of serum layer/Total height) × 100
Prior to centrifugation (1100 × g, 5 min), the emulsion samples were heated at 80 °C for 30 min to determine the emulsion stability. The ES was then calculated by using as follows:
Pasting properties
Rapid Visco Analyzer (Model: RVA-4, Newport Scientific Pvt., Ltd. Australia) was employed to evaluate pasting characteristics of the bean flours. The flour suspensions with a total weight of 29 g were then used to record the viscosity profiles of the flours. The suspensions were subjected to heating from 50 to 95 °C at 6 °C min−1 (following a 1 min equilibration period at 50 °C), held at 95 °C for 5 min, cooled from 95 to 50 °C at 6 °C min−1 and again held at 50 °C for 2 min (Nasrin et al. 2015).
Thermal properties
The thermal properties of different moth bean flours were analyzed with a differential scanning calorimeter, (DSC-1 STAR System, Mettler-Toledo, Switzerland). Before DSC analysis, flour samples (14 mg) were kept in aluminum pans, sealed and incubated at room temperature for 1 h. Thermal transitions were then characterized as To (transition onset temperature), Tp (transition peak temperature) and ΔH (transition enthalpy). The triplications were made for each sample which was scanned from 20 to 110 °C at a heating rate of 10 °C min−1. An empty aluminum pan was utilized as the reference. Thermal transitions were acquired and recorded as thermograms (Henshaw et al. 2003).
Field emission scanning electron microscopy (FE-SEM)
FE-SEM (Hitachi High Technologies Corporation, Tokyo, Japan) was conducted by applying accelerating voltages in the range of 1–7 kV and was used to examine the morphology of the moth bean flours. The particle size of flours in images was measured by using ImageJ software (Schneider et al. 2012).
Statistical analysis
All estimations were performed in triplicate for each sample, and the results obtained were reported as the mean ± standard error. Statistical analysis was then conducted utilizing a statistical analysis software (SPSS Inc., Chicago, IL, USA) and Tukey’s multiple range test was utilized for determining significant differences (P < 0.05) among the mean values.
Results and discussion
Proximate composition
The proximate compositions of moth bean flours (raw, sprouted and cooked) are shown in Table 1. The raw moth bean flour were reported to have 3.47, 3.38, 0.38, 6.00, 23.63 and 63.17 g 100 g−1 ash, moisture, crude lipid, crude fiber, protein and carbohydrate contents, respectively. The ash, moisture and crude protein content of raw moth bean flour were found remarkably higher compared to the sprouted and cooked moth bean flour. On the other hand, crude lipid and fiber contents of sprouted moth bean flour (0.60 g and 7.00 g 100 g−1) were found substantially elevated in comparison to the raw (0.38 g and 6.00 g 100 g−1) and cooked moth bean flour (0.35 g and 6.70 g 100 g−1). Carbohydrate content was found higher in cooked moth bean flour (75.29 g 100 g−1) followed by sprouted (69.93 g 100 g−1). The results obtained were found similar to that reported by Seena and Sridhar (2005) for the composition of Canavalia legume flour.
The current investigation revealed that a significant decrease was noticed in the ash content of the treated samples with a significant decrease observed for the cooked samples (P < 0.05). The variations in ash content subsequent to soaking for a particular time was because of the loss of minerals due to rootlet; however, the decrease in ash content may be because of the draining out of macro and micro elements from the flour throughout soaking and cooking. Comparable observation have been accounted for in past investigations of processed brown rice that were pre-germinated (Ohtsubo et al. 2005), and microwave cooked germinated legumes (Khatoon and Prakash 2006).
Moisture content was found decreased following the different processing treatments, but a significant decrease was observed in sprouted moth bean only (P < 0.05). It is known that during germination, legumes absorb the surrounding water for the commencement of metabolic process. Dry legumes ingest water quickly; however, it can be affected by the legume structure. The increment in water absorption over a time period is because of the expanding cell number inside the seed getting to be hydrated. On the contrary, Khatoon and Prakash (2006) reported a higher moisture content for germinated legumes such as Bengal, green and horse gram.
Lipid content was found increased for the sprouted moth bean sample but no significant differences in the lipid content of raw and cooked moth bean samples were observed. On the contrary, it has been reported by previous studies that as the germination time increased, it led to an increase in the fat content of soybeans (Dhaliwal and Aggarwal 1999), mung bean, pea and lentil seeds and some legume seeds (Ghavidel and Prakash 2006). The elevation of crude lipid content of sprouted seeds can be endorsed to increase in essential fatty acids due to increase in lipase activity. An increment in lipolytic activity during sprouting causes hydrolysis of triacylglycerols to glycerol and constituent fatty acids.
The sprouted and cooked moth bean samples were found to have a significantly increased content of crude fiber. This indicates that the total dietary fiber amount was affected by the germination process. As the concentration of dietary fiber in sprouted moth bean increases, the sprouted beans are suitable for consumption due to the increased fiber intake. Martín-Cabrejas et al. (2009) revealed that the impact of germination on the concentration of total dietary fiber was reliant on the legume type. The soaked barley, mung bean, peanut and wheat have been reported to have decreased amount of total dietary fiber, however, it was conversely increased in soaked rice and soybean. The process of cooking reduces the insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) content of lentil (Dueñas et al. 2015). Similarly, due to the cooking process, peas demonstrated reduction in SDF (Wang et al. 2008), lentils (Wang et al. 2009) and because of the polysaccharide solubilization that comprises the SDF fraction. The process of sprouting was noted to produce an increase of IDF (34%) and total dietary fiber (TDF) (26%) content of dark beans compared to the raw bean. Furthermore, sprouted lentil noted a decrease in TDF (25%) with a marked decrease of IDF (23%). Similarly, during the germination process an increase in SDF content have been described previously by Martín-Cabrejas et al. (2008). The elevated levels of the crude fiber may be due to the synthesis of new cells and due to by increasing the structural carbohydrates like cellulose and hemicellulose of the plant during sprouting (Wang et al. 2009). The increased crude fiber was noted to be mainly due to the alterations in the polysaccharides found in the cell wall, like cellulose, glucose and mannose.
The sprouted and cooked moth bean found significantly decreased protein content. Similarly, germinated and non-germinated legumes (kidney, peanut, soybean, and mung) and the different types of rice (Barrio, brown, black, red and milled) and germinated pigeon pea (Megat Rusydi et al. 2011) have been reported to have reduced protein content. On the contrary, germinated brown rice found increased protein content (Ohtsubo et al. 2005). Germination of beans enhances the proteolytic activeness, which causes the storage proteins hydrolysis (Ghavidel and Prakash 2007). Decrease in protein content after sprouting and cooking; could be due to the increase in level of protease during sprouting. Increased proteolysis activity during seed sprouting tends to the breakdown of reserve proteins for the allocation of nitrogen resources (free amino acids) to for sprouting process. Cooking causes softening and rupture of tissues that leads to the leaching out water soluble proteins and loss of heat liable protein fractions, which may cause decrease in crude protein content (Wang et al. 2008).
Physical properties
The weight and volume for moth bean (100 seeds) was found to be 3.89 g and 3.0 mL, respectively. The density of moth bean was reported to be 1.30 g mL−1 and their husk content was found to be 8.39 (% w/w). Likewise, the hydration and swelling capacity were accounted for 0.04 g and 0.04 mL seed−1. The hydration and swelling index of moth bean were observed to be 1.00 and 0.39, respectively. Composition of seed, cell wall structure and compactness of cell in seed are responsible for the water absorption capacity of the seed (Kaur et al. 2009). The cooking time for moth bean was 32 min. Physical properties of moth bean have been found to be similar with the properties of Indian black gram (UG 1017) belonging to same family, subfamily and genus of moth bean but different species. The physical properties of black gram have been reported to be as follows: weight of 100 seeds (3.8 g), volume (3.1 mL), density of seed (1.23 g mL−1), hydration capacity (4.1 g 100 seed), hydration index (1.09), swelling capacity (3.8 mL, 100 seed), swelling index (1.22) and minimum cooking time (43 min) (Singh et al. 2004). The density of moth bean was found to be 1.3 g mL−1. Similar result have been reported in dry bean cultivars (1.18–1.36 g mL−1) (Deshpande and Campbell 1992). The longer cooking time due to the slower water uptake has been reported in cotyledon with higher density (Seena and Sridhar 2005).
Hunter color properties
The color of the moth beans and bean flours are displayed in Table 2. L* values of the seed were observed to be significantly different (P < 0.05) for the raw and processed beans, with the raw and cooked beans having the lowest (41.98 and 40.15) and the sprouted beans having the highest (55.65) values. The magnitude of L* values indicated the respective degrees of lightness of the different beans. The raw moth beans had an a* value of 8.99 which was the most noteworthy. The a* values were found to differ significantly for the cooked and sprouted beans. The sprouted moth beans were reported to have the highest b* value of 22.95 which suggests the larger intensity of yellowness of their seed coat. This value varied significantly (P < 0.05) from raw and cooked moth beans. In terms of the chroma value, no significant differences were observed for the raw, sprouted and cooked bean flour sample. Sprouted moth bean had the highest hue angle value (78.91). The chroma value illustrates the color intensity of a sample whereas the hue angle determines the ability of an average person to distinguish that color. The chroma values of bean flours showed a similar pattern as was observed for the whole beans. However, chroma values of flours had a small range, which showed that the color differences were smaller in flours than those observed in whole beans. The color of raw and processed beans and their respective flours varied depending on the presence and amount of flavonoids (flavonols, anthocyanins, and condensed tannins) (Sharma et al. 2015). Sprouted seed showing more L* values indicates more lightness and this may be due to the leaching out of tannins from the seed coat because of soaking during sprouting process. Cooked moth bean flour was noted with lower L* value because of Maillard reaction while cooking of bean. The color differences could be attributed to the color pigments of the seed coat. Raw moth bean flour reported slightly higher b* value which indicates their higher amount of ash content (Shevkani et al. 2015).
Least gelation concentration
The gelation properties of the bean flours were determined at various concentrations (2–30 g 100 mL−1) as shown in Table 3. Partial gelation was visible at a concentration of 12 g 100 mL−1 and a complete gelation was noticeable at a concentration of 14 g 100 mL−1 for the sprouted and cooked bean flours respectively. The partial gelation for raw moth bean flour began at 24 g 100 mL−1, and a complete gelation was noticed at 26 g 100 mL−1.
The raw moth bean flours were found to exhibit higher gelation concentration than the sprouted and cooked flours. LGC for moth bean was reported to be 26% (w/v; raw) and 14% (w/v; sprouted and cooked), respectively. The sprouting and cooking of beans may be responsible for the increasing gelation capacity of flours as these processes might be influencing factors for gel formation due to adjustments in the qualities of proteins, carbohydrates, and lipids. This was found similar to that previously reported by Benítez et al. (2013).
Emulsion and foaming properties
The emulsifying and foaming characteristics of raw and processed moth bean flours have been demonstrated in Table 4. Cooked moth bean flour was found to have the least foaming capacity (5.88 mL, 100 mm−1) with zero foaming stability. The highest values for foaming capacity and stability were observed in the sprouted (23.94, 11.08 mL, 100 mL−1) and raw (29.62, 6.27 mL, 100 mL−1) moth bean flours. For emulsion capacity raw moth bean flour displayed significant contrasts (P < 0.05) with sprouted bean flours, whereas cooked moth bean flour was found to be unable to form emulsions. Foaming stability of sprouted moth bean flour was remarkably (P < 0.05) higher in comparison to the raw moth bean flour.
Emulsifying capacity (EC) was noted higher in raw bean flour than sprouted and cooked bean flour. Similarly, emulsifying activity was found reduced in germinated legume flours, with a more drastic reduction for jack bean (25%) as reported by Benítez et al. (2013). The EC in present study was higher than in chickpea and bean, despite the fact that it has been reported to be lower than that in lentil, green gram and cowpea (Ghavidel and Prakash 2006). Sprouted bean flour was showing higher ES than the raw bean flours and cooked bean flour. ES of raw and sprouted bean flour was observed to be 61.20% and 80.36%, respectively. After germination, the decreased observed in EC might be ascribed to protein concentration changes, which were found reduced for all legumes (cowpea, dolichos, jack bean and mucuna). They may also be attributed to the hydrophobicity/hydrophilicity proportion and structural constraints of proteins through which they can unfurl so as to create a film or skin surrounding the scattered oil droplets (Boye et al. 2010). A similar trend has been observed in the case of foaming capacity and foaming stability. It has been found that the foaming capacity of moth bean flour was 29.62% (raw), 23.94% (sprouted) and 5.88% (cooked). Foaming capacity of moth bean flour was recorded as 6.27% (raw), 11.08% (sprouted) and 0% (cooked). In the present study, raw moth bean was observed to have 29.62% foam capacity (FC) which was lower in comparison to the other raw legumes (54–90%) (Benítez et al. 2013). During germination, the observed variations in FC may be the results of interactions of the various elements of the flours such as proteins that have an effect on their physicochemical properties. Furthermore, the results obtained were found different in comparison to studies done previously which demonstrated an increase in EA and FC values following germination for green gram, lentil, cowpea and sorghum (Elkhalifa and Bernhardt 2010; Ghavidel and Prakash 2006).
Pasting properties
The results of pasting viscosities are shown in Table 4. The raw moth bean flour was found to have remarkably (P < 0.05) larger viscosity followed by the cooked and sprouted moth bean flour. The pasting properties of the flours associated with the amylose content, chains of amylopectin and swelling properties of the flours. There was no significant difference noted in the peak viscosity of sprouted and cooked moth bean flour. The raw moth bean flour was observed with highest peak viscosity as compared to sprouted and cooked moth bean flours, it indicates the higher water absorption and swelling capacity of raw moth bean flour before rupture (Ghumman et al. 2016). The peak viscosity revealed that there was no significant difference between sprouted and cooked moth bean flour. Sprouted moth bean flour had significantly higher breakdown viscosity than cooked moth bean flour. For pasting time, the cooked moth bean flour demonstrated highest pasting time accompanied by the raw and sprouted moth bean flour respectively. The pasting temperature for raw moth bean flour was recorded at 79.70 °C, whereas sprouted and cooked flours did not show any pasting temperature.
The least temperature needed for cooking and during heating at which there was an increase in the viscosity in the samples were indicated by pasting temperature. The lowest breakdown viscosity of moth bean sprouted flour (MBSF) and moth bean cooked flour (MBCF) flours showed their higher thermal stability. MBCF indicated a lower tendency for retrogradation due to their lowest final and setback viscosity. The viscosity was found to increase during the cooling (from 95 to 50 °C) period suggesting the realignment of the chains of amylose (Flores-Farías et al. 2000).
Thermal properties
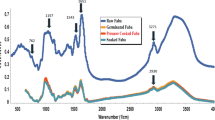
The transition onset temperature of raw moth bean flour (62.23 °C) was significantly lower (P < 0.05) than sprouted moth bean (63.48 °C) and cooked moth bean flour (63.24 °C) (Fig. 1). The similar onset temperatures for sprouted and cooked moth bean flour samples lead us to believe that starch gelatinization was initiated in both flours samples at similar temperatures. T0 values revealed that there was no significant difference between sprouted and cooked moth bean flours. It was revealed that sprouted moth bean flour (70.17 °C) was having significantly higher (P < 0.05) transition peak temperature than raw (67.33 °C) and cooked moth bean flour (68.83 °C) transition peak temperature. Td had been associated to the amino acid composition as well as protein structure and conformations involved (Sharma et al. 2015). However, as heating progressed, gelatinization of the more significant population of starch granules was initiated in the sprouted moth bean flour followed by cooked and raw moth bean flour, with the peak gelatinization and conclusion temperatures displaced to higher values. Similarly, the conclusion temperature was observed significantly higher in sprouted moth bean flour (76.54 °C). The results obtained are found similar to former research done by Wani et al. (2013) where the kidney bean flours demonstrated To, Tp, Tc, and ΔH values of 63.0 °C, 67.2 °C, 70.5 °C and 4.9 J g−1, respectively. The significantly higher ΔH value of raw moth bean indicates that a greater thermal energy was needed for starch gelatinization because double bond helices are strongly associated inside the granules (Sharma et al. 2015). Lower ΔH values obtained for sprouted and cooked moth bean flour can be attributed to some extent to starch modification and protein denaturation, which may have taken place during the processing of the seed. Variations in the thermal profile of bean are also known to depend upon the amylose content, distribution of amylopectin branch chain, lipid complex amylose chain and proteins present in it (Rui et al. 2011).
FE-SEM
Microstructure of the moth bean flours were visualized by FE-SEM (Fig. 2a–f). The flour granules were variable in size, shape and appearance for raw, sprouted and cooked moth bean. The particle size of raw moth bean flour was found to range between 81.93 and 507.54 µm2. On the other hand, the particle size of sprouted and cooked moth bean flour was found to range between 83.05–716.09 µm2 and 148.59–4837.23 µm2 respectively. Particle size was reported to be highest in cooked moth bean flour. Cooking causes the swelling of granules resulting in larger size granules. The raw moth bean flour granule surfaces were observed smooth. Generally, it is known that the starch granular size was bigger than that of proteins and lipids. Hence, the bigger globular structures are reported to be starch granules, which were shown to have different sizes and shapes: spherical and ovoid (Romano et al. 2015). The surface of the sprouted and cooked (Fig. 2d, f) flour granules were found rough as compared to the raw moth bean flour granule surface (Fig. 2b). The cooked moth bean flour granules were ruptured, swelled and not as smooth as the raw moth bean flour granule.
Conclusion
This investigation exhibited variations in the physicochemical and functional properties of seeds and flours of raw, sprouted and cooked moth bean. Sprouting of moth bean led to an increase in the emulsion and foaming stability. The lowest breakdown viscosity of MBSF and MBCF flours indicated their high thermal stability. Sprouting and cooking of beans were responsible for the increasing gelation capacity of flours. It can be concluded that the processing of moth bean led to a change in their physiochemical and functional properties which is indicated by changes in the fractions of beans. The results obtained are essential for developing various food products by where the moth bean flours can be utilized in parallel to the other bean flours. The moth bean flour can have potential applications for developing gluten-free products, which have demonstrated an enormous marketing potential lately.
References
Ajibola CF, Malomo SA, Fagbemi TN, Aluko RE (2016) Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. Food Hydrocoll 56:189–200. https://doi.org/10.1016/j.foodhyd.2015.12.013
AOAC (2000) Official method of analysis of AOAC international. Association of Official Analytical Chemists, Gaithersburg
Benítez V, Cantera S, Aguilera Y, Mollá E, Esteban RM, Díaz MF, Martín-Cabrejas MA (2013) Impact of germination on starch, dietary fiber and physicochemical properties in non-conventional legumes. Food Res Int 50:64–69. https://doi.org/10.1016/j.foodres.2012.09.044
Boye J, Zare F, Pletch A (2010) Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int 43:414–431. https://doi.org/10.1016/j.foodres.2009.09.003
Deshpande S, Campbell C (1992) Genotype variation in BOAA, condensed tannins, phenolics and enzyme inhibitors of grass pea (Lathyrus sativus). Can J Plant Sci 72:1037–1047. https://doi.org/10.4141/cjps92-130
Dhaliwal Y, Aggarwal R (1999) Composition of fat in soybeans as affected by duration of germination and drying temperature. J Food Sci Technol 36:266–267
Dueñas M, Martínez-Villaluenga C, Limón RI, Peñas E, Frias J (2015) Effect of germination and elicitation on phenolic composition and bioactivity of kidney beans. Food Res Int 70:55–63. https://doi.org/10.1016/j.foodres.2015.01.018
Elkhalifa AEO, Bernhardt R (2010) Influence of grain germination on functional properties of sorghum flour. Food Chem 121:387–392. https://doi.org/10.1016/j.foodchem.2009.12.041
Flores-Farías R, Martínez-Bustos F, Salinas-Moreno Y, Chang YK, Hernandez JG, Ríos E (2000) Physicochemical and rheological characteristics of commercial nixtamalised Mexican maize flours for tortillas. J Sci Food Agric 80:657–664. https://doi.org/10.1002/(sici)1097-0010(20000501)80:6%3c657:aid-jsfa576%3e3.0.co;2-j
Ghavidel RA, Prakash J (2006) Effect of germination and dehulling on functional properties of legume flours. J Sci Food Agric 86:1189–1195. https://doi.org/10.1002/jsfa.2460
Ghavidel RA, Prakash J (2007) The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci Technol 40:1292–1299. https://doi.org/10.1016/j.lwt.2006.08.002
Ghumman A, Kaur A, Singh N (2016) Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol 65:137–144. https://doi.org/10.1016/j.lwt.2015.07.075
Gupta N, Shrivastava N, Singh PK, Bhagyawant SS (2016) Phytochemical evaluation of moth bean (Vigna aconitifolia L.) seeds and their divergence. Biochem Res Int 2016:1–6. https://doi.org/10.1155/2016/3136043
Hamid S, Muzaffar S, Wani IA, Masoodi FA, Bhat MM (2016) Physical and cooking characteristics of two cowpea cultivars grown in temperate Indian climate. J Saudi Soc Agric Sci 15:127–134. https://doi.org/10.1016/j.jssas.2014.08.002
Han JJ, Janz JA, Gerlat M (2010) Development of gluten-free cracker snacks using pulse flours and fractions. Food Res Int 43:627–633. https://doi.org/10.1016/j.foodres.2009.07.015
Henshaw FO, McWatters KH, Akingbala JO, Chinnan MS (2003) Thermal properties of cowpea flour: a study by differential scanning calorimetry. Food/Nahrung 47:161–165. https://doi.org/10.1002/food.200390038
Jain AK, Kumar S, Panwar J (2009) Antinutritional factors and their detoxification in pulses—a review. Agric Rev 30:64–70
Kaur S, Singh N, Sodhi NS, Rana JC (2009) Diversity in properties of seed and flour of kidney bean germplasm. Food Chem 117:282–289. https://doi.org/10.1016/j.foodchem.2009.04.002
Khatoon N, Prakash J (2006) Nutrient retention in microwave cooked germinated legumes. Food Chem 97:115–121. https://doi.org/10.1016/j.foodchem.2005.03.007
Martín-Cabrejas MA, Díaz MF, Aguilera Y, Benítez V, Mollá E, Esteban RM (2008) Influence of germination on the soluble carbohydrates and dietary fibre fractions in non-conventional legumes. Food Chem 107:1045–1052. https://doi.org/10.1016/j.foodchem.2007.09.020
Martín-Cabrejas MA, Aguilera Y, Pedrosa MM, Cuadrado C, Hernández T, Díaz S, Esteban RM (2009) The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem 114:1063–1068. https://doi.org/10.1016/j.foodchem.2008.10.070
Megat Rusydi M, Noraliza C, Azrina A, Zulkhairi A (2011) Nutritional changes in germinated legumes and rice varieties. Int Food Res J 18:703–713
Nasrin TAA, Noomhorm A, Anal AK (2015) Physico-chemical characterization of culled plantain pulp starch, peel starch, and flour. Int J Food Prop 18:165–177. https://doi.org/10.1080/10942912.2013.828747
Neto VQ, Narain N, Silva J, Bora P (2001) Functional properties of raw and heat processed cashew nut (Anacardium occidentale, L.) kernel protein isolates. Food/Nahrung 45:258–262. https://doi.org/10.1002/1521-3803(20010801)45:4%3c258:aid-food258%3e3.0.co;2-3
Ohtsubo K, Suzuki K, Yasui Y, Kasumi T (2005) Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J Food Compos Anal 18:303–316. https://doi.org/10.1016/j.jfca.2004.10.003
Reyes-Bastidas M, Reyes-Fernández E, López-Cervantes J, Milán-Carrillo J, Loarca-Piña G, Reyes-Moreno C (2010) Physicochemical, nutritional and antioxidant properties of tempeh flour from common bean (Phaseolus vulgaris L.). Food Sci Technol Int 16:427–434. https://doi.org/10.1177/1082013210367559
Romano A, Giosafatto C, Masi P, Mariniello L (2015) Impact of dehulling on the physico-chemical properties and in vitro protein digestion of common beans (Phaseolus vulgaris L.). Food Funct 6:1345–1351
Rui X, Boye JI, Ribereau S, Simpson BK, Prasher SO (2011) Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Res Int 44:2497–2504. https://doi.org/10.1016/j.foodres.2011.01.008
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671. https://doi.org/10.1038/nmeth.2089
Seena S, Sridhar K (2005) Physicochemical, functional and cooking properties of under explored legumes, Canavalia of the southwest coast of India. Food Res Int 38:803–814. https://doi.org/10.1016/j.foodres.2005.02.007
Sharma S, Singh N, Virdi AS, Rana JC (2015) Himalayan kidney bean germplasm: grain-flour characteristics, structural-functional properties and in vitro digestibility of starches. Food Res Int 77:498–505. https://doi.org/10.1016/j.foodres.2015.08.030
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Siddhuraju P, Becker K (2005) Nutritional and antinutritional composition, in vitro amino acid availability, starch digestibility and predicted glycemic index of differentially processed mucuna beans (Mucuna pruriens var. utilis): an under-utilised legume. Food Chem 91:275–286. https://doi.org/10.1016/j.foodchem.2004.02.044
Siddiq M, Ravi R, Harte J, Dolan K (2010) Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours. LWT Food Sci Technol 43:232–237. https://doi.org/10.1016/j.lwt.2009.07.009
Singh N, Kaur M, Sandhu KS, Sodhi NS (2004) Physicochemical, cooking and textural characteristics of some Indian black gram (Phaseolus mungo L.) varieties. J Sci Food Agric 84:977–982. https://doi.org/10.1002/jsfa.1744
Świeca M, Gawlik-Dziki U, Kowalczyk D, Złotek U (2012) Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of Lens culinaris sprouts. Sci Hort 140:87–95. https://doi.org/10.1016/j.scienta.2012.04.005
Tharanathan R, Mahadevamma S (2003) Grain legumes-a boon to human nutrition. Trends Food Sci Technol 14:507–518. https://doi.org/10.1016/j.tifs.2003.07.002
Wang N, Hatcher DW, Gawalko EJ (2008) Effect of variety and processing on nutrients and certain anti-nutrients in field peas (Pisum sativum). Food Chem 111:132–138. https://doi.org/10.1016/j.foodchem.2008.03.047
Wang N, Hatcher D, Toews R, Gawalko E (2009) Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT Food Sci Technol 42:842–848. https://doi.org/10.1016/j.lwt.2008.10.007
Wani IA, Sogi DS, Gill BS (2013) Physicochemical and functional properties of flours from three Black gram (Phaseolus mungo L.) cultivars. Int J Food Sci Technol 48:771–777. https://doi.org/10.1111/ijfs.12025
Acknowledgements
The author acknowledged scholarship donor Social Justice and Special Assistance Department, Government of Maharashtra, India and Asian Institute of Technology, Thailand to conduct this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Medhe, S., Jain, S. & Anal, A.K. Effects of sprouting and cooking processes on physicochemical and functional properties of moth bean (Vigna aconitifolia) seed and flour. J Food Sci Technol 56, 2115–2125 (2019). https://doi.org/10.1007/s13197-019-03692-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03692-y