Abstract
In this research, the effect of different milling processes on pulse quality parameters has been investigated. Chickpea and navy bean seeds were milled using a laboratory-scale roller mill with four different streams: middling 1, middling 2 and 3, break, and straight grade (SG) flours and a Ferkar mill. The effect of mill type on particle size of chickpea flours was different from navy bean flours. The smallest particle size (30 µm) was determined in Ferkar-milled chickpea flour. The highest starch and lowest protein contents were found in break flours independent of pulse type. The highest starch damage was also observed in break flours. Oil absorption capacities of Ferkar flours were higher than roller-milled streams, whereas middling 2 and 3 flours had higher oil emulsion capacities. Foaming stability of flours decreased over time; however, roller-milled streams showed higher foam stabilities than Ferkar-milled flours from navy bean. The highest pasting viscosities were found in break flours of both pulses. Mill type did not change the rapidly digestible starch. However, the highest slowly digestible starch contents were determined in Ferkar-milled flour for all pulse types. Resistant starch of chickpea and navy bean flours ranged from 14–22% to 16–28%, respectively. No significant difference was observed for any of the pulse flours on protein digestibility or quality. The findings may provide a better understanding of functional and nutritional properties of chickpea and navy bean flours produced by different milling processes and their suitability to create different food formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, consumers and the food industry have demonstrated a growing interest in pulse ingredients and their use in traditional cereal-based foods alone or blended with cereals. Pulses are good sources of protein, carbohydrate, dietary fiber, vitamins, and mineral with low lipid contents (with the exception of chickpeas, 5–7%) [1]. Environmental and genetic factors may have different impacts on the nutritional value of pulses [2]. To increase the consumption of pulse products, the food industry seeks ways to use pulse ingredients in the development of value-added and healthy food products [3]. Kabuli chickpeas, also called garbanzo beans, have a salmon white color and small to large seed size (>100–<50 seeds/oz) [4]. Kabuli chickpeas are a good source of carbohydrate (60–70%), protein (21–27%), fiber (4–11%) and lipids (5–7%) [5]. Navy beans, on the other hand, have a smaller seed size and contain approximately 1.5% lipid. Pulses, in general, are known for their nutritional health benefits and may reduce incidence of diabetes, cardiovascular diseases, and cancer [5].
Milling plays a crucial role in turning pulse seeds into ingredients. There are no standards for pulse flours; therefore, producers may use different milling processes which can affect their functional and nutritional properties. Previously, it was reported that roller and pin milling yield smaller particle size and more narrow particle size distribution relative to hammer and stone milling [6]. The effects of particle size of pulse flours on baking quality, textural properties, nutritional properties, and pasting properties have been studied with different pulses and milling processes [1, 6,7,8]. The particle size of flours affected their composition. For instance, lentil flours with smaller particle size had lower protein content [8]. However, pulse flours with finer particle size showed improved bread scores [6].
Milling technologies and screen sizes used affect particle size and size distribution of pulse flours. For instance, both particle size and the type of mill (hammer mill versus cryo-milling) had an impact on starch digestibility of sorghum, and smaller particle size and frictional heat from hammer mill increased starch digestibility [9]. Particle size may have an impact on digestion kinetics of protein, but yield no significant differences on protein digestibility—in vitro—of peas or cowpea [10, 11], whereas both protein digestibility and quality—in vivo—of lupin have demonstrated increases with decreasing particle size [12]. In addition, not only particle size but also compositional characteristics may influence pulse flour characteristics, as coarse lentil flour behaved differently in cookie dough than coarse navy bean and pinto bean due to its different water absorption capacity [1]. The goal of this study was to investigate the impacts of different milling processes on the functional and nutritional properties of chickpea and navy bean flours. To this end, chickpea and navy bean seeds were milled using a laboratory scale Bühler (roller mill) and Ferkar mill after hammer milling. Four different streams; middling, break, and straight grade (SG) flours from the roller mill and one flour from the Ferkar mill were assessed for physicochemical, functional, pasting properties, and in vitro starch and protein digestibility and quality.
Material and methods
Materials
Kabuli chickpeas (CDC Orion) and navy beans (Nautica) were harvested in the 2018 crop year and were obtained from Reisner Farm Ltd. (Limerick, SK) and Hensall Co-op Ltd. (Hensall, ON), respectively.
Milling
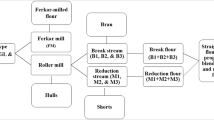
Pre-breaking of each pulse type was performed with a hammer mill (Model 120-B, Jacobson Machine Works, Inc., Minneapolis, MN) fitted with an 8/64″ (3.18 mm) screen. After hammer milling, the samples were milled on a Ferkar multipurpose knife mill with 140 µm screen (Ferkar 5 Model, KFM, d.o.o., Slovenia) or a Bühler laboratory roller mill (MLU 202, Bühler Group, Switzerland) in duplicate. A single flour was produced from the Ferkar mill. The mill flow diagram of the Bühler laboratory roller mill is presented in Online Resource. Straight grade (SG) flour was composed of all six flour streams. In addition, stream blends of the break flours (B1 + B2 + B3), middling flour 1 (M1) and the blends of middling flour 2 and 3 (2 M + 3 M) were produced.
Chemical analysis
Moisture, starch, and crude protein contents (N × 6.25) of all flours were determined according to AACC Methods 44-15.02, 76-13.01 and a combustion nitrogen analysis method using the LECO FP-828 (LECO Corp., St. Joseph, MI), respectively [13, 14]. Damaged starch analysis was performed according to the AACC Method 76-31.01 with a Megazyme starch damage assay kit (K-SDAM, Megazyme, Wicklow, Ireland) [13].
Particle size analysis
Particle size distributions of flours in terms of volume weighted mean (VWM) and uniformity were determined by laser diffraction with dry dispersion using the Malvern Mastersizer 2000 with Scirocco 2000 accessory (Malvern Instruments Inc, Westborough, MA).
Functional properties
Foaming capacity (FC) and stability (FS)
Foaming capacity and stability analyses were performed according to the method described by Sathe et al. [15] with modifications. Briefly, 100 g of flour dispersion (2% w/w, db) was sheared using a homogenizer (13,500 rpm, 1 min) and poured into a graduated cylinder (250 mL). Foaming capacity was determined by the percent of volume increase after shearing. The height of the foam was recorded after 10, 30, 60 and 120 min and expressed as a percentage of the initial foam volume to determine foaming stability.
Oil absorption capacity (OAC)
OAC was determined according to the method of Wang et al. [16]. Briefly, a filter paper (Cat. 28313-080, VWR) was cut in 4.5 cm diameter, folded and inserted into a 20 cc syringe barrel. Samples (0.5 g) were weighed into a test tube (16 × 100 mm). The syringe with folded filter paper and the test tube containing the sample were then weighed. Canola oil (1.5 mL) was added to the test tube, which was vortexed for 5 s every 10 min for a total of 20 min. The tube containing sample and oil was then inverted into the syringe with filter paper seating inside at the bottom, and then the whole assembly was placed into a 50 mL conical centrifuge tube for centrifugation at 600×g for 25 min. Free oil passed through the filter paper upon centrifugation and was collected in the conical centrifuge tube, with solids retained in the syringe. The syringe assembly after centrifugation was then weighed. A blank filter paper was included in each batch. OAC was calculated using Eq. (1):
where W1 = weight of the sample before oil addition (g); W2 = weight of the syringe assembly (syringe barrel, filter paper, test tube and sample) (g); W3 = weight of the syringe assembly after centrifugation (g); W4 = weight of oil absorbed by the blank filter paper after centrifugation (g); mc = initial moisture content of the sample (%).
Oil emulsion capacity (OEC)
OEC was measured according to Wang, Maximiuk [17]. In brief, a pulse flour suspension (0.40% w/v, db) was homogenized for 30 s in a 500 mL glass jar using a PowerMax AHS 250 homogenizer fitted with a 10 × 105 mm saw tooth generator probe at setting 1. Then, the glass jar containing the sample suspension (75 mL) was attached onto a BF-30 homogenizer. Canola oil (25 mL) was delivered into the glass jar using a Masterflex pump (Cole-Parmer, Vernon Hills, IL). The suspension, together with 25 mL oil, was blended at 6000 rpm using the BF-30 homogenizer for 30 s, at which time oil was delivered continuously into the chamber with the pump at a rate of 1.0 mL/s. The entire process for emulsion formation and collapse was recorded by measuring the electrical resistance with a digital multimeter, where the emulsion break point was indicated by a sudden increase in electrical resistance. At break point, oil addition was stopped and the total amount of oil emulsified was calculated. OEC was expressed as mL oil/g sample, db.
Pasting properties
Evaluation of the pasting profile of flours was performed according to AACC 76-21.01 (STD1, 13 min profile) using an RVA 4500 (Perkin-Elmer, Waltham, MA) [13].
Mixolab properties
Mixolab assessment was determined using the Mixolab 2 (CHOPIN Technologies, France) according to the AACC Method 54-60.01 with modifications [13]. Samples were run at constant water absorption at 14% moisture basis. A water absorption of 55% was used for chickpea and navy bean flour samples.
In vitro starch digestibility
In vitro starch digestibility in pulse flours was determined according to the method described by Englyst et al. [18]. The analysis was done under controlled enzymatic hydrolysis followed by colorimetric measurement of the glucose released. Rapidly digestible starch (RDS) and slowly digestible starch (SDS) were measured following incubation with porcine pancreatic alpha-amylase and amyloglucosidase at 37 °C in a water bath. RDS is the glucose released after 20 min and SDS is the glucose released after a further 100 min incubation. Resistant starch (RS) was measured indirectly by calculating the starch that is not hydrolysed after 120 min incubation.
Amino acid (AA) composition and amino acid score (AAS)
Amino acids were acid hydrolyzed (AOAC 982.30) with the additional performic acid and sodium metabisulfite procedures utilized for methionine and cysteine (AOAC 994.12), with the exception of alkaline hydrolysis for tryptophan (ISO 13904) [19, 20]. Detection of amino acids was through UV or fluorescence utilizing AccQ-Tag (Waters Corporation, Milford, MA, USA) precolumn derivatization and reverse-phase HPLC (Shimadzu Corporation, Kyoto, Japan). No precolumn derivatization was required for tryptophan, which was previously described [21].
Each indispensable amino acid for the milled flours was ratioed on a protein basis, relative to the reference pattern for children ages 2–5 [22], to obtain amino acid scores (AAS). The single most limiting amino acid was selected as the AAS using Eq. (2).
In vitro protein digestibility (IVPD)-corrected amino acid score (IVPDCAAS)
Protein digestibility was assessed in vitro following the procedures outlined by Hsu et al. [23] with updated enzymatic preparation and regression expression from Tinus et al. [10]. Sample preparation of 1 mg/mL of nitrogen in Milli-Q water was solubilized for 1 h at 37 °C prior to adjusting pH to 8.0 ± 0.05 with 1 M NaOH or HCl. A multi-enzyme solution containing of 3.1 mg/mL chymotrypsin (C4129; bovine pancreas ≥ 40 units/mg protein), 1.6 mg/mL trypsin (T0303; porcine pancreas 13,000–20,000 BAEE units/mg protein) and 1.3 mg/mL protease (P5147; Streptomyces griseus ≥ 15 units/mg solid) prepared in Milli-Q water was also adjusted to pH to 8.0 ± 0.05 at 37 °C and transferred to an ice water bath (0–4 °C). Utilizing the following regression equation, IVPD was calculated using Eq. (3):
where the initial and final pH—after the addition of 1 mL of multi-enzyme solution—was determined over a 10-min period. The product from the IVPD and the AAS are then used to calculate IVPDCAAS using Eq. (4):
Data analysis
Milling of flours was performed in duplicate and experiments were carried out in duplicate analysis (n = 2 × 2), except for IVPD (n = 2 × 3) and amino acid hydrolysis/analysis (n = 2 × 1). Data were analyzed by one-way analysis of variance (ANOVA) with Tukey’s and Games–Howell post hoc test (p < 0.05) using IBM SPSS Statistics for Windows (version 27, Armonk, NY). Protein digestibility and quality data were analyzed using GraphPad Prism (version 9.3.0, San Diego, CA), with two-way ANOVA conducted for the comparison of pulse type and milling on IVPD, AAS and IVPDCAAS, and one-way ANOVA for AA composition, with Tukey’s post-hoc test (p < 0.05).
Results and discussion
Physicochemical properties
The physicochemical properties of chickpea and navy bean flours are given in Table 1. The protein content of chickpea and navy bean flours was 19–23% and 23–31%, respectively. The protein content of the Ferkar-milled flours for both pulse types was higher than that of break flour, but lower than that of middling 2 and 3 flour of roller milling. In contrast, the highest starch content for both pulse types was found in break flours and the lowest was in middling 2 and 3 flours. In addition, damaged starch content ranged from 0.9 to 1.4% and was the highest in break flours. Increasing starch damage was associated with smaller particle size in pea flours [24]. However, there was no relationship between particle size and protein, starch, and damaged starch content of flours. VWM of chickpea and navy bean flour ranged from 30–67 µm to 57–70 µm, respectively. The Ferkar-milled flour of chickpea had the smallest VWM (30 µm) of all flours. The effect of milling type on different pulses could be associated with seed size [25]. The uniformity of particle size was the highest (2.3) for the Ferkar-milled flour of navy beans. Since roller milling involves a series of rolls that cause the reduction in size and screen/sieves which aid hull separation, as a single stage mill, Ferkar mill resulted in a wider particle size distribution for navy bean flour, but not for chickpeas. It was hypothesized that thinner seed coat of Kabuli chickpeas might be the cause of the more uniform particle size compared to navy beans [26]. The uniformity of particle size of chickpea flours ranged from 0.7 to 0.9, while it was 0.7–2.3 in navy bean flours.
Functional properties
Oil absorption (OAC) and emulsion capacities (OEC) of chickpea and navy bean flours from different milling processes were determined (Table 1). The OAC values of Ferkar flours (0.43 g oil/g) were higher than those of roller mill streams (0.24–0.31 g oil/g) in each pulse type. In an earlier study, commercial navy bean flours with fine grind (131 µm) yielded lower OAC (1.23 g oil/g) than regular grind flour (248 µm; 1.47 g oil/g) [6]. Similarly, OAC of chickpea and navy bean flours was found to be less than 0.5 g oil/g with particle size less than 70 µm. The OEC values of chickpea and navy bean flours ranged from 281–319 mL oil/g to 307–407 mL oil/g, respectively. The highest OEC values were observed in roller mill flour middling 2 and 3 for both pulses. It is reported that OAC and OEC values may be associated with the characteristics of protein fraction [27]. Therefore, the higher protein content of middling 2 and 3 flours may have contributed to higher OEC values of flours.
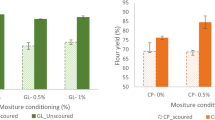
The foaming properties of chickpea and navy bean flours are presented in Fig. 1. Foams can be stabilized by proteins, which form layers along the gas–liquid interface and reduce the surface tension [24]. The foaming capacity (FC) of chickpea flours ranged between 25 and 30%, but no significant differences were found. Similarly, FC of navy bean flours ranged from 25 to 34%. There was no significant difference in FC regarding mill type. In chickpea flours, foaming stability (FS) values decreased from 85–90% (10 min) to 36–41% (120 min). Between milling processes, FS values were similar in each time period. However, FS values of navy bean flours produced by Ferkar milling were significantly lower than the roller mill flours in 60 and 120 min periods. A major drop in FS values was observed in the Ferkar-milled flour of navy beans, which decreased from 90 to 30% in 120 min.
Pasting properties
The pasting properties of chickpea and navy bean flours milled by Ferkar and roller mills are presented in Table 2. Since milling can cause damage to starch structures, starch gelatinization and pasting properties of flours are affected by the mill type [28]. It was found that pasting temperatures of navy bean flours (82–84 ℃) were slightly higher than chickpea flours (76–77 ℃). Similarly, pasting temperatures of navy bean and Kabuli chickpea were reported as 83.5 and 75 ℃, respectively [29, 30]. Higher pasting temperatures may indicate the interactions between starch and other components (lipid, protein, etc.). Amylose–lipid complexes or starch–protein interactions may restrict the swelling of starch granules during the pasting process [29, 31]. In addition, navy bean flours had higher pasting viscosities than chickpea flours, which could be associated with relatively higher protein content of navy bean flours. In both pulses, the Ferkar mill resulted in lower pasting viscosities (peak, trough, final, and setback viscosities) than SG flour from roller mill, which is a combination of all streams. To understand the effect of milling process, the physicochemical properties and pasting properties may be evaluated together. Flours produced by Ferkar milling had lower damaged starch content, but higher OAC than SG flour of roller milling. Only chickpea flour produced by Ferkar milling had smaller particle size (30 µm) than the SG stream. Previous research on the particle size effect on Ferkar-milled flours of navy beans (136, 265, 312, 506 µm) showed that finer flours had higher final viscosities [6]. However, no consistent trend associated with particle size and pasting viscosity was observed in this study due to a smaller particle size range of all flours (≤70 µm). Higher pasting viscosity of break flours could be related to higher starch and lower protein contents despite higher damaged starch content. Furthermore, lower pasting viscosities of roller-milled flours were determined in the middling 2 and 3 stream, which had the highest protein content among roller-milled flours. These findings emphasize the restrictive effect of protein network on starch pasting properties.
Mixolab properties
The performance characteristics of chickpea and navy bean flour were examined using Mixolab characteristics of dough samples (Table 3) which provides information about the dough development time, protein weakening, stability during baking, starch gelatinization and retrogradation [32]. Initial peak consistency (C1) values of chickpea and navy bean flours ranged from 0.16–0.43 to 0.68–1.86 Nm, respectively. The middling 2 and 3 flours of both pulses had the highest dough consistency. A longer time to C1 (1.8 min) and higher stability (9.5 min) were found in the Ferkar flour of chickpea samples which had the smallest particle size (30 µm) among the rest of the samples. In addition, higher lipid content of chickpea flours (4–5%) could be one of the reasons of higher stability values due to the formation of lipoprotein complexes between starch, protein, and other hydrophobic constituents [33, 34]. The C2 torque is a measure of proteins weakening when subjected to the dual constraint of mixing and heating. Navy bean flours showed lower dough strength and an increase in weakening of protein network with higher CS–C2 values (0.2–0.8 Nm) compared to chickpea (0.06–0.15 Nm) [35]. For navy bean flours, C3 and C4 were measured at constant times 30 and 32 min, respectively, due to lack of stable peak or trough. In general, C3 indicates the starch gelatinization, C4 defines the hot gel stability, and C5 shows starch retrogradation in the cooling phase [35]. C3 values of chickpea and navy bean flours ranged between 0.86–0.96 and 0.76–0.89, respectively. Ferkar-milled chickpea flour and middling 2 and 3 navy bean flours exhibited the highest torque during the heating stage (C3) and the torque obtained after cooling at 50 ℃ (C5). Interactions of hydrocolloids with starch and swelling power of starch may affect the maximum viscosity [36].
In vitro starch digestibility
Disruption of starch granule structure during different milling processes may affect starch digestibility [24]. In vitro starch digestibility of chickpea and navy bean flours from different milling processes is shown in Table 4. There was no significant difference in the rapidly digestible starch (RDS) values of flours milled from either pulse milled by Ferkar or roller mill. Different flours from the roller mill also did not cause significant difference in RDS values. RDS values of chickpea and navy bean were 10% and 6–7%, respectively. Slowly digestible starch (SDS) values of Ferkar-milled flours were significantly higher than roller-milled flours. However, roller-milled flours were similar within pulse type. In both pulse types, the highest resistant starch (RS) values were found in the break flours and the the lowest values in the middling 2 and 3 flours. This finding showed that starch molecules, after multiple milling steps (e.g., middling 2 and 3), were more susceptible to enzymatic digestion. Since the particle size of all flours can be classified as superfine (<80 µm), the relationship between particle size and starch digestibility was not correlated, whereas starch digestibility of lentil flours with smaller particle size had lower RS values compared to coarse flours [3].
Amino acid composition
The quantity of all amino acids (AA) is presented as the relative abundance in the milled flour (Table 5). Within all chickpea flours, AAs ranged from 0.157 to 3.370 (g/100 g sample), and within all navy bean flours from 0.191 to 4.072 (g/100 g sample). Glutamine was the most abundant AA within both pulses, whereas tryptophan was the least abundant in chickpea and cysteine and the least abundant in navy bean. In general, Ferkar, roller middling 1 and SG flours of chickpea and navy bean led to similar AA profiles (p > 0.05). Only histidine and tryptophan were stable across both pulse types and milling fractions. Roller break flours in both pulses produced flours with a significantly lower abundance of AA, whereas roller middling 2 and 3 produced flours with a greater abundance of AA. These changes correspond to the overall protein levels in these fractions (Table 1), where protein was 2.41 and 3.78% higher in the roller middling flour—relative to roller break—for chickpea and navy bean, respectively. Tryptophan was found to be the most limiting AA for chickpea in this study, similar to previous findings for chickpeas subjected to thermal treatments [37, 38]. Threonine has also been shown to be the most limiting AA without thermal treatment in chickpea [37, 39, 40]. Notably, the milling of chickpea may explain whether threonine is limiting, but not tryptophan, as indicated between the differences observed in roller break and roller middling 2 and 3 flours. Similarly, this effect may also be observed in the methionine content of navy bean, but not cysteine, as observed in the significantly greater content in the middling 2 and 3 flour. Cysteine and methionine are routinely found to be limiting in navy bean and other common beans [41,42,43,44,45], which can persist after cooking, baking or extrusion [46]. Relative to other studies, it cannot be dismissed that genetic and environmental factors may be responsible for differences in AA composition of chickpea [47] or common beans [41, 48], in addition to differences in AA hydrolysis and analysis procedures [49].
In vitro protein digestibility and quality
Protein digestibility and quality as assessed by in vitro methods are presented in Table 6. No significant differences were observed in IVPD, AAS, or IVPDPCAAS in both the pulse and the milling treatments. The IVPD of chickpea and navy bean flours was within a narrow range of 76.16–76.52% and 73.29–73.87%, respectively. Studies utilizing the same or similar in vitro digestion procedures found comparable IVPD of 76–78% for chickpea [39, 40, 50], and 71% in navy bean [42]. Chickpea AAS ranged from 0.84 to 0.91 and corresponded to the greatest change on protein quality, with a minimum mean IVPDCAAS observed in the roller break flour of 64.05%, and the maximum mean in the Ferkar or straight grade flours of 69.43 and 68.02%. Likewise navy bean AAS ranged from 0.81 to 0.90 and corresponded to the greatest impact on protein quality; however, maximum mean IVPDCAAS was found in the roller middling 2 and 3 flour and the minimum mean protein quality in the straight grade flour of 66.23 and 59.28%. Untreated chickpea flours have been shown to have similar IVPDCAAS utilizing the same IVPD procedures and amino acid reference pattern—within the range of those in this study—of 64.99 and 69.38% [39, 40]. Protein quality assessed by relative nutritive value in three navy bean cultivars reported values between 64 and 78% [41]. Previous IVPDCAAS of raw navy beans has not been established—to the knowledge of the authors—although it may be ascertained that based on earlier reported amino acid composition and IVPD [42], values fall within the range found in this study. The addition of thermal treatments may be expected to reduce protein quality of navy beans and other common beans [44, 46]—relative to raw—as demonstrated in baked (60.95%), cooked (54.86%), and extruded (55.33%) flours.
Conclusions
Ferkar and roller mills affected the physicochemical, functional, pasting, thermo-functional properties, and starch digestibility of chickpea and navy bean flours. The effect of different milling processes on particle size and size distribution varied according to pulse type and no consistent effect was observed. Since all flours had fine particle size, not all characteristics of flours were different. Differences in OAC were found according to the mill type. Foaming properties after 60 min for navy bean flours differed for Ferkar flours. Among roller-milled flours, break flours had higher starch content, damaged starch, and pasting viscosities. RDS values of all flours were similar for each pulse. However, Ferkar milling resulted in higher SDS values for both pulse types. Although particle size of all flours was similar, single step (i.e. Ferkar) or multi-step (i.e. Roller) milling processes caused different functionalities in pulse flours. While amino acid composition fluctuated between mill types for both pulses, the milling processes did not significantly alter the most limiting amino acid and protein quality estimates. This finding indicates that the particle size of flours may not be the only parameter to predict the final product quality. The findings from the evaluation of different milling techniques on the characteristics of chickpea and navy bean flours provide insight to food manufacturers for new food formulations using pulse flours.
References
Zucco F, Borsuk Y, Arntfield SD (2011) Physical and nutritional evaluation of wheat cookies supplemented with pulse flours of different particle sizes. LWT-Food Sci Technol 44(10):2070–2076
Guldiken B, Konieczny D, Wang N, Hou A, House JD, Tu K, Rosendahl S, Lavier M, Nickerson MT (2021) Effect of variety and environment on the physicochemical, functional, and nutritional properties of navy bean flours. Eur Food Res Technol 247:1–12
Kathirvel P, Yamazaki Y, Zhu W, Luhovyy BL (2019) Glucose release from lentil flours digested in vitro: the role of particle size. Cereal Chem 96(6):1126–1136
McKay K, Miller P, Jenks B (2002) Growing chickpea in the northern great plains. NDSU Extension Service, North Dakota State University of Agriculture and Applied Science, and U.S. Department of Agriculture. https://mccc.msu.edu/wp-content/uploads/2018/12/ND_2002_Growing-Chickpea-in-the-Northern-Great-Plains.pdf. Accessed 1 July 2020
Xu Y, Thomas M, Bhardwaj HL (2014) Chemical composition, functional properties and microstructural characteristics of three kabuli chickpea (Cicer arietinum L.) as affected by different cooking methods. Int J Food Sci Technol 49(4):1215–1223
Bourré L, Frohlich P, Young G, Borsuk Y, Sopiwnyk E, Sarkar A, Nickerson MT, Ai Y, Dyck A, Malcolmson L (2019) Influence of particle size on flour and baking properties of yellow pea, navy bean, and red lentil flours. Cereal Chem 96(4):655–667
Borsuk Y, Arntfield S, Lukow OM, Swallow K, Malcolmson L (2012) Incorporation of pulse flours of different particle size in relation to pita bread quality. J Sci Food Agric 92(10):2055–2061
Ahmed J, Taher A, Mulla MZ, Al-Hazza A, Luciano G (2016) Effect of sieve particle size on functional, thermal, rheological and pasting properties of Indian and Turkish lentil flour. J Food Eng 186:34–41
Mahasukhonthachat K, Sopade PA, Gidley MJ (2010) Kinetics of starch digestion in sorghum as affected by particle size. J Food Eng 96(1):18–28
Tinus T, Damour M, Van Riel V, Sopade PA (2012) Particle size-starch–protein digestibility relationships in cowpea (Vigna unguiculata). J Food Eng 113(2):254–264
Nguyen GT, Gidley MJ, Sopade PA (2015) Dependence of in-vitro starch and protein digestions on particle size of field peas (Pisum sativum L.). LWT-Food Sci Technol 63(1):541–549
Kim JC, Mullan BP, Heo JM, Hansen CF, Pluske JR (2009) Decreasing dietary particle size of lupins increases apparent ileal amino acid digestibility and alters fermentation characteristics in the gastrointestinal tract of pigs. Br J Nutr 102(3):350–360
AACC (2010) AACC approved methods of analysis, 11th edn. Minnesota, Cereals and Grains Association
Williams P, Sobering D, Antoniszyn J (1998) Protein testing methods at the Canadian Grain Commission. In: Wheat protein, production and marketing, proceedings of the wheat protein symposium. University of Saskatchewan Press: Saskatoon, SK, pp 37–47
Sathe SK, Deshpande SS, Salunkhe DK (1982) Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J Food Sci 47(2):491–497
Wang N, Maximiuk L, Fenn D, Nickerson MT, Hou A (2020) Development of a method for determining oil absorption capacity in pulse flours and protein materials. Cereal Chem 97(6):1111–1117
Wang N, Maximiuk L (2015) Development of an improved electrical resistance method for determining emulsifying capacity of pulse and soy materials 1. Cereal Chem 92(3):253–257
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:S33-50
AOAC (2003) Official method of analysis, 17th edn. Association of Official Analytical Chemists Inc., Virginia
ISO (2016) Animal feeding stuffs Determination of tryptophan content, International Organization for Standardization.
Nosworthy MG, Franczyk A, Zimoch-Korzycka A, Appah P, Utioh A, Neufeld J, House JD (2017) Impact of processing on the protein quality of pinto bean (Phaseolus vulgaris) and buckwheat (Fagopyrum esculentum Moench) flours and blends, as determined by in vitro and in vivo methodologies. J Agric Food Chem 65(19):3919–3925
FAO/WHO (1991) Protein Quality Evaluation-Report of the Joint FAO/WHO Expert Consultation; Food and Nutrition Paper 51. Food and Agriculture Organization of the United Nations, The World Health Organization. Rome, Italy
Hsu HW, Vavak DL, Satterlee LD, Miller GA (1977) A multienzyme technique for estimating protein digestibility. J Food Sci 42(5):1269–1273
Kaiser AC (2019) Hammer and Roller Milling of Yellow Split Pea. Doctoral dissertation. North Dakota State University of Agriculture and Applied Science. Fargo, North Dakota
Marshall DR, Mares DJ, Moss HJ, Ellison FW (1986) Effects of grain shape and size on milling yields in wheat. II. Experimental studies. Aust J Agric Res 37(4):331–342
Wood JA, Knights EJ, Choct M (2011) Morphology of chickpea seeds (Cicer arietinum L.): comparison of desi and kabuli types. Int J Plant Sci 172(5):632–643
Patrascu L, Vasilean I, Banu I, Aprodu I (2017) Functional properties of pulse flours and their opportunities in spreadable food products. Quality Assur Saf Crops Foods 9(1):67–78
Hasjim J, Li E, Dhital S (2013) Milling of rice grains: effects of starch/flour structures on gelatinization and pasting properties. Carbohyd Polym 92(1):682–690
Stone AK, Nosworthy MG, Chiremba C, House JD, Nickerson MT (2019) A comparative study of the functionality and protein quality of a variety of legume and cereal flours. Cereal Chem 96(6):1159–1169
Gujska E, Reinhard WD, Khan K (1994) Physicochemical properties of field pea, pinto and navy bean starches. J Food Sci 59(3):634–636
Li L, Yuan TZ, Setia R, Raja RB, Zhang B, Ai Y (2019) Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem 276:599–607
Dubat A (2010) A new AACC international approved method to measure rheological properties of a dough sample. Cereal Foods World (CFW) 55(3):150
Mironeasa S, CodinĂ GG, Mironeasa C (2012) The effects of wheat flour substitution with grape seed flour on the rheological parameters of the dough assessed by Mixolab. J Texture Stud 43(1):40–48
Tiwari BK, Singh N (2012) Pulse chemistry and technology. Royal Society of Chemistry, London
Awolu OO, Magoh AO, Ojewumi ME (2020) Development and evaluation of extruded ready-to-eat snack from optimized rice, kersting’s groundnut and lemon pomace composite flours. J Food Sci Technol 57(1):86–95
Zhang D, Mu T, Sun H (2017) Comparative study of the effect of starches from five different sources on the rheological properties of gluten-free model doughs. Carbohyd Polym 176:345–355
El-Adawy TA (2002) Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Hum Nutr 57(1):83–97
Nosworthy MG, Medina G, Franczyk AJ, Neufeld J, Appah P, Utioh A, Frohlich P, Tar’an B, House JD (2020) Thermal processing methods differentially affect the protein quality of Chickpea (Cicer arietinum). Food Sci Nutr 8(6):2950–2958
Bai T, Nosworthy MG, House JD, Nickerson MT (2018) Effect of tempering moisture and infrared heating temperature on the nutritional properties of desi chickpea and hull-less barley flours, and their blends. Food Res Int 108:430–439
Wang S, Nosworthy MG, House JD, Niefer S-H, Nickerson MT (2020) Effect of barrel temperature and feed moisture on protein quality in pre-cooked Kabuli chickpea, sorghum, and maize flours. Food Sci Technol Int 26(3):265–274
Koehler HH, Chang CIH, Scheier G, Burke DW (1987) Nutrient composition, protein quality, and sensory properties of thirty-six cultivars of dry beans (Phaseolus vulgaris L.). J Food Sci 52(5):1335–1340
Chang KC, Harrold RL (1988) Changes in selected biochemical components, in vitro protein digestibility and amino acids in two bean cultivars during germination. J Food Sci 53(3):783–787
Wu W, Williams WP, Kunkel ME, Acton JC, Huang Y, Wardlaw FB, Grimes LW (1996) Amino acid availability and availability-corrected amino acid score of red kidney beans (Phaseolus vulgaris L.). J Agric Food Chem 44(5):1296–1301
Boye J, Wijesinha-Bettoni R, Burlingame B (2012) Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br J Nutr 108(S2):S183–S211
Jafari M, Rajabzadeh AR, Tabtabaei S, Marsolais F, Legge RL (2016) Physicochemical characterization of a navy bean (Phaseolus vulgaris) protein fraction produced using a solvent-free method. Food Chem 208:35–41
Nosworthy MG, Medina G, Franczyk AJ, Neufeld J, Appah P, Utioh A, Frohlich P, House JD (2018) Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 10(6):671
Chavan JK, Kadam SS, Salunkhe DK, Beuchat LR (1987) Biochemistry and technology of chickpea (Cicer arietinum L.) seeds. Crit Rev Food Sci Nutr 25(2):107–158
Mensack MM, Fitzgerald VK, Ryan EP, Lewis MR, Thompson HJ, Brick MA (2010) Evaluation of diversity among common beans (Phaseolus vulgaris L.) from two centers of domestication using “omics” technologies. BMC genomics 11(1):1–11
Rutherfurd SM, Gilani GS (2009) Amino acid analysis. Curr Protoc Protein Sci 58(1):11–19
Baik BK, Han IH (2012) Cooking, roasting, and fermentation of chickpeas, lentils, peas, and soybeans for fortification of leavened bread. Cereal Chem 89(6):269–275
Funding
Financial support was provided through the AgriScience Program (Canadian Pulse Science Research Cluster) under the Canadian Agricultural Partnership.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
This confirms that there is no competing interests for any of the authors.
Compliance with ethical standards
The above body of work did not involve human or animal testing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guldiken, B., Franczyk, A., Boyd, L. et al. Physicochemical, nutritional and functional properties of chickpea (Cicer arietinum) and navy bean (Phaseolus vulgaris) flours from different mills. Eur Food Res Technol 248, 1847–1858 (2022). https://doi.org/10.1007/s00217-022-04010-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04010-1