Abstract
The effect of the partial substitution (0, 10, 15, and 20%) of wheat flour with resistant starch (RS) on dough rheology and structure, and on the quality and staling rate of bread was evaluated. The results from farinograph, extensograph, alveograph, oscillatory rheological tests, and from confocal laser scanning microscopy, indicated that the substitution up to 15% of flour with RS slightly affected the dough structure, weakening it through dilution of gluten protein. Bread made with 15% of RS had specific volume, crumb moisture, and firmness values similar to those of the control bread (without RS), indicating very good quality. During storage, the RS breads had higher crumb moisture, lower firmness, and a lower retrogradation rate than the control bread. The lower retrogradation rate, in conjunction with the higher crumb moisture and high water-retention capacity of RS, was responsible for lower crumb firmness in bread containing up to 15% RS. Using wheat flour of high quality helped to minimize the deleterious effect of RS on the dough and provided high-fiber bread with high quality and low staling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistant starch (RS) is defined as a starch fraction, or the products of its degradation, that does not undergo any action by the gastrointestinal tract digestive enzymes of a healthy human being (Englyst et al. 1992), and which has a physiologic behavior similar to that of conventional dietary fibers.
The interest in the use of RS for the development of bakery products has increased in recent years due to its association with health benefits related to diabetes, obesity, cardiovascular diseases, and colon cancer (Higgins 2014; Sharma et al. 2008). This starch fraction has some advantages over conventional fibers when added to food products. Due to its white color, neutral flavor, and small particle size, RS can be used without significantly altering the sensory characteristics of the product (Sharma et al. 2008, 2016), and can be used either alone or combined with other fibers in different food products. However, the amount of RS used to replace flour depends on the starch type being used, the food type, and the desired fiber level (Sharma et al. 2016).
In breadmaking, the partial replacement of wheat flour with fibers may alter the rheological behavior of the dough, the fermentation time, and the bread quality (Ronda et al. 2014; Rosell and Santos 2010). The amount of flour to be substituted is directly related to changes that can occur in breadmaking performance, such as dilution of gluten proteins, alteration of the water absorption characteristics of the flour, and interaction of fiber with other components in the formulation (Cauvain and Young 1999).
Many studies about the effect of RS on bread’s quality and staling rate have been published. However, the results are controversial. Some studies have shown that RS improves the machinability of dough, positively influencing the breadmaking performance (Sanz-Penella et al. 2010) and resulting in bread with high volume, uniform crumb size, and neutral taste (Sharma et al. 2008), as well as a soft and smooth texture (Gómez et al. 2013; Sanz-Penella et al. 2010). On the other hand, it has also been reported that RS decreases the development time and stability of dough (Ozturk et al. 2009), weakens the gluten (Rosell et al. 2010), decreases the specific volume of bread, and increases crumb firmness (Altuna et al. 2016; Rosell and Santos 2010; Ozturk et al. 2009).
Bread staling is a complex phenomenon, mainly caused by water migration, amylopectin retrogradation, and gluten-starch interactions that happen during storage (Gray and Bemiller 2003). These events result in a loss of crispiness in the crust, a loss of water-holding capacity, an increase in crumb firmness, and a loss of organoleptic properties in loaves (Oliveira et al. 2014). Studies have reported that RS decreases the amylopectin retrogradation rate (Sanz-Penella et al. 2010) or behaves as an inert ingredient in the system (Almeida et al. 2013). On the other hand, Rosell and Santos (2010) reported that RS contributed to increasing the amylopectin retrogradation rate.
These conflicting results may be due to the different formulations and breadmaking methods used, differences that may influence the viscosity, emulsification, and water-retention capacity of dough (Curti et al. 2014). Some studies have used RS in combination with other dietary fibers (Almeida et al. 2013; Rosell and Santos 2010), enzymes (Altuna et al. 2016), and technological additives (Rosell and Santos 2010). The use of emulsifiers, enzymes, ascorbic acid, and hydrocolloids can minimize the deleterious effects of RS on dough (Almeida et al. 2013; Altuna et al. 2016; Gómez et al. 2013). Therefore, there are still not sufficient data to allow for a full understanding of the effect of RS on dough structure, bread quality, and staling.
The aim of this study was to systematically investigate the effect of RS on dough rheology and the structure, quality, and the staling rate of bread using a formulation without any technological additive.
Materials and methods
Materials
Wheat flour with 72% extraction rate was kindly donated by the Anaconda Mill (Curitiba, Brazil). The wheat flour contained 0.68% ash, 1.66% fat, 12.13% protein, which were determined according to the American Association of Cereal Chemists methods (AACC 2000). Hi-Maize 260®, kindly donated by Ingredion (São Paulo, Brazil) is a high amylose corn starch with 60% of RS, which had the following thermal properties determined by Differential Scanning Calorimetry (DSC): (tonset = 111.58 °C; tpeak = 126.40 °C; tconclusion = 141.24 °C; ΔH = 8.42 J/g). The other ingredients used to prepare the bread were obtained from a local market (São José do Rio Preto, Brazil).
Methods
Preparation of flour-resistant starch blends
Wheat flour based on 14% moisture was blended with RS in ratios of 100:0, 90:10, 85:15 and 80:20 (wheat flour: RS, w/w). The 100:0 sample was used as a control, and the other samples were named RS10, RS15, and RS20, respectively.
Rheological characteristics
Farinographic, extensographic, and alveographic properties of the wheat flour blends were determined according to AACC methods 54–21, 54–10, and 50–30A (AACC 2000), respectively, using a Brabender 810101 farinograph (Brabender, Duisberg, Germany), a Brabender 860000 extensograph (Brabender, Duisberg, Germany), and a Chopin MA-95 alveograph (Chopin Technologies,Villeneuve-la-Garenne, France).
Oscillatory rheological tests of the wheat flour-RS dough were carried out using an AR 2000EX rheometer (TA Instruments, Newcastle, USA), with serrated parallel plate geometry (40 mm in diameter and a fixed gap of 1 mm). The temperature was controlled at 30 °C by a Peltier system. The doughs were prepared without yeast in a Brabender 810101 farinograph (Brabender, Duisberg, Germany). The amount of water and development time used for preparing the doughs were those obtained from farinograph tests. Storage (G′), loss (G″), and complex (G*) moduli were evaluated by running frequency sweeps from 1 to 100 rad/s with a maximum strain of 0.2, which was found to be in the linear viscoelasticity region that was evaluated after strain sweeps at fixed frequency. The loss tangent (tan δ) was calculated as the ratio between G″ and G′. The results were analyzed using the Rheology Advantage Instrument Control AR software, v. 5.7.1 (TA Instruments, Newcastle, USA).
Confocal laser scanning microscopy of dough
Wheat flour-RS dough samples were prepared in a Brabender 810101 farinograph (Brabender, Duisberg, Germany) as described by Peighambardoust et al. (2006). The amount of water and development time used for preparing the doughs were those obtained from the farinograph tests. The method described by Van de Velde et al. (2003) with modifications was used for obtaining confocal scanning-laser microscope images. Dough samples (± 1 g) were immediately frozen in liquid nitrogen, cut (3–5 mm thickness) with a microtome (MEV, Slee, Mainz, Germany) at − 18 °C, and put on the glass slides, which were dipped in a fixative solution of 4% paraformaldehyde and 1% glutaraldehyde in 0.05 M phosphate buffer (pH 6.8). After resting for 1 h at room temperature, the glass slides were colored with a solution of 0.01% fluorescein isothiocyanate (FITC) and 0.1% eosin in deionized water. FITC colors the starch granules (green), while eosin colors proteins (red). Around 1 h after coloring, the glass slides were viewed under an LSM 710 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany) with an Ar/Kr laser-light source. The excitation wavelength was 568–625 nm and a 100 UV oil immersion objective lens was used.
Breadmaking
The bread samples were produced following the method described by El-Dash (1978), with modifications. The bread dough formulations were made up of 100% wheat flour:RS blends in different ratios (100:0; 90:10, 85:15, 80:20), 3% sugar, 3% dehydrated yeast (Saccharomyces cerevisiae), 3% maize oil, 1.75% salt and ~ 60% water depending on the amount determined in the farinograph tests. The doughs were placed in baking pans (15 × 8 cm) and proofed at 32 °C for 1 h before baking at 180 °C for 15 min in an industrial oven (Pasiani, Itajobi, Brazil). After baking, the loaves were allowed to cool at room temperature for 2 h, packaged in low density polyethylene bags (25 × 14 × 0.6 cm, JPR Packaging, São Paulo, Brazil), sealed and stored at room temperature for 0, 3, 5, 7, and 10 days for staling studies.
Specific volume of bread
The bread volume was measured 2 h after baking using the seed displacement test in a bread volumeter according to the AACC method 10–05 (AACC 2000). Three bread loaves were used to measure loaf volume of each formulation. The specific volume of the bread was calculated as the ratio between volume and weight (cm3/g).
Color of crumb and crust
The CIELAB system was used to determine the bread crumb and crust color. The color coordinate L*, a*, and b* values were determined using a Colorflex 45/0 colorimeter (HunterLab, Reston, USA) with D65 illuminant and 10° angle. Samples of bread crust cut as 5 cm diameter discs and bread crumb slices 1.25 cm thick, removed from the center of the bread were used for analysis.
Crumb and crust moisture content
Crumb and crust moisture contents of the bread stored at room temperature for 0, 3, 5, 7, and 10 days were analyzed according to the AACC method 44–40 (AACC 2000).
X-ray diffraction of bread crumb
The bread crumb samples, stored at room temperature for 0, 3, 5, 7, and 10 days, were left for 10 days in a desiccator with a saturated solution of BaCl2 (0.9 aw) for moisture stabilization. The X-ray diffraction patterns were determined using a benchtop MiniFlex 300 X-ray diffractometer (Rigaku, Tokyo, Japan) equipped with Cu Kα monochromatic radiation (λ = 0.1542 nm). The scanning speed was 1°/min at 40 kV and 30 mA. The relative crystallinity was quantitatively estimated based on the relationship between the peak and total areas following the method described by Nara and Komiya (1983).
Thermal analysis
Calorimetric measurements of the bread crumbs stored at room temperature for 0, 3, 5, 7, and 10 days and then lyophilized were determined using a Pyris 1 differential scanning calorimeter (DSC) (Perkin Elmer, Fremont, USA). Bread crumb samples (3 mg) were weighed in aluminum DSC pans, mixed with 9 μL of deionized water and sealed. The pans were kept at room temperature for 2 h and scanned at a rate of 10 °C/min from 25 °C to 150 °C. An empty aluminum pan was used as a reference. The enthalpy (ΔH) was calculated using the Pyris 1 software, v. 10.1 (Perkin Elmer, Fremont, USA).
Amylopectin recrystallization rate
The melting enthalpy data of the loaves stored at room temperature for 0, 3, 5, 7, and 10 days were used to estimate the Avrami model parameters according to the non-linear regression Eq. 1.
in which: θ: recrystallization fraction still to occur; ∆H0, ∆H∞, ∆Ht: retrogradation enthalpy (J/g) at ‘0’ zero time (fresh bread), ‘\( \infty \)’ final time, and ‘t’ time t; K: is a constant rate (usually used 1/k =time constant) to compare amylopectin retrogradation rate; n: exponent of Avrami (Armero and Collar 1998).
The k and n values were used to calculate the half-life time (t1/2) (Ronda et al. 2014) according to Eq. 2.
Firmness of the bread crumb
The firmness of the bread crumbs was determined using a TA-XT2i Texture Analyzer (Stable Microsystems, Surrey, UK) following the AACC method 74–09 (AACC 2000). Samples of bread crumb (25 mm thickness and 36 mm diameter) were obtained using a metal molder. The maximum force (in Newtons) needed to compress a sample of bread crumb to 40% of its original height using a probe of 36 mm in diameter at 5 s intervals between compressions was determined and 25% of this force was defined as the firmness value of the crumb. Ten replicates of bread crumb from each formulation were analyzed and averaged. The Exponent software, v. 5.010.0 (Stable Microsystems, Surrey, UK) was used for processing data.
Resistant starch content in bread
The RS content in the loaves was determined using the method described by Goñi et al. (1996). Bread crumb samples were lyophilized, triturated to pass through a 1 mm sieve and defatted. Defatted samples (100 mg) were incubated at 40 °C for 60 min with a solution (0.2 mL) containing 20 mg of pepsin to removing protein. A solution of tris-maleate containing 40 mg of pancreatic α-amylase EC 3.2.1.1 (A3176 Sigma) (1 mL, pH 6.9) was added and the mixture was incubated at 37 °C for 16 h. The hydrolysate was centrifuged and the residue solubilized with 4 M KOH, and incubated with amyloglucosidase EC 3.2.1.3 (A9913 Sigma) (80 μL) at 60 °C for 45 min. The free glucose content was measured using a Liquiform glucose-oxidase–peroxidase analysis kit (Labtest, Lagoa Santa, Brazil) and the RS content was calculated by multiplying mg of glucose by 0.9.
Statistical analysis
All determinations were performed in triplicate, with the exception of the oscillatory rheological tests of the dough and texture analysis of the bread that were performed twice and ten replicates, respectively. Analysis of variance (ANOVA) was applied using Statistica software, v. 7.0 (StatSoft Inc., Oklahoma, USA) and the differences were compared using the t test with Tukey’s adjustment (p <0.05). Pearson correlation and nonlinear regression (Avrami model) were also carried out with the Statistica 7.0 software. The Pearson correlation coefficient determined the following: Strong correlation: (r ≥ 0.9, p > 0.05); medium correlation (0.6 ≤ r < 0.9, p > 0.05), and weak correlation (r < 0.6, p > 0.05).
Results and discussion
Rheological characteristics
The farinographic, extensographic, and alveographic results are shown in Table 1. From the farinograph, the dough sample where the flour had not been substituted by RS had high water absorption (WA), development time (DT), stability (S), and a low mixing tolerance index (MTI) with values of 58.0%, 10.3 min, 17.2 min, and 10 BU, respectively. These parameters varied from 66.0 to 72.5%, 6.4 to 7.3 min, 14.2 to 15.3 min, respectively, and from 27.5 to 40.0 BU for the dough samples with RS substitution. WA substantially increased as the RS concentration increased indicating that, even with the lower amounts of protein and damaged starch in the flour-RS blends, the RS contributed to increase in WA. WA of the RS used in this work, determined at 30 °C, was 7% (data not shown). High WA values for blends of flour and RS have also been reported earlier (Fu et al. 2008; Sanz-Penella et al. 2010). The substitution of flour with RS, independent of the concentration, significantly decreased the DT and S of dough, while the MTI increased with increasing RS content when compared to the control. The RS15 sample with an intermediate level of RS had the lowest S when compared to the other samples. The S and MTI indicate how much processing a flour can undergo following the dough’s DT. According to Cauvain and Young (1999), flours with DT between 6.1 and 8.0 min are classified as medium-strong flours, while ones with S between 10.1 and 15.0, and MTI between 0 and 50, are classified as strong flours. Based on these parameters, all of the flour-RS blends, no matter the RS concentration, had adequate farinographic characteristics for baking (Table 1).
From the extensographic data, dough made with unsubstituted flour (no RS) showed characteristics of a strong dough, with resistance to extension at constant deformation (R50) and extensibility (E) of 630.0 BU and 149.5 mm, respectively, at the final rest time (135 min). R50 and E values ranged from 570.0 to 465.0 BU and from 132.5 to 121.0 mm, respectively, for the dough samples substituted with RS. The rest time was important for the RS15 and RS20 samples, which needed the highest rest time (135 min) in order to reach maximum resistance. This implies that an excessive level of free water in the dough can increase mobility of the other molecules, resulting in a lower R50 (Salvador et al. 2006). From the alveographic data, dough made from flour that had not been substituted with RS had 93.6 mm of tenacity (P), 77.5 mm of extensibility (L), and 278.9 × 10−4 J of energy (W), while P increased, and L deceased, in dough samples with increasing levels of substituted RS. In general, the control and RS10 samples had a better ratio between tenacity and extensibility (P/L). On the other hand, the W values of the RS10 and RS15 dough samples decreased in relation to the control dough, while W increased for RS20, reaching 317.7 × 10−4 J. However, the W value alone is not enough to determine baking quality. The RS20 dough also had the highest P/L ratio (4.0). This value was high enough to potentially hinder dough expansion during fermentation (Table 1).
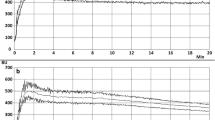
The results from the oscillatory rheological tests are presented in Table 1 and Fig. 1. The mechanical spectra showed that G′ and G″ moduli increased only slightly over a wide frequency range, revealing the existence of a network with a behavioral characteristic like that of a structured system in the samples (Fig. 1). All of the samples had G′ higher than G″, indicating that the dough samples were predominantly of an elastic character (Fig. 1a). The RS caused a decrease in gel consistency (Fig. 1b), with the RS15 and RS20 doughs affected to the greatest degree. However, both of these doughs had similar behavior. These results confirm the slight weakening of the dough due to the dilution of gluten protein caused by the substitution of flour with RS. Different from the starch present in flour, it seems that RS worked as an inert ingredient and that it did not interact with other dough components, since lower responses of G′ and G″ were obtained for the samples containing RS in comparison to the control dough.
Mechanical spectra: a Storage moduli (G′) (closed symbol) and loss moduli (G″) (open symbol) of dough from flour-RS blends: control (closed square, open square), RS10 (closed circle, open circle), RS15 (closed up-pointing triangle, open up-pointing triangle), RS20 (closed left-pointing triangle, open left-pointing triangle); b complex moduli (G*): control (closed square), RS10 (closed circle), RS15 (closed up-pointing triangle), RS20 (closed left-pointing triangle); c phase angle (tan δ): control (closed square), RS10 (open circle), RS15 (closed up-pointing triangle), RS20 (open left-pointing triangle)
The power-law model was applied to estimate the empiric rheological parameters (k and n). The model fitted very well to the data (R2 ≥ 0.962) (Table 1). The consistency indexes (k′, k″, and k*) decreased with increasing RS concentration. These results indicate that the RS slightly decreased the consistency of the dough. The exponents of the power law (n′, n″, and n*) can be related to the relaxation exponent nr. True gels should present n′ = n″ = nr. Gels with nr approaching 1 are purely viscous, whereas nr approaching 0 suggests a purely elastic gel (Albano et al. 2014). Table 1 shows that n′ and n″ were lower than 0.211, suggesting that the doughs had a strong elastic character, although the differences between n′ and n″ for each sample increased with increasing addition of RS, indicating an increasing departure from the behavior of a true gel system.
The phase angle (tan δ) provides information about the structural order (molecular interactions) of a material: highly structured materials have low tan δ values (Albano et al. 2014). The tan δ of all the dough samples was relatively low. Practically no difference was observed in tan δ for the control and RS15 doughs over the whole frequency range (Fig. 1c), whereas RS10 and RS20 doughs had slightly higher and slightly lower tan δ values, respectively, than the control dough. These results suggest that the dough samples, independent of their RS content, were well structured.
Confocal laser scanning microscopy of the dough
The CSLM micrographs (Fig. 2) display the protein phase (red) and starch granules (green), while air pockets are represented in black. The control and RS15 dough samples displayed starch granules embedded in an elongated fibril protein network, as previously observed by Peighambardoust et al. (2006) for dough prepared with NaCl. However, in the control dough, the gluten network was more homogeneous (Fig. 2a) than in the RS15 dough (Fig. 2b), which displayed a more dispersed phase (protein) surrounding the starch granules. This could have occurred due to the dilution of the flour and consequent weakening of the dough gluten by RS.
Bread characteristics
Specific volume, color parameters, moisture, firmness, and RS content of bread made from flour with and without RS substitution are shown in Table 2. The control, RS10, and RS15 specific volumes were similar, while there was a decrease of 25% in the specific volume of the RS20 bread. These results agree with the rheological characteristics of the dough samples (Fig. 1), as different elastic properties can be affected by the addition of dietary fibers. A correlation between the difference between G′ and G″ and the specific volume of bread was found: the lower the difference (G′–G″), the larger the specific volume of the bread (Khatkar and Schofield 2002). The RS20 bread had the highest G′- G″ value, and the smallest specific volume. Decreased bread specific volumes were found by Altuna et al. (2016), Rosell and Santos (2010), and Ozturk et al. (2009) when they incorporated RS into their formulations. According to these authors, the RS diluted the gluten network, resulting in less expandable doughs. On the other hand, Gómez et al. (2013) reported that the substitution of wheat flour with up to 12.5% of RS did not affect the bread’s specific volume, probably due to the additives used in its formulation (a combination of emulsifiers, enzymes, and oxidants).
Bread without substitution showed L*, a*, and b* values of 76.16, 0.91, and 18.78, respectively, for the crumb, while the crust of the bread showed values of 58.85, 14.41, and 36.03, respectively. The bread crumb color is influenced by the color of the formulation ingredients and, in white wheat bread, should have a creamy shade and a yellowish color (Cauvain and Young 1999). For the bread crumb, values of L* and b* were slightly affected by the addition of RS, independent of the concentration. In contrast, a*, which is associated with red coloring, decreased with increasing RS content (Table 2). The color of the crust affects the acceptability of the bread. It should be golden, bright, and homogeneous (Cauvain and Young 1999). The higher the concentration of RS, the higher the L* and the lower the a* values (Table 2). This could be due to the protein dilution and lower amount of wheat flour starch in the flour-RS blends, which decreased the amount of reducing sugar and amino acids available for the Maillard reaction during baking, resulting in slightly lighter and less reddish bread crusts. Sharma et al. (2016) reported that cookies and muffins prepared by substitution of wheat flour with RS were lighter in color compared to those made without substitution, attributing this to the dilution of protein.
After 2 h of baking the moisture contents of the bread crumb samples were similar to each other, being approximately 43% (Table 2), which is an acceptable value for fresh bread (Shah et al. 2006).
The firmness of the control, RS10, and RS15 bread samples was very similar, while that of the RS20 bread was double that of the control bread. As expected, these results indicated a direct relation with the specific volume of the bread samples. Gómez et al. (2013) and Sanz-Penella et al. (2010) also reported that RS did not affect bread firmness when it was used to substitute up to 12.5% and 30% of wheat flour. However, in contrast to our present study, the authors used flour improvers in their formulations that may have masked the effect of RS on firmness. On the other hand, Altuna et al. (2016), Rosell and Santos (2010), and Ozturk et al. (2009) found loaves to be firmer when RS was used, even with the addition of additives to the formulation. These results confirm the importance of using flour with high protein content and quality for breadmaking when it will be partially substituted with fibers.
The control bread contained only 1.21% RS, agreeing with Goñi et al. (1996) who reported that the RS level in white bread ranges from 1 to 2.5%. On the other hand, the RS levels in the RS10, RS15, and RS20 loaves were 3.86, 5.93, and 11.11%, respectively. RS levels higher than 5% in food products can ensure the starch’s functional effect (Sharma et al. 2008) and contribute to the recommended daily intake of 14.0–33.6 g of dietary fiber, which is reported in the Dietary Guidelines for Americans (USDA 2015) as the necessary amount for maintaining good health.
Generally, for a formulation without any chemical additives, a wheat flour of high quality and an adequate amount of RS (up to 15%) resulted in breads of very good quality. At these RS concentrations, bread with characteristics similar to those of the control bread, but with increased fiber contents, were obtained.
Bread staling
Moisture content of the bread
Moisture content of the crumb and crust of bread, made with and without substitution of flour with RS, as a function of storage time, is shown in Fig. 3a. Bread’s water content is related to its softness, and high moisture can contribute to delayed bread staling (Ruan et al. 1996). A moisture gradient is formed during storage. The crust, with relatively low moisture, easily absorbs water from the crumb (Ruan et al. 1996). In our study, moisture migrated from the crumb to the crust for all the bread samples, independent of the RS concentration. However, the decrease in moisture content in the control bread crumb was more accentuated than in breads containing RS. During the whole storage period, the crumb of the loaves formulated with RS retained more moisture than that of the control loaf. The higher moisture gradient in the control loaf at the beginning of storage potentially contributed to these results. There was a medium correlation (r = 0.80, p > 0.05) between the moisture content of the bread crumb and the WA of the flour-RS blends.
Moisture content (a) of crumbs (closed symbol) and crust (open symbol) of the bread obtained from flour-RS blends as a function of storage time: Control (closed square, open square); RS10 (closed circle, open circle); RS15 (closed up-pointing triangle, open up-pointing triangle); RS20 (closed left-pointing triangle, open left-pointing triangle), and X-ray pattern and relative crystallinity of the crumbs of the bread obtained from flour-RS blends during storage at 0 day (b) and at 10 day (c); Enthalpy change (d) and firmness (e) of the bread crumbs as a function of storage time: control (closed square); RS10 (closed circle); RS15 (closed up-pointing triangle); RS20 (closed left-pointing triangle)
X-ray diffraction of bread crumb
The X-ray diffraction pattern and relative crystallinity of the crumb of the bread made with flour-RS blends during storage at day 0 and day 10 are shown in Fig. 3b, c. Starch exhibits different X-ray diffraction patterns depending on its crystalline structure. The B-type crystalline pattern displays peaks at 5.6°, 14.9°, 17°, and 22° in 2θ, and is characteristic of tuber-derived, high amylose, and retrograded starches, while the V-type pattern is characterized by peaks around 7.4°, 12.9°, and 20.1°, which correspond to amylose–lipid complexes (Tian et al. 2009). The diffractograms of the crumbs from the control and RS-substituted fresh loaves at day 0 (Fig. 3b) displayed a V-pattern with a peak around 20° in 2θ, which is characteristic of the amylose–lipid complex formed between starch and endogenous and exogenous lipids during baking (Primo-Martín et al. 2007). Although the RS did not gelatinize during baking, the peaks relative to the intact starch granules (B-type pattern) contained in fresh bread crumb with added RS were not observed, probably due to the small amount of RS in the crumb samples.
During storage, gelatinized starch molecules re-associate and form a new crystalline order (Primo-Martín et al. 2007). In the present study, the bread crumb displayed peaks with high resolution around 15°, 17°, 20°, and 22° in 2θ (Fig. 3c), suggesting a mixture of V- and B-types patterns. As expected, the relative crystallinity of the fresh control bread crumb was low (11.27%), increasing along with RS levels. There was a significant increase in the relative crystallinity of bread crumb during storage as a function of amylopectin recrystallization. Independent of the storage day, the relative crystallinity increased when the RS content of the bread was increased. As presented above, the RS used in this work had a high gelatinization temperature (Tp = 126.40 °C), which was not reached during baking, meaning that the RS could contribute to the relative crystallinity of bread.
Amylopectin recrystallization rate in the bread crumb
The enthalpy change during storage of the bread crumb was similar for each formulation, independent of RS concentration (Fig. 3d). There was a progressive increase in ΔH until the third day of storage, followed by an incrementally lower rate of increase until the tenth day. This behavior could be explained by the reassociation of amylopectin molecules during storage. The addition of RS, however, significantly decreased the extent of amylopectin recrystallization over the whole storage period, independent of concentration, inducing a slower retrogradation rate. This occurred due to the lower amount of gelatinized starch ready to retrograde during storage of the bread samples containing substituted RS.
In order to understand amylopectin recrystallization in the bread crumb, the Avrami equation was used (Table 3). The k and n parameters of the Avrami model are related to the amylopectin crystallization rate. High values for these constants indicate a quick retrogradation rate, which is more perceptible with the increase of k (Armero and Collar 1998). However, low values for the ΔH0 and ΔH∞ parameters also contribute to a slow retrogradation rate (Curti et al. 2014). We found that the Avrami exponent (n) did not vary for the control, RS10, and RS15 breads, which suggested that the recrystallization of amylopectin molecules in the crumb of these loaves was similar. On the other hand, there was a perceptible increase in the n value for the RS20 loaf compared to the other samples. A higher amount of crystals in a sample leads to a higher n value (Armero and Collar 1998). The higher RS concentration in this sample, which did not gelatinize during baking, may have contributed to this result. Indeed, there was a strong correlation (r = 0.95, p > 0.05) between relative crystallinity and Avrami number for the samples stored for 10 days. On the other hand, there was a progressive decrease in the k constant, and the ΔH∞, as the RS level increased, as well as an increase in t½. In other words, the longer the half-life, the slower the rate of amylopectin recrystallization (Ronda et al. 2014). These results confirmed a lower retrogradation rate in loaves with RS added. This probably occurred due to the decreased amount of gelatinized amylopectin ready for recrystallization during storage in the loaves with RS added.
Firmness of the bread crumb
There was a progressive increase in the firmness of the bread crumb with storage time (Fig. 3e), increasing from the third to seventh day, independently of the sample being examined. In the first three days, the control, RS10, and RS15 loaves were similar in terms of firmness, with values close to 2 N. After that, the RS15 bread was the least firm, reaching values below 8 N on the tenth day. The RS20 bread was the firmest during the entirety of the time in storage. These results confirm that amylopectin retrogradation is not solely responsible for bread staling, an idea that has been extensively postulated (Gray and BeMiller 2003; Ronda et al. 2014). Indeed, the moisture content of the crumb, and the interactions between gluten proteins and starch, are important factors that affect changes in the texture of bread. In this study, there was no evidence of any starch-gluten interactions.
There was a strong negative correlation (r = − 0.90, p > 0.05) between crumb firmness and moisture throughout the storage period. The moisture delayed the bread staling rate since it affected the gluten network (Curti et al. 2014), led softer bread crumb (Ronda et al. 2014). The moisture content of the RS-substituted bread crumb was approximately 45%, while the control loaf had the lowest moisture content (Fig. 3a). In addition, crumb moisture was positively correlated with RS level in the samples (0.88, p > 0.05). In accordance with this, higher water absorption capacity of the flour-RS blends (Table 1) contributed to the higher moisture levels observed in the crumb of the RS-substituted loaves. The lower retrogradation rate and the higher water retention of the loaves with added RS seems to have been responsible for lower firmness in these loaves, with the exception of the RS20 bread. The highest amount of RS (20%) in the flour-RS blend negatively affected the dough, and resulted in the lowest specific volume of the bread and highest firmness.
Conclusion
Resistant starch acted as an inert ingredient, diluted the flour proteins and weakened the dough, which slightly compromised the gluten network. However, the use of high quality flour allowed for the substitution of up to 15% of flour with RS and produced bread with high fiber content as well as technological quality similar to that of the control bread. RS decreased the amylopectin recrystallization rate during storage which, in conjunction with the high water-retention capacity of RS, was responsible for decreasing the bread staling rate. Therefore, the results demonstrates the fiber content and staling rate of bread may be improved through the addition of significant amounts of RS, without negatively impacting the bread’s quality.
References
AACC (2000) Approved methods of the American Association of Cereal Chemists, 10th ed. St. Paul
Albano KM, Franco CML, Telis VRN (2014) Rheological behavior of Peruvian carrot starch gels as affected by temperature and concentration. Food Hydrocoll 40:30–43
Almeida EL, Chang YK, Steel CJ (2013) Dietary fibre sources in bread: influence on technological quality. LWT Food Sci Technol 50:445–453
Altuna L, Romano RCO, Pileggi RG, Ribotta PD, Tadini C (2016) Torque measurement in real time during mixing and kneading of bread dough with high content of resistant maize starch and enzymes. Int J Food Eng 12:719–728
Armero E, Collar C (1998) Crumb firming kinetics of wheat breads with antistaling additives. J Cereal Sci 28:165–174
Cauvain SP, Young LS (1999) Technology of breadmaking. Aspen Publishers, Gaithersburg
Curti H, Carini E, Tribuzio G, Vittadini E (2014) Bread staling: effect of gluten on physico-chemical properties and molecular mobility. LWT Food Sci Technol 59:418–425
El-Dash AA (1978) Standardized mixing and fermentation procedure for experimental baking test. Cereal Chem 55:336–446
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:33–50
Fu L, Tian JC, Sun C, Li C (2008) RVA and farinograph properties study on blends of resistant starch and wheat flour. Agric Sci China 7:812–822
Gómez AV, Buchner D, Tadini CC, Añón MC, Puppo MC (2013) Emulsifiers: effects on quality of fibre-enriched wheat bread. Food Bioprocess Technol 6:1228–1239
Goñi I, Garcia-Diz L, Mañas E, Saura-Calixto F (1996) Analysis of resistant starch: a method for foods and food products. Food Chem 56:445–449
Gray JA, BeMiller JN (2003) Bread staling: molecular basis and control. Compr Rev Food Sci Food Saf 2:1–21
Higgins JA (2014) Resistant starch and energy balance: impact on weight loss and maintenance. Crit Rev Food Sci 54:1158–1166
Khatkar BS, Schofield JD (2002) Dynamic rheology of wheat flour dough. I. Non linear viscoelastic behaviour. J Sci Food Agric 82:827–829
Nara S, Komiya T (1983) Studies on the relationship between water-saturated state and crystallinity by the diffraction method for moistened potato starch. Starch/Staerke 35:407–410
Oliveira DS, Telis-Romero J, Da-Silva R, Franco CML (2014) Effect of a Thermoascus aurantiacus thermostable enzyme cocktail on wheat bread quality. Food Chem 143:139–146
Ozturk S, Koksel H, Ng PKW (2009) Farinograh properties and bread quality of flours supplemented with resistant starch. Int J Food Sci Nutr 60:449–457
Peighambardoust SH, Van Der Goot AJ, Van Vliet T, Hamer RJ, Boom RM (2006) Microstructure formation and rheological behaviour of dough under simple shear flow. J Cereal Sci 43:183–197
Primo-Martín C, Van Nieuwenhuijzen NH, Hamer RJ, Van Vliet T (2007) Crystallinity changes in wheat starch during the bread-making process: starch crystallinity in the bread crust. J Cereal Sci 45:219–226
Ronda F, Quilez J, Pando V, Ross YH (2014) Fermentation time and fiber effects on recrystallization of starch components and staling of bread from frozen part baked bread. J Food Eng 131:116–123
Rosell CM, Santos E (2010) Impact of fibers on physical characteristics of fresh and staled bake of bread. J Food Eng 98:273–281
Rosell CM, Santos E, Collar C (2010) Physical characterization of fiber enriched bread doughs by dual mixing and temperature constraint using the Mixolab. Eur Food Res Technol 231:535–544
Ruan R, Almaer S, Huang VT, Perkins P, Chen P, Fulcher RG (1996) Relationship between firming and water mobility in starch-based food systems during storage. Cereal Chem 73:328–332
Salvador A, Sanz T, Fiszman SM (2006) Dynamic rheological characteristics of wheat flour–water doughs. Effect of adding NaCl, sucrose and yeast. Food Hydrocoll 20:780–786
Sanz-Penella JM, Wronkowska M, Soral-Smietana M, Collar C, Haros M (2010) Impact of the addition of resistant starch from modified pea starch on dough and bread performace. Eur Food Res Technol 231:499–508
Shah AR, Shah RK, Madamwar D (2006) Improvement of the quality of whole wheat bread by supplementation of xilanase from Aspergillus foetidus. Biores Technol 97:2047–2053
Sharma A, Yadav BS, Ritika BS (2008) Resistant starch: physiological roles and food applications. Food Rev Int 24:193–234
Sharma S, Singh N, Katyal M (2016) Effect of gelatinized-retrograded and extruded starches on characteristics of cookies, muffins and noodles. J Food Sci Technol 53:2482–2491
Tian YQ, Li Y, Jin ZY, Xu XM, Wang JP, Jiao AQ, Yu B, Talb T (2009) β-cyclodextrin: a new approach in bread staling. Thermoch Acta 489:22–26
USDA (2015) U.S. Department of Health and Human Services and U.S. Department of Agriculture, 2015–2020 Dietary Guidelines for Americans. 8th ed. Retrieved Aug. 18, 2017, from: http://health.gov/dietaryguidelines/2015/guidelines/
Van de Velde F, Weinbreck F, Edelman MW, Van Der Linden E, Tromp RH (2003) Visualization of biopolymers mixtures using confocal scanning laser microscopy (CSLM) and covalent labelling techniques. Colloids Surf B Biointerfaces 31:159–168
Acknowledgements
The authors thank the São Paulo Research Foundation—FAPESP—(Grants Nos. 2013/17725-8 and 2009/53990-2) and National Council for Scientific and Technological Development—CNPQ (Grant No. 306320/2012-6) in Brazil for financial support. The authors also thank Dr Patricia S. L. Vilamaior for technical support on confocal microscopy analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Barros, J.H.T., Telis, V.R.N., Taboga, S. et al. Resistant starch: effect on rheology, quality, and staling rate of white wheat bread. J Food Sci Technol 55, 4578–4588 (2018). https://doi.org/10.1007/s13197-018-3393-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3393-6