Abstract
This study focused on the changes of physicochemical and microbiological properties and aroma compounds of freshly-squeezed orange juice during storage at different temperatures. Aroma compounds were analyzed by solid-phase microextraction–gas chromatography–mass spectrometry (SPME–GC–MS). The results showed that the total aerobic plate counts of orange juice stored at room temperature and 37 °C was far more than 4 °C. Totally 33 aroma compounds were determined in these orange juices. Significant differences on the aroma compounds in orange juices stored at different temperatures were observed in the present study. Most of the terpenes decreased at 4 °C after 15 days’ storage, while 10 and 8 terpenes increased during storage at room temperature and 37 °C. α-Terpineol and p-vinylguaiacol were the only off-flavor compounds found in juice stored at 4 °C and room temperature at late storage respectively. While terpinen-4-ol, 4-ethylguaiacol and p-vinylguaiacol were found in juice stored at 37 °C at late storage. α-Terpineol was the only off-flavor compound found in orange juice stored at 4 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Citrus fruit is one of the most important commercial fruits in the world. And it is the most economically relevant and extensively grown fruit tree crop in the world and their fruits are an important source of secondary metabolites for nutrition, health, and industrial applications (Rodríguez et al. 2017). Orange juice is the most popular juice in the world because it has aroma, attractive flavor and color, as well as its health benefits. (O’Neil et al. 2011). Orange flavor is perhaps widely recognized and accepted in the food and beverage industry around the world (Kelebek and Selli 2011). The flavor of fresh orange juice is due to the complex combination of a large number of volatile compounds which includes esters, aldehydes, alcohols, ketones, and terpenes et al. (Perez-Cacho and Rouseff 2008a). Changes of physicochemical and microbiological parameters and aroma compounds of the orange juice may occur during storage. Odors and flavors are the major determinants of fruit quality, but these traits are often genetically complex and difficult to score (Galili et al. 2002). Storage time, temperature and microbial contamination had a significant effect on the flavor of fruit juice (Perez-Cacho and Rouseff 2008a).
Freshly-squeezed orange juice is the sensory standard for the taste of orange juice. The concentration of volatile compounds in juice depends on temperature, processing methods, storage time, heat treatment and the amount of volatile compounds added to the juice and so on (Perez-Cacho and Rouseff 2008b). Wibowo et al. (2015) reported that the volatile had different changes at different storage temperatures and it has great influence on storage temperature. They found that more volatiles changed at higher temperatures than that at 20 °C and the increase of terpenes and sulphur compounds was observed at higher storage temperatures (Wibowo et al. 2015). Additionally, some off-flavors may be formed due to complex chemical reactions. The emergence of fresh and unique flavor was caused by natural combination of volatile compounds of sugar, acid and phenolic compounds, and it is a balanced system (Kelebek and Selli 2011).
It is difficult to preserve the fresh orange juice because of temperature, light exposure and other storage environment. At present, the judgement of the quality of orange juice is performed mainly according to the appearance, color, acidity and microbial detection. However, the operation of microbial detection is more complex and time consuming. Aroma index is one of the important qualities of citrus juice, and this indicates that the quality may be judged from the changes of these compounds. The aroma compounds in orange juice and its changes in processing orange juices during storage have been investigated widely, while the changes of these compounds in freshly-squeezed orange juice stored at different temperatures have seldom been reported. Hence, it is interesting to investigate the changes of aroma compounds and physicochemical and microbiological properties of orange juice during storage. This study aimed to study the changes of aroma compounds and physicochemical and microbiological properties of freshly-squeezed orange juice during storage until spoilage.
Materials and methods
Reagents and reference samples
The reagent acetic acid, sodium hydroxide, phenolphthalein and sodium chloride were of analytical reagent grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The water used in the study was purified using Millipore-Q system (Millipore Corp., Saint-Quentin, France). Standards of n-paraffins (C6–C25) were purchased from Sigma Chemical Company (Saint Louis, MO, USA). Aroma standards, particularly 1-hexanol, β-elemene, α-thujene, α-terpinene, copaene, isoamyl acetate, benzyl acetate, were gifts from Shenzhen Boton Flavors & Fragrances Co., Ltd. (Shenzhen, China). 3-Carene, d-limonene, terpinolene, α-terpineol, citral, decanal, octanal, β-myrcene, β-phellandrene, nerol, germacrene D, 2-hexenal, α-pinene, valencene, δ-cadinene, γ-terpinene, terpinen-4-ol, camphene, alloaromadendrene, β-gurjunene, cubebene, caryophyllene and p-vinylguaiacol were obtained from Sigma Chemical Company (Saint Louis, MO, USA).
Plant material
Mature navel oranges were purchased from Zigui City, Hubei Province, P. R. China. The oranges were harvested in December. The harvested citrus fruits were washed and then dried. The pulp was obtained by hand separation from the peels and was extracted into juice by using a centrifugal juice extractor. Then the juices were packed in 500-mL sterilized bottles and stored at 4 °C, room temperature and 37 °C, respectively. The physicochemical and microbiological properties and aroma compounds of the orange juice were detected everyday until significant deterioration occurred.
Physicochemical and microbiological properties
The pH values of the juices were detected using a pH meter. The total titratable acid was measured by titration using standardized 0.1 M sodium hydroxide. The total soluble solids were measured as °Brix using a saccharometer. Microbiological analysis was performed according to the Chinese National Standard (GB 4789.2-2016) by measurement of the total bacteria amounts which was indicated as total aerobic plate counts (TPC). TPC was determined by spread plating samples on nutrient agar and incubating at 36 °C for 48 h. Microbial colonies were counted and reported as log CFU/g of fresh weight.
Sensory analysis
Sensory analysis was performed according to the previous study (Ren et al. 2015). Ten assessors (eight females and two males) from College of Food Science and Technology, Huazhong Agricultural University. Most of the assessors experienced in GC–olfactometry. All the assessors were trained according to the guidelines of the ISO 8586-1 (1993) and have passed the screening tests, in order to familiarize the aroma of the orange juice and to improve their ability to recognize, identify and quantify the sensory attributes of the juice. Then they were familiarized with the flavor of the orange juice and instructed to agree on a common list of five descriptors: fruity, orange, grassy, floral, and sour. Descriptive analysis of the orange juices was performed as follows: Five milliliter of the orange juices was placed in a 10 mL coded flask. Then about 2 cm of the extremity of the fragrance blotter paper (142 mm × 6 mm) was immersed in the sample for 0.5 min and then presented to the assessors. The intensity of each descriptor was tested on a scale of 1–9 (1 = very weak intensity, 3 = weak intensity, 5 = moderate intensity, 7 = strong intensity, and 9 = very strong intensity) (Selli et al. 2008). And the sensory profile analysis results were plotted in a column chart.
Extraction of aroma compounds
A solid-phase microextraction (SPME) manual device equipped with 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, Bellfonte, PA, USA) was used to extract aroma compounds from orange juices. The fiber was conditioned in a GC injector port at 270 °C for 1 h before use. Afterward, 10 mL of orange juices with 3.6 g of NaCl was placed in a 20 mL vial containing a microstirring bar. The samples were equilibrated at 40 °C for 15 min and extracted using the DVB/CAR/PDMS fiber for 40 min at the same temperature with continuous stirring. After volatiles were extracted, the fiber was inserted into the GC injection port to desorb the analytes for 5 min. Each analytical sample was analyzed in triplicate.
GC–MS analysis of volatile compounds
Volatile compounds were subjected to GC analysis on an Agilent 6890 N GC coupled to mass spectrometer (5975B) and equipped with a J&W HP-5MS fused silica capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). Mass spectral ionization was set at 230 °C. The mass spectrometer was operated in an electron ionization mode at a voltage of 70 eV. The flow rate of helium on the HP-5 column was 1.2 mL/min. A 0.75 mm liner was used. Analysis was conducted in a splitless mode. Injector temperature was 250 °C. The column was initially maintained at 40 °C for 3 min; temperature was then increased from 40 to 160 °C at 3 °C/min, maintained at 160 °C for 2 min, and finally increased to 220 °C at a rate of 8 °C/min. Temperature was maintained at 220 °C for 3 min.
The compounds detected by GC–MS analysis were identified by comparing the obtained mass spectra and retention indices (RI) with those of authentic standards and published data and by comparing the corresponding mass spectra with the MS library of Wiley7.0 and Nist05. RIs were calculated using a mixture of n-paraffin C6–C25 as standards. Volatile compound contents were expressed as the GC peak areas.
E-nose analysis
The E-nose analysis was conducted using a FOX4000 Alpha M.O.S. (France) E-nose system. A static headspace (HS-100) autosampler was used for sample introduction. Three chambers of 18 sensors (chamber 1 contains LY2/LG, LY2/G, LY2/AA, LY2/GH, LY2/gCTL and LY2/gCT; chamber 2 contains T30/1, P10/1, P10/2, P40/1, T70/2 and PA/2; chamber 3 contains P30/1, P40/2, P30/2, T40/2, T40/1 and TA/2) were used for the measurement of the odor characteristics of samples.
Static headspace was generated in a 10 mL vial using 2 g of samples. Headspace (2500 mL) carried by air (150 mL/min) was injected into the E-nose. Sensor resistance was measured during 120 s at the rate of one acquisition every 1 s. All samples were run in seven repetitions. Radar graph and data for PCA were obtained using the built in software for E-nose analysis. Alpha Soft (version. 12.4) software was used for data processing.
Statistical analysis
The average values and standard deviations of the intensity of each descriptor obtained from the ten assessors were calculated and reported. Significant differences of physicochemical and microbiological properties and aroma compounds in orange juices obtained in triplicate analysis were determined by one-way ANOVA using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Correlations between sensory data and chemical compounds were computed using partial least square (PLS) regression analysis in XLSTAT 2010 (Addinsoft, New York, NY, USA).
Results and discussion
Physicochemical and microbiological properties
The changes in physicochemical properties of orange juices during storage at different temperatures are shown in Fig. 1. As shown in Fig. 1a, the pH of the orange juice decreased first, then increased, and finally tended to be stable during storage at 4 °C. The pH of freshly-squeezed orange juice varied between 3.3 and 4.3 (Parish 1998). The changes of pH value were relatively flat. It increased slightly and then decreased slightly during storage at room temperature. The pH value initially decreased and then increased during storage at 37 °C. As shown in Fig. 1b, the total acid contents of the orange juice were unstable during the storage. The content of the total soluble solids content of the orange juice decreased first and then increased during storage at 4 °C (Fig. 1c). And it increased when storage at room temperature and 37 °C.
The changes in total acidity in fruit juices during storage have been observed earlier. Li et al. (2009) found that the total acid of longan juice decreased first and then increased after 50 days’ storage at 4 °C. Qian et al. (2014) found that the total acid of mandarin fruit juice increased greatly. It decrease after 5 weeks’ of storage at 5 °C was observed. Total acid also increased greatly in pasteurized processed and fresh commercial-squeezed orange juices during 4 days’ storage at 5 °C (Baldwin et al. 2012). And they reported that the heating process in thermal pasteurization might have caused an increase in the total acids (Baldwin et al. 2012). The main reason of the increase of the total acid in juice might be the generation of acids from carbohydrate according to the oxidation (Fischer and Bipp 2005).
The changes in total aerobic plate counts (TPC) of orange juices stored at different temperatures are shown in Fig. 1d. Similarly, total aerobic plate counts of the orange juices stored at room temperature and 37 °C were only measured for 4 days. TPC in orange juices stored at 4 °C were less than 10 CFU/g in the first 5 days’ storage. While it increased gradually from the sixth day. While a trend of decreasing followed by increasing microbial populations took place in the first few days of storage of unpasteurized orange juice at low temperatures (Eleftheriadou et al. 1998). TPC increased significantly during the storage at room temperature and 37 °C, and it exceeded the TPC standard of 10 000 CFU/g in DB33/533-2005 (Hygienic standard and regulation for squeezed fresh fruit and vegetable juices of Zhejiang Province in China). While TPC in orange juices stored at 4 °C didn’t exceed this criteria during the 15 days’ storage. Microbiological contaminations can change the characteristic aroma of orange juices or produce specific off-flavors (Gocmen et al. 2005).
Sensory evaluation
The flavors of the orange juices were evaluated by ten panelists using five descriptors (grassy, floral, fruity, orange, and sour), which were considered to be the most efficient sensorial characteristics of juices. The average aroma intensity scores of the orange juices on the column chart are shown in Fig. 2. The significant decrease on the mean scores of each sensory descriptor of the orange juices stored at different temperatures was observed. Orange was the strongest flavor of the orange juices at the first few days’ storage, followed by sour. It is interesting that sour became to the main flavor of the orange juices stored at 4 °C and 37 °C at the late stage of the storage. The reason might be the growth of lactic acid bacteria in orange juice which could give rise to butter-milk or a vinegar odor (Hays and Riester 1952). Sensory scores decreased rapidly with the increase of storage temperature. It is reported that the overall aroma of orange juices changes in aroma compounds have been observed in orange juices when stored at higher temperatures than refrigerated (4–6 °C) for up to 16 weeks, but it did not change dramatically if they are stored at refrigerated (4–6 °C) temperatures (Perez-Cacho and Rouseff 2008a). And this will result in the decrease of sensory quality of the orange juice.
Aroma compounds
The aroma compounds of orange juices that stored at different temperatures were analyzed everyday during the whole storage until significant spoilage occurred. The results are shown in Table 1 (part of the data was not shown) and Table 2. Totally 33 aroma compounds were determined in these orange juices.
Terpenes were the most abundant compounds in the orange juices, accounting for 97.8% of the total peak area. These compounds played an important role in the odor of oranges (Plotto et al. 2004). In which, D-limonene was the predominant compound and accounting for 93% of the total peak area. Although the concentration of orange juice is high, it may not be the most important flavor contributor to orange juice. It has a ‘lifting effect’ for other aroma compounds, which is similar with ethanol in wine (Perez-Cacho and Rouseff 2008b), while Mastello et al. (2015) found that it has a citric and mint ordor. It is also considered by the industry to be an important compound in odor models of orange juice (Torres et al. 2009). Octanal, 2-hexenal and decanal were the three aldehydes detected in freshly-squeezed orange juice. Octanal and decanal are considered to be the major constituents of aroma active substance in orange juice (Perez-Cacho and Rouseff 2008b). Octanal has an orange-like odor (Petersen et al. 1998). The decrease of these two aldehydes during storage was observed in the present study. The reason for the losses of these two aldehydes might be partly due to the reaction of these aldehydes with amino acids according to a nonenzymatic browning reaction (Ayhan et al. 2002). While Petersen et al. (1998) proposed that the significant decrease of these aldehydes might be a result of an oxidation of the aldehyde to the corresponding carboxylic acid.
The aroma compounds of orange juice stored at 4 °C were detected daily during storage (Table 1). Totally 8 aroma compounds disappeared during the 15 days’ storage. In which, octanal was the only compound detected in freshly-squeezed orange juice, and it was not found at the followed storage. α-Thujene disappeared at the early storage of the orange juice. While 2-hexenal, α-terpinene, β-phellandrene, decanal, citral and selinene were not found at the late storage. In total 16 aroma compounds were detected in the whole storage, while the content of most of these compounds declined after 15 days’ storage. Similar results were also found in Ryo and Barringer’s study that they found a majority of volatiles decreased significantly in the first week stored at 5 °C because only the free and not bound aroma compounds can exist in the headspace and they could occur chemical reactions and oxidation (Ryo and Barringer 2015). Moshonas and Shaw found that the volatile compounds dropped dramatically in 9 weeks and the water-soluble volatiles lost 70%, while oil-soluble compounds lost 30% of the original concentration (Moshonas and Shaw 2000). And Ryo and Barringer found that about 30% of oil and water-soluble volatiles were lost in the process (Ryo and Barringer 2015). The destruction of most orange juice volatiles is proposed to be the cause of an acidcatalyzed reaction (Petersen et al. 1998; Berlinet et al. 2005). α-Terpineol, cubebene, β-gurjunene and tetradecanal were the four new formed compounds during storage in this study. The total peak area of the aroma substances increased first and then decreased. Changes in aroma compounds in juices during storage could occur by rearrangement, hydrogenation or dehydrogenation of other components (Njoroge et al. 1996). Tetradecanal is an aliphatic aldehyde which has a floral and waxy flavor with a low odor threshold (Chisholm et al. 2003; Lu et al. 2011). The aliphatic aldehydes are commonly known to derive from lipid oxidation (Sérot et al. 2004). Increase in some terpenes can be linked to the oxidative reaction and/or acid-catalysed hydration-dehydration reactions of other terpenes as suggested by Perez-Cacho and Rouseff (2008a). The formation ways of several terpenes in juices have been clarified, such as p-cymene from α-terpinene, γ-terpinene and limonene (Njoroge et al. 1996), α-terpineol from D-limonene and linalool (Haleva-Toledo et al. 1999). While the explicit formation ways of many other specific terpenes, such as cubebene and β-gurjunene, are still unknown.
The aroma compounds of orange juice stored at room temperature were also detected everyday during the whole storage. As shown in Table 2, only 2-hexenal, α-thujene, sabinene, octanal and citral disappeared during the storage. Totally 18 aroma compounds were found in the whole storage. Be different from the results obtained from the juices stored at 4 °C, the content of 11 aroma compounds increased significantly after 4 days’ storage. The reason might be that the relative high temperature promoted the release of these volatiles. Nerol, p-vinylguaiacol, cubebene and β-gurjunene were the four new formed aroma compounds in orange juice stored at room temperature, and their concentrations increased during the storage.
With regard to the aromas in orange juice stored at 37 °C, 2-hexenal, sabinene and octanal disappeared on day 1, and α-thujene, citral and γ-muurolene disappeared on day 3. Totally 19 aroma compounds were found in the whole storage, and eight of them increased during this storage. 1-Hexanol, terpinen-4-ol, nerol, 4-ethylguaiacol, p-vinylguaiacol, cubebene, β-gurjunene and tetradecanal were the 8 new formed compounds.
Temperature, storage light exposure, oxygen content, and container sorption or chemical contamination are the factors that affected the aroma compounds in orange juice. Among these, storage temperature was the most important factor (Graumlich et al. 1986). Significant differences on the aroma compounds in orange juices stored at different temperatures were observed in the present study. Most of the terpenes decreased at 4 °C after 15 days’ storage, while 10 and 8 terpenes increased at room temperature and 37 °C. Wibowo et al. (2015) also found that terpenes (terpene hydrocarbons and terpene alcohols) in orange juice stored at 20 and 28 °C increased during shelf-life. The α-pinene is an important aroma-active compound in orange juice. A significant decrease of α-pinene occurred through polymerisation and evaporation was observed in previous study (Njoroge et al. 1996), and its degradation was accelerated by light exposure (Bacigalupi et al. 2013).
The significant decrease of this compound was also observed in other studies (Averbeck and Schieberle 2011; Wibowo et al. 2015). The α-pinene content in freshly recombinant orange Juice decreased by 50% during storage at 20 °C for 16 weeks (Averbeck and Schieberle 2011). It is reported that terpenes may undergo a series of oxidative hydration-dehydratation reactions which results in the formation of alcohols (e.g., terpinen-4-ol, α-terpineol) under the acidic conditions (pH ~ 3.8) in orange juice (Rouseff and Naim 2000). These terpenes can also be converted into other terpenes under acid conditions by means of hydration, dehydration, rearrangements and cyclization reactions (Clark and Chamblee 1992). Therefore, these chemical reactions of particular terpenes can give rise to the formation and deformation of other terpenes (Wibowo et al. 2015). In addition, the absorption of aromas by packaging materials was another responsible factor for the decrease of some terpenes in model citrus juices (Lebossé et al. 1997).
1-Hexanol, terpinen-4-ol and 4-ethylguaiacol were the three compounds detected only in juice stored at 37 °C. And α-terpineol was the only compound detected in juice stored at 4 °C. Decanal was the only aldehyde detected in all the samples, while it disappeared in juice stored at 4 °C at late storage. 2-Hexenal was not found in juices stored at room temperature and 37 °C, and it disappeared at late storage of juices stored at 4 °C. Octanal was also not found in juices stored at 37 °C and it disappeared in juices stored at 4 °C and room temperature at late storage. Decanal and octanal contribute to the typical characteristic of citrus note, and the reduction of these compounds during storage could result in the reduction of the typical orange flavor of orange juices.
Citral is a monoterpene aldehyde and it can decompose rapidly during storage at acidic condition according to a series of cyclization and oxidation reactions (Djordjevic et al. 2008). The degradation rates of citral were much faster at higher temperature (35 °C) compared to at room temperatures (He et al. 2018). Similar result was also obtained in this study that citral degraded more rapidly at 37 °C than that at room temperature.
Off-flavors are the main factor affecting consumers’ acceptability. α-Terpineol, terpinen-4-ol, 4-ethylguaiacol and p-vinylguaiacol were the four off-flavors detected in orange juices when stored at different temperatures. α-Terpineol was the only off-flavor compound found in juice stored for longer period at 4 °C. While it was found in commercial lemon, grapefruit and orange juices before and after accelerated storage at 45 °C for 2 weeks, and its increase was both temperature- and time-dependent (Haleva-Toledo et al. 1999). Terpinen-4-ol, 4-ethylguaiacol and p-vinylguaiacol were found in juice stored at 37 °C at late storage. This indicates that off-flavor compounds might be formed more easily at high temperature than that at relatively low temperature. It was also found that the concentration of these off-flavor compounds increased with the extended storage. And α-terpineol was more easily formed at relatively low temperature with long-term storage. It has a musty, stale, or piney off-flavor when added to orange juice (Tatum et al. 1975), and it was a product of acid catalyzed hydration of d-limonene. The formation of α-terpineol was also observed in orange juice after pasteurization (Pérez-López and Carbonell-Barrachina 2006a), and its formation was closely related with the orange juice pH (Haleva-Toledo et al. 1999). Additionally, the relativity between α-terpineol, oxidized taste/odour and bitterness was observed in previous study (Petersen et al. 1998). And it was recommended as an indicator of shelf-life of orange juice because the increase of this compound was linear with the extension of storage time (Askar et al. 1973). While this compound was only found in juice stored at 4 °C at the late storage, and was not found in juices stored at room temperature and 37 °C. The reason might be that the storage time used in the present study was short. Terpinen-4-ol was also an off-flavor compound degraded from d-limonene and linalool. Through the determination of the stability of d-limonene and linalool, and formation of α-terpineol and terpinen-4-ol, it provided a basis for further analysis of the quality of mandarin juice (Pérez-López et al. 2006b).
p-Vinylguaiacol contribute off-flavor to juice stored at room temperature. It was found in orange juices stored for 2 days at room temperature and 37 °C, while it was not detected in the freshly-squeezed orange juice and juice stored at 4 °C. This indicated that this compound might not be formed under low temperature. This compound can be formed from an odorless precursor ferulic acid. Walsh et al. (1997) also found that the concentration of p-vinylguaiacol remained unchanged and never exceeded its aroma threshold when stored at 4 °C for up to 16 weeks, while its concentration increased dramatically and exceeded its aroma threshold after only 6 weeks when stored at 40 °C. Similar result was also found in the present study that the content of p-vinylguaiacol in juice stored at 37 °C for 4 days was about ten times higher than that in juice stored at room temperature. And p-vinylguaiacol increased with the extension of storage time. This compound was also found in Hamlin sweet oranges existed as a bound volatile compound (Ren et al. 2015). The formation of p-vinylguaiacol in orange juice during storage might due to the hydrolysis of glycosidically bound volatile compound under its mild acidic condition.
Electronic nose analysis
The FOX4000 Alpha M.O.S. (France) E-nose system was used to detect and analyze the juice samples.
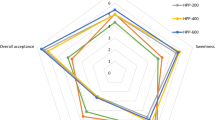
The result showed that the parallel detection of each test sample constituted a separate ethnic group, which indicated that the electronic nose analysis had a good reproducibility (Fig. 3a, b). From the PCA diagram (Fig. 3b), it can be seen that the contribution rates of the first principal component and the second principal component were 81.251% and 9.79% respectively. The detection data of the aroma components at 4 °C at early storage was basically separated with no overlap. The detection data of the aroma components at room temperature and 37 °C was also separated. There were great differences among the samples stored at 37 °C, which could be distinguished clearly and there is no overlap with a more obvious distance. It indicated that the aroma of the orange juice was significantly different during storage at different temperatures, and it could be separated according to the electronic nose analysis.
E-nose analysis of freshly-squeezed orange juice stored at different temperatures. a DFA analysis, b PCA analysis and c FINGERPRINT chart. 1: 0 day, 2: 4 °C for 1 day, 3: 4 °C for 2 days, 4: 4 °C for 3 days, 5: 4 °C for 4 days, 6: 4 °C for 5 days, 7: 4 °C for 6 days, 8: 4 °C for 7 days, 9: 4 °C for 8 days, 10: 4 °C for 9 days, 11: 4 °C for 10 days, 12: 4 °C for 11 days, 13: 4 °C for 12 days, 14: 4 °C for 13 days, 15: 4 °C for 14 days, 16: 4 °C for 15 days, 17: room temperature for 1 day, 18: room temperature for 2 days, 19: room temperature for 3 days, 20: room temperature for 4 days, 21: 37 °C for 1 day, 22: 37 °C for 2 days, 23: 37 °C for 3 days, 24: 37 °C for 4 days
In the DFA analysis, the differentiation of the orange juices was more obvious at 4 °C, although the difference in longer storage was not obvious in PCA analysis. The orange juices stored at room temperature for different days were obviously separated, and there was a great distance between the samples. This showed that the aroma components of the orange juice changed obviously at room temperature and there was a clear distinction between them. The orange juices stored at 37 °C were more distinct, and there was a great distance between the samples. This indicated that the aroma components of the juices changed greatly at 37 °C and they also had obvious distinction. Through the combination of PCA analysis and DFA analysis, the juices at different temperatures could be identified accurately. The distinction of DFA analysis was better than that of PCA.
The sensitivity and selectivity of sensors is an important aspect of any E-nose system. As the recognition ability of an E-nose system depends on the ability of the gas sensors to produce different modes for different substances (Mamat et al. 2011). As shown in Fig. 3c, the difference was obvious that the detection of aromas changed in the orange juices at 4 °C in LY2/g CTL, LY2/GH, LY2/AA, LY2/G, LY2/LG. The aromas at room temperature and 37 °C changed in the detection of LY2/g CTL, LY2/GH, LY2/AA, LY2/G. The response values on the rest of the sensor were not different. Therefore, the establishment of the fingerprint database of the orange juices is conducive to the detection of fresh orange juice stored at different temperatures. It is conducive to the detection of fresh degree of the freshly-squeezed orange juice, and to identify and control the quality of the orange juice.
The changes of aroma components in freshly-squeezed orange juice stored at 4 °C, room temperature and 37 °C were detected and analyzed according to PCA and DFA using electronic nose. The difference of aroma components at 4 °C for different days was not very significant due to the quality decreased slowly. The basic distinction was observed through the analysis of electronic nose detection, while the division is general. During storage at room temperature and 37 °C, the quality of the juice changed rapidly, and the change of the aroma composition was obvious. It provides a reference and experimental data for the application of electronic nose detection in the quality inspection and control in the production of freshly-squeezed orange juice.
Partial least square regression analysis
The PLS regression analysis was carried out with the 33 volatile compounds detected in the orange juices at different temperatures during the storage and the 5 sensory descriptors (fruity, grassy, floral, orange, and sour), which could describe the relativity between sensory data and chemical compounds detected by instrument. As shown in Fig. 4, the five flavors were located closely to 0-day juice which was the freshest, and it was in accordance with the result of sensory analysis. While only citral, 2-hexenal, α-thujene, octanal, α-terpinene and γ-terpinene were close to these five flavors. The juices stored at 4 °C for different days were separated from each other, and they were farther away from the five sensory attributes with the extension of storage. The samples stored at room temperature and 37 °C were relatively concentrated and far away from the five flavors. And many aroma compounds, such as 3-carene, β-elemene, caryophyllene and the four off-flavors, were situated around. This indicated that the characteristic flavor of the juices altered significantly during the storage at relatively high temperatures.
PLS regression loading plots of sensory data correlating with chemical compounds. L-1: 4 °C for 1 day, L-2: 4 °C for 2 days, L-3: 4 °C for 3 days, L-4: 4 °C for 4 days, L-5: 4 °C for 9 days, L-6: 4 °C for 15 days, R-1: room temperature for 1 day, R-2: room temperature for 2 days, R-3: room temperature for 3 days, R-4: room temperature for 4 days, H-1: 37 °C for 1 day, H-2: 37 °C for 2 days, H-3: 37 °C for 3 days, H-4: 37 °C for 4 days. The number 1–33 represents the corresponding aroma compounds listed in Tables 1 and 2
Conclusion
TPC increased significantly during the storage at room temperature and 37 °C. While they were less than 10 CFU/g in the first 5 days’ storage at 4 °C. A significant decrease of the mean scores of each sensory descriptor of the orange juices stored at different temperatures was found during storage, and the sensory scores decreased rapidly with the increase of storage temperature. Totally 8 aroma compounds disappeared and most of the terpenes decreased at 4 °C after 15 days’ storage. α-Terpineol, cubebene, β-gurjunene and tetradecanal were the four new formed compounds at 4 °C. Only 2-hexenal, α-thujene, sabinene, octanal and citral disappeared, and 11 aroma compounds increased significantly after 4 days’ storage at room temperature. Totally 6 aroma compounds disappeared, and 8 new were formed in juice stored at 37 °C for 4 days’. α-Terpineol was the only off-flavor compound in juice stored at 4 °C and was found after long storage. Terpinen-4-ol, 4-ethylguaiacol and p-vinylguaiacol were three off-flavor compounds formed in juice stored at 37 °C for longer storage. This indicates that off-flavor compounds in juice might be formed more easily at high temperature and long-term storage.
References
Askar A, Bielig HJ, Treptow H (1973) Aroma changes in organge juice. II. Aroma changes during manufacture and storage of bottled orange juice. Dtsch Lebensm-Rundsch 69:162–167
Averbeck M, Schieberle P (2011) Influence of different storage conditions on changes in the key aroma compounds of orange juice reconstituted from concentrate. Eur Food Res Technol 232:129–142. https://doi.org/10.1007/s00217-010-1366-8
Ayhan Z, Zhang QH, Min DB (2002) Effects of pulsed electric field processing and storage on the quality and stability of single-strength orange juice. J Food Protect 10:1623–1627. https://doi.org/10.4315/0362-028X-65.10.1623
Bacigalupi C, Lemaistre MH, Boutroy N, Bunel C, Peyron S, Guillard V, Chalier P (2013) Changes in nutritional and sensory properties of orange juice packed in PET bottles: an experimental and modelling approach. Food Chem 141:3827–3836. https://doi.org/10.1016/j.foodchem.2013.06.076
Baldwin EA, Bai J, Plotto A, Cameron R, Luzio G, Narciso J, Manthey J, Widmer W, Ford BL (2012) Effect of extraction method on quality of orange juice: hand-squeezed, commercial-fresh squeezed and processed. J Sci Food Agric 10:2029–2042. https://doi.org/10.1002/jsfa.5587
Berlinet C, Ducruet V, Brillouet JM, Reynes M, Brat P (2005) Evolution of aroma compounds from orange juice stored in polyethylene terephthalate (PET). Food Addit Contam 22:185–195. https://doi.org/10.1080/02652030500037860
Chisholm M, Wilson M, Gaskey G (2003) Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle). Flavour Frag J 18(2):106–115. https://doi.org/10.1002/ffj.1172
Clark BC Jr, Chamblee TS (1992) Acid-catalyzed reactions of citrus oils and other terpene-containing flavors. In: Charalambous G (ed) Off-flavors in food and beverages. Elsevier Science Publishers, New York, pp 229–285
Djordjevic D, Cercaci L, Alamed J, McClements DJ, Decker EA (2008) Stability of citral in protein-and gum arabic-stabilized oil-in-water emulsions. Food Chem 106(2):698–705. https://doi.org/10.1016/j.foodchem.2007.06.033
Eleftheriadou M, Quantick P, Nolan M, Akkelidou D (1998) Factors affecting quality and safety of fresh squeezed orange juice (FSOJ). Dairy Food Environ Sanit 18(1):14–23
Fischer K, Bipp HP (2005) Generation of organic acids and monosaccharides by hydrolytic and oxidative transformation of food processing residues. Bioresour Technol 96:831–842. https://doi.org/10.1016/j.biortech.2004.07.003
Galili G, Galili S, Lewinsohn E, Tadmor Y (2002) Genetic, molecular, and genomic approaches to improve the value of plant foods and feeds. Crit Rev Plant Sci 21(3):167–204. https://doi.org/10.1080/0735-260291044232
Gocmen D, Elston A, Willians T, Parish M, Rouseff RL (2005) Identification of medicinal off-flavors generated by Alicyclobacillus species in orange juice using GC-Olfactometry and GC/MS. Lett Appl Microbiol 40:172–177. https://doi.org/10.1111/j.1472-765X.2004.01636.x
Graumlich TR, Marcy JE, Adams JP (1986) Aseptically packaged orange juice and concentrate: a review of the influence of processing and packaging conditions on quality. J Agric Food Chem 34(3):402–405. https://doi.org/10.1021/jf00069a004
Haleva-Toledo E, Naim M, Zehavi U, Rouseff RL (1999) Formation of α-terpineol in citrus juices, model and buffer solutions. J Food Sci 64(5):838–841. https://doi.org/10.1111/j.1365-2621.1999.tb15923.x
Hays GL, Riester DW (1952) The control of “off-odor” spoilage in frozen concentrated orange juice. Food Technol 6:386–389
He F, Qian YL, Qian MC (2018) Flavor and chiral stability of lemon-flavored hard tea during storage. Food Chem 239:622–630. https://doi.org/10.1016/j.foodchem.2017.06.136
Kelebek H, Selli S (2011) Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J Sci Food Agric 91:1855–1862. https://doi.org/10.1002/jsfa.4396
Lebossé R, Ducruet V, Feigenbaum A (1997) Interactions between reactive aroma compounds from model citrus juice with polypropylene packaging film. J Agric Food Chem 45:2836–2842. https://doi.org/10.1021/jf960957e
Li J, Miao S, Jiang Y (2009) Changes in quality attributes of longan juice during storage in relation to effects of thermal processing. J Food Qual 10:48–57. https://doi.org/10.1111/j.1745-4557.2008.00235.x
Lu F, Zhang JY, Liu SL, Wang Y, Ding YT (2011) Chemical, microbiological and sensory changes of dried Acetes chinensis during accelerated storage. Food Chem 127(1):159–168. https://doi.org/10.1016/j.foodchem.2010.12.120
Mamat M, Samad SA, Hannan MA (2011) An electronic nose for reliable measurement and correct classification of beverages. Sensors 11:6435–6453. https://doi.org/10.3390/s110606435
Mastello RB, Capobiango M, Chin ST, Monteiro M, Marriott PJ (2015) Identification of odour-active compounds of pasteurised orange juice using multidimensional gas chromatography techniques. Food Res Int 75:281–288. https://doi.org/10.1016/j.foodres.2015.06.014
Moshonas MG, Shaw PE (2000) Changes in volatile flavor constituents in pasteurized orange juice during storage. J Food Qual 23:61–71. https://doi.org/10.1111/j.1745-4557.2000.tb00196.x
Njoroge SM, Ukeda H, Sawanmura M (1996) Changes in volatile oil composition of yuzu (Citrus junas Tanka) cold-pressed oil during storage. J Agric Food Chem 44:550–556. https://doi.org/10.1021/jf950284k
O’Neil CE, Nicklas TA, Rampersaud GC, Fulgoni VL (2011) One hundred percent orange juice consumption is associated with better diet quality, improved nutrient adequacy, and no increased risk for overweight/obesity in children. Nutr Res 31(9):673–682. https://doi.org/10.1016/j.nutres.2011.09.002
Parish ME (1998) Coliforms, Escherichia coli and Salmonella serovars associated with a citrus-processing facility implicated in a salmonellosis outbreak. J Food Protect 61(8):280–284. https://doi.org/10.4315/0362-028X-61.3.280
Perez-Cacho PR, Rouseff R (2008a) Processing and storage effects on orange juice aroma: a review. J Agric Food Chem 56(21):9785–9796. https://doi.org/10.1021/jf801244j
Perez-Cacho PR, Rouseff R (2008b) Fresh squeezed orange juice odor: a review. Crit Rev Food Sci 48:681–695. https://doi.org/10.1080/10408390701638902
Pérez-López AJ, Carbonell-Barrachina ÀA (2006) Volatile odour components and sensory quality of fresh and processed mandarin juices. J Sci Food Agr 86:2404–2411. https://doi.org/10.1002/jsfa.2631
Pérez-López AJ, Saura D, Lorente J, Carbonell-Barrachina ÁA (2006) Limonene, linalool, α-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. Eur Food Res Technol 222:281–285. https://doi.org/10.1007/s00217-005-0055-5
Petersen MA, Tonder D, Poll L (1998) Comparison of normal and accelerated storage of commercial orange juice-changes in flavor and content of volatile compounds. Food Qual Prefer 9:43–51. https://doi.org/10.1016/S0950-3293(97)00027-X
Plotto A, Margaría CA, Goodner KL, Goodrich R, Baldwin EA (2004) Odour and flavour thresholds for key aroma components in an orange juice matrix: terpenes and Aldehydes. Flavour Frag J 19(6):491–498. https://doi.org/10.1002/ffj.1470
Qian Z, Wang H, Liu T, Jia Y, Prasad KN, Qu H, Duan X, Jiang Y (2014) Changes in quality attributes of mandarin with and without leaf during refrigerated storage. J Food Process Pres 38:11–20. https://doi.org/10.1111/j.1745-4549.2012.00731.x
Ren JN, Tai YN, Dong M, Shao JH, Yang SZ, Pan SY, Fan G (2015) Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem 185:25–32. https://doi.org/10.1016/j.foodchem.2015.03.142
Rodríguez A, Peris JE, Redondo A, Shimada T, Costell E, Carbonell I, Rojas C, Peña L (2017) Impact of D-limonene synthase up- or down-regulation on sweet orange fruit and juice odor perception. Food Chem 217:139–150. https://doi.org/10.1016/j.foodchem.2016.08.076
Rouseff R, Naim M (2000) Citrus flavor stability. In: Risch SJ, Ho C-T (eds) Flavor chemistry. Industrial and Academic Research, vol 756. American Chemical Society, Washington, pp 101–121
Ryo S, Barringer SA (2015) Effect of storage, oxygen and concentration on the levels of key volatiles in processed orange juice. J Food Process Pres 39:495–507. https://doi.org/10.1111/jfpp.12255
Selli S, Canbas A, Varlet V, Kelebek H, Prost C, Serot T (2008) Characterization of the most odor-active volatiles of orange wine made from a Turkish cv. Kozan (Citrus sinensis L. Osbeck). J Agric Food Chem 56:227–234. https://doi.org/10.1021/jf072231w
Sérot T, Baron R, Knockaert C, Vallet J (2004) Effect of smoking processes on the contents of 10 major phenolic compounds in smoked fillets of herring (Cuplea harengus). Food Chem 85(1):111–120. https://doi.org/10.1016/j.foodchem.2003.06.011
Tatum JH, Nagy S, Berry RE (1975) Degradation products formed in canned single-strength orange juice during storage. J Food Sci 40(4):707–709. https://doi.org/10.1111/j.1365-2621.1975.tb00536.x
Torres RG, Ponagandla NR, Rouseff RL, Goodrich-Schneider RM, Reyes-de-Corcuera JI (2009) Effects of dissolved oxygen in fruit juices and methods of removal. Compr Rev Food Sci F 8:409–423. https://doi.org/10.1111/j.1541-4337.2009.00090.x
Walsh M, Rouseff R, Naim M (1997) Determination of furaneol and p-vinylguaiacol in orange juice employing differential UV wavelength and fluorescence detection with a unified solid phase extraction. J Agric Food Chem 45(4):1320–1324. https://doi.org/10.1021/jf960435z
Wibowo S, Grauwet T, Kebede BT, Hendrickx M, Van LA (2015) Study of chemical changes in pasteurised orange juice during shelf-life: a fingerprinting-kinetics evaluation of the volatile fraction. Food Res Int 75:295–304. https://doi.org/10.1016/j.foodres.2015.06.020
Acknowledgements
This study was supported by the National key Reasearch and Development Program of China (2017YFD0400101), National Natural Science Foundation of China (Program No. 31671824), the Major Scientific and Technological Innovation Project in Hubei Province (2015ABA035, 2016ABA112).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Ren, JN., Fan, G. et al. Changes of aroma compounds and qualities of freshly-squeezed orange juice during storage. J Food Sci Technol 55, 4530–4543 (2018). https://doi.org/10.1007/s13197-018-3389-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3389-2