Abstract
Rheological characteristics of Desi ghee were investigated at 18, 24, 30 and 36 °C. The steady shear properties were evaluated by varying the shear rate from 0.01 to 100 s−1 and the dynamic shear properties were studied by varying strain and frequency sweep from 0.01 to 100% and 0.1 to 100 rad s−1, respectively. At the four selected temperatures, the ghee samples displayed non-Newtonian shear thinning behavior with flow behavior index (n) ranging from 0.224 to 0.911. As the shear rate increased from 0 to 100 s−1, the values of dynamic viscosity decreased from 54 to 8.14, 20.01 to 1.05, 1.33 to 0.295, and 3.02 to 0.0025 Pa s at 18, 24, 30 and 36 °C, respectively. Out of four rheological models (Power-law or Ostwald-de Waele, Herschel–Bulkley, Casson, and Bingham model) fitted to the shear rate and stress data, the Ostwald model was found to be superior in predicting the shear rate-stress data at 18 °C, whereas Ostwald-de Waele and Herschel–Bulkley models predicted all the data points over the temperature range of 24–30 °C, as observed by the values of coefficient of determination (R2), standard deviation (SD), and relative deviation percentage (Rd). The value of activation energy (EA), as calculated from Arrhenius type equation, was found to be 1.98 × 106 kJ mol−1 over the entire temperature range. The study also revealed that the magnitudes of dynamic shear viscosity (η*) were higher than those of the steady shear viscosity (η) at the four temperatures, indicating that the Cox–Merz rule was not applicable to the ghee samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk is highly perishable and often converted into various stable food products by employing various value addition techniques as it is well established that conversion of liquid milk into various high value food products is more profitable than the sale of liquid milk (Aneja et al. 2015). One such value added product of Indian origin is Desi ghee. Desi ghee is among the most popular traditional milk products in Indian sub-continent. As per Food Safety and Standards Authority of India (FSSAI) regulation (2011), ghee is defined as the pure clarified fat derived solely from milk, curd, butter or cream to which no coloring agent or preservative is added. The CODEX Standard (2011) for milk fat products states that ghee is a product exclusively obtained from milk, cream or butter by adopting processes which result in almost total removal of water and solids-not-fat (SNF) with a typical flavor and texture. In the present study, Desi ghee refers to the ghee processed from milk, butter or cream obtained from indigenous (Desi) cow of Indian origin. Desi ghee is regarded as an element component of diet, being a rich source of energy, essential fatty acids and fat-soluble vitamins like vitamin A, D, E, and K. The Indian Ayurveda system of Medicine describes Desi ghee as capable of increasing mental and physical strength, improving vision and voice, complementing digestion as well as supporting sound functioning of liver and kidney. Consumption of Desi ghee promotes human longevity, and when consumed with medicinal and aromatic plants and herbs, it allows its functional components to pass into deeper tissue levels enhancing their bioavailability and efficacy in human body (Suryakumari et al. 2014). The Indian medicine system has been strongly recommending potential use of Desi ghee for maintaining good physical and mental health as well as treatment of various health disorders like indigestion and improper functioning of vital organs. Ghee is especially liked for its convenience, since it can be added to hot food and served without further cooking. Desi ghee is considered as one of the vital ingredients in food, pharmaceutical and nutraceutical applications.
Ghee consists of a mixture of higher softening-point fats, especially triglycerides (triacylglycerols) in crystalline form, dispersed in a liquid with lower softening-point fats (Gupta et al. 2015). In the process of ghee manufacturing, cream undergoes a phase inversion during churning where fat-globule membranes rapture, globules coalesce and oil leaks out to form a continuous phase. This continuous phase contains crystal aggregates along with remnants of damaged and intact fat globules and the rheological property of ghee is due to this 3-dimensional network of fat crystals that are intimately associated with a continuous oil phase.
Use of Desi ghee for garnishing or in course of preparation and processing involves alteration of its rheological properties. Therefore, it is crucial to understand the rheological properties of Desi ghee for its optimum utilization. Besides, rheological properties affect the sensory quality, shelf-life, stability, bioavailability and overall texture of a product during product development. The rheological characteristics of a sample matrix at different temperatures and shear rates provides an indication of extractability and release kinetics for valuable bioactive and flavor components from the sample matrix. Rheology of a substance can be used primarily to map down the possible ways in which sample matrix might behave when subjected to any change in external environment that may be in form of pressure, force, temperature etc., which is essentially the fundamental driving phenomenon behind most of the extraction processes. However, there is no such literature regarding rheological characterization of Desi ghee so far that can put light towards extractability potential of Desi Ghee. Therefore, the objectives of the present study are (1) to investigate the rheological characteristics of Desi ghee when subjected to varying conditions of shear rate and strain at four temperatures (18, 24, 30 and 36 °C), (2) to evaluate the best-fitted rheological model to predict the experimental data, and (3) to analyze the temperature dependency of the ghee in the above température range. The study will be helpful to develop process technology for formulation of ghee based food products. Further, it is expected that the study will indicate extractability-behavior of aroma or flavor bearing component(s) when ghee is subjected to an external force (shear) or thermal treatment during its processing and storage.
Materials and methods
Sample preparation
Milk (4.3% fat, 8.5% solid-not-fat, 3.1% protein, 1.5% lactose and 0.6% ash) collected from a particular cow of Indian origin from Krishna Farm, Karnal, India was used for preparation of ghee by Creamery-Butter method as described by Ganguli and Jain (1973). The cream separated from the milk was ripened up to 0.9% acidity and was churned to obtain butter which was further clarified at 80–110 °C to obtain ghee. Prior to rheological analysis, the samples were heated in an oven at 80 °C for 5 min to destroy any crystal memory. The heated ghee samples were removed from the oven and allowed to cool down to 4 °C by subjecting to fast cooling at 10 °C min−1 in order to prevent crystal formation and were stored at 4 °C (Wiking et al. 2009).
Rheological measurements
A dynamic rotational and oscillatory rheometer (MCR-302, Anton Paar, Austria) with parallel plate type geometric assembly was used to measure rheological properties of the ghee samples. The proximate distance between the two plates was maintained at 0.5 mm during experiments. Special care was taken to minimize the effect of work softening and air inclusion in the samples while loading on the plate.
Steady shear properties
Flow behavior
Controlled shear rate mode was used to study the flow behavior of ghee samples at four temperatures, i.e., 18, 24, 30 and 36 °C, considering the melting and softening range of ghee in account as reported by Ramesh and Bindal (1987). The ghee sample (1.0 ml) was placed between the plates and measurement was started immediately. The shear rate γ was increased from 0.01 to 100 s−1 to study variation in shear stress τ and dynamic shear viscosity η. During the shearing, total 25 data points were generated keeping time duration ramp-wise decrease. Each measurement was replicated three times.
The experimental values of shear rate and shear stress data were fitted to four rheological models using Rheoplus Software (32V3.62, Anton-Paar, Austria) through non-linear regression analysis.
Power-law or Ostwald-de Waele:
Herschel–Bulkley model:
Bingham model:
Casson model:
where, τ is the shear stress (Pa), γ is the shear rate (s−1), n is the flow behavior index (dimensionless), k is the consistency index (Pa sn), \( \tau_{0} \) is the yield stress (Pa), and η is the infinite shear viscosity (Pa s) (Steffe 1996).
The goodness of fit of the developed models was evaluated by determining coefficient of determination R2, standard deviation SD and relative deviation percentage Rd. The value of Rd was calculated by using following expression (Srimagal et al. 2016).
where, τexp.i and τpre.i are the experimental and predicted values of shear stress (Pa), and N is the number of data points. Generally, a model is considered acceptable, if the value of Rd is below 10% (Das 2005).
Effect of temperature on dynamic viscosity
Viscosity directly affects flavor release potential as well as flavor perception and sensory. Therefore, apparent viscosity η of the ghee samples was determined within temperature range of 18–36 °C. The heating rate was maintained constant at 1 °C min−1, keeping the shear rate constant at 50 s−1 which is the shear rate in mouth (Bourne 2002). The apparent viscosity measured at this specified shear rate (γ = 50 s−1) was symbolized as η50 for description of the effect. The temperature dependency of the apparent viscosity η50 at the specified shear rate was described by the following Arrhenius type equation.
where, η50 is apparent viscosity (Pa s) at shear rate 50 s−1, η0 is a constant (Pa s), EA is activation energy (kJ mol−1), R is universal gas constant (8.3145 kJ mol−1 K−1) and T is absolute temperature (K). The fitness of the predicted model was evaluated by calculating R2, SD and Rd values as described in “Flow behavior” section.
Dynamic shear properties
Strain sweep
The samples were subjected to dynamic oscillatory tests with strain sweep from 0.01 to 100% at 6.28 rad s−1 to identify linear viscoelastic region. The time duration for data points was decreased logarithmically so as to increase accuracy and adaptability of the samples to increasing strain; giving longest time to generate first data points and subsequently shortening the duration with progress of the sweep.
Frequency sweep
Frequency sweep tests were conducted in range of 0.1 to 100 rad s−1 in order to plot mechanical spectra within the linear viscoelasticity regions identified for the four selected temperatures. Storage modulus G′ and loss modulus G″ were determined as a function of frequency. Loss tangent tan δ, a dimensionless number giving clear indication of whether a material behaves as solid or liquid-like, was calculated according to the following equation (Steffe 1996).
The overall response of the samples to the sinusoidal strain was calculated from complex modulus G* and complex viscosity η* as below (Yilmaz et al. 2011).
where, ω is the angular frequency (rad s−1).
Plots of ω versus G′ and G″ were subjected to non-linear regression and the magnitudes of intercepts (K′, K″ and K*), slopes (n′, n″ and n*) and R2 were determined from the raw data according to the following equations (Yilmaz et al. 2011).
Applicability of Cox–Merz rule
Correlations between values of oscillatory shear parameters (complex viscosity η* and angular frequency ω) and steady shear parameters (apparent viscosity η and shear stress γ) were established by using Cox–Merz rule (Juszczak et al. 2004). The Cox–Merz rule is used to predict steady shear viscosity from complex shear viscosity and vice versa (Steffe 1996).
Results and discussion
Steady shear properties
Flow behavior
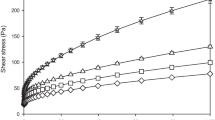
The flow curves (shear stress σ and shear rate γ) for the ghee samples at the four selected temperatures are shown in Fig. 1. The values of σ increased from 8.14 to 54, 1.05 to 20.01, 0.295 to 13.3, and 0.002 to 3.02 Pa at 18, 24, 30 and 36 °C, respectively with increase in the values of γ from 0 to 100 s−1. From the figure, it is clear that the slopes of the flow curves decline gradually indicating that the flow behavior of the ghee samples tend to shift from pseudo-plastic to Newtonian nature with increase in temperatures and shear rates. The shift towards Newtonian flow became more apparent with increase in temperature as evident from the flow curves at 30 and 36 °C. This behavior might be due to the temperature induced reduction in flow resistance in the fat rich sample matrix as observed for soybean, sunflower, olive and rapeseed oils (Santos et al. 2005). The authors reported that soybean, sunflower, olive and rapeseed oils exhibited the Newtonian behavior at shear rates higher than 10 s−1 when temperature increased from 10 to 80 °C. The pseudo-plastic nature is often displayed by many biological materials that are structurally phase stable when subjected to an external force (Citerne et al. 2001; Mossaz et al. 2010). This highly temperature dependent structural behavior indicates that ghee could be modified easily for extraction of selective components such as flavor compounds like lactones as well as bio-active compounds like conjugated linoleic acid by careful selection of extraction parameters comparative to structurally complex, dense packed sample matrix.
Figure 2 shows the shear rate γ and dynamic viscosity η relationship of the ghee samples at the four selected temperatures T. The value of η decreased with increase in value of γ at all four temperatures. Similarly, the value of η decreased from 54 to 8.14, 20.01 to 1.05, 1.33 to 0.295 and 3.02 to 0.0025 Pa s at 18, 24, 30 and 36 °C, respectively as value of γ increased from 0 to 100 s−1. The decreasing trend in the value of η of the samples with increasing value of γ and T might be due to the reduction in fluid resistance of the sample matrix. This shear thinning, pseudo-plastic behavior and viscosity independency at higher shear rate is common phenomenon with fat rich biological materials. Similar results were reported for milkweed oil (O’kuru and Carriere 2002), beef tallow, grease, poultry fat and yellow grease (Goodrum et al. 2003), canola, corn, grapeseed, hazelnut, olive, soybean, and sunflower oils (Kim et al. 2010), and rapeseed oil (Liu et al. 2012). Selective change in viscosity of Desi Ghee under shearing can be applied to control and enhance migration of flavor components due to decreased inhibition as reported in similar studies by Lubbers and Butler (2010) for dairy custards and De Roos (2000) for stirred aqueous solutions with carboxymethylcellulose (CMC).
Rheological modelling
Table 1 describes the rheological and statistical parameters of the four models (Ostwald-de Waele or Power-law, Bingham, Herschel–Bulkley and Casson) fitted to the experimental data. The values of flow behavior index n for all the samples at the four selected temperatures were found to be less than unity (n < 1), indicating non-Newtonian shear thinning behavior of the ghee samples. However, for all the samples, as the temperature increased from 18 to 36 °C, the values of n increased from 0.224 to 0.911 for the selected models. Thus, it inferred that the flow behavior of ghee experienced a shift from non-Newtonian to slightly Newtonian behavior with increase in temperature as shown in Fig. 1. However, reverse was observed for the consistency coefficient k which decreased with increase in température. The values of k using the four selected models for all the samples decreased from 14.07 to 0.046 as the temperature increased from 18 to 36 °C (Table 1). This behavior could be explained on the basis of subsequent reduction of fluid viscosity and shifting of the flow behavior from yield pseudo-plastic to Newtonian nature with increase in temperature. Similar trends were reported for unrefined pollock oil (Sathivel et al. 2008) and salmon oil (Huong and Sathivel 2008) in earlier studies.
Table 1 also shows that the values of yield stress τ0, as calculated using the four rheological models, decreased from 5.115 to 0.001 Pa with increase in the température from 18 to 36 °C. The reason behind low yield stress might be due to weaker network and soft gel matrix of the ghee sample at higher temperature which makes Desi Ghee sample matrix extractive compared to other compact matrices.
The estimated statistical parameters such as coefficient of determination R2, standard deviation SD and relative deviation percentage Rd were compared in order to judge the best-fitted rheological model. As shown in Table 1, Ostwald and Herschel–Bulkley model have R2 values 0.928 and 0.958, 0.934 and 0.962, 0.908 and 0.927, 0.041 and 0.739, respectively at 18, 24, 30 and 36 °C. Similarly, the values of SD for the two models at the four selected temperatures are 3.742 and 2.845, 0.616 and 1.175, 0.687 and 0.612, and 0.765 and 0.399 respectively. The values indicated that Ostwald and Herschel–Bulkley models fit the experimental data at lower temperatures as indicated by the higher R2, lower SD and lower Rd values. The values of R2 and SD for Bingham and Casson model ranged between 0.370 to 0.898 and 11.109 to 0.465, respectively at the four selected temperatures. The estimated R2 values for all the models at 36 °C were low, the highest being 0.739 for Hershel-Bulkley model. At 18 °C, despite \( R^{2}_{Hershel - Bulkley} > R^{2}_{ostwald} \), the negative value of τ0 was estimated by Herschel–Bulkley model which had no rheological significance and hence, was not considered suitable for use at this temperature. Similar negative values of τ0 were reported by some authors. Yilmaz et al. (2011) obtained negative yield stress values at 10 and 20 °C for processed cheese whereas Taghizadeh and Razavi (2009) observed negative yield stress values for pistachio butter at 45 and 65 °C while fitting Herschel–Bulkley model. In view of the above, it is obvious that ghee samples were highly temperature dependent. Among the four selected models, no model was suitable to predict the experimental data accurately at higher temperatures. Ostwald model showed the best-fitting of the data at 18 °C, whereas Ostwald and Herschel–Bulkley models were fitted accurately within the temperature range of 24–30 °C comparatively.
Effect of temperature on dynamic viscosity
Effect of temperature on dynamic viscosity η50 was analyzed by using Arrhenius type expression. Figure 3 shows the effect of temperature on the value of η50 at γ = 50 s−1 (shear rate in mouth) with heating rate 1 °C min−1.The figure shows that increase in temperature from 18 to 36 °C led to 10 times decline in η50 value (0.5–0.04 Pa s).
The declining trend in the values of η50 associated with temperature might be due to the increased intermolecular distances, caused by thermal expansion and high kinetic energy, among the constituting particles which are basically composed of saturated triglycerides and fatty acids. Similar observations were reported by Nik et al. (2005) for coconut, canola, sunflower, corn and superlein oils and Franco and Nguyen (2011) for olive, peanut and soybean oils.
Linear regression analysis was applied to logarithmic form of Eq. (6) in order to determine the parameters associated with the Arrhenius expression. The estimated value of Arrhenius model constant η0 and activation energy EA were 0.443 Pa s and 1.983 × 106 kJ mol−1, respectively. The high value of R2 (0.985), low value SD (0.0258) and Rd (1.243) indicated that the Arrhenius model could accurately predict the temperature dependency of the viscosity data for the ghee samples. Similar decreasing trend in viscosity with increase in temperature was reported for fat rich rapeseed and salmon oil by Liu et al. (2012) and Huang and Sathivel (2008), with the values of EA as 29.0 and 51.3 kJ mol−1, respectively. Temperature directly affects the intermolecular bonding, partition coefficient and aroma release kinetics as reported by Seuvre et al. (2008) in dairy custard.
Structurally, responsible flavor components in Desi ghee are a mixture of free fatty acids (FFAs), lactones and carbonyl compounds present in close association with triglycerides and other fat molecules. Flavor of Desi ghee is released when there is a temperature beyond the melting range of these compounds with subsequent change of viscosity (Ganguli and Jain 1973).
Viscoelastic properties
Amplitude sweep test
The storage modulus G′ means the magnitude of the energy stored in a material or recoverable per cycle of deformation, whereas loss modulus G″ expresses the energy that is lost from a material at viscous dissipation per cycle of deformation. Linear viscoelastic region (LVR) denotes the conditions in which the applied external force will not alter the phase stability or lead to structural breakdown of a material. Figure 4 shows the variation of G′ and G″ with respect to the increasing strain for the ghee samples at the four selected temperatures. The figure could be divided into three phases i.e., (1) stable phase/linear viscoelastic region, where values of G′ and G″ remained constant with increase in strain; (2) transition phase, where values eventually reached cross-over flow point (G′= G″), and (3) inversion phase, where G″> G′. The figure shows that the ghee samples exhibited narrow range of LVRs at the four selected temperatures. The calculated upper limit values of LVRs for the four selected temperatures are presented in Online Resource-1 (ESM-1).
For the entire range of strain sweep, the values of G′ and G″ decreased with increasing strain value from 0 to 100% at the four selected temperatures. Hyun et al. (2002) observed similar behavior while studying the rheological properties of polysaccharide fluids such as polyvinyl alcohol, hyaluronic acid, borax and Xanthin gum. Citerne et al. (2001) studied the strain sweep for peanut butter and observed the similar behavior.
However, despite the value of G′ being greater in magnitude than the G″ at initial stage (Fig. 4.), the samples displayed narrow linearity range which was indicative of it being a soft material, owing to high fat content comparative to peanut butter and other similar products. While analyzing Fig. 4, it was observed that the values of G′ and G″ were practically constant in a very narrow linear viscoelastic region (LVR) where the values of strain were varied from 0.01 to 0.1% at 18 and 24 °C. However, such region was not observed at higher temperatures. The cross-over points, where G′ = G″, as obtained, were 162.63, 12.6, 3.72 and 4.23 Pa, respectively at 18, 24, 30 and 36 °C. As shown in the figure, the decline in the magnitude of G′ displayed gradual breakdown of the matrix which was indicative of spreadable nature of the ghee samples. Bruno and Moresi (2004) studied the variation of G′ and G″ of fat rich sausages with respect to temperature and shear strain and observed the similar behavior.
Frequency sweep test
Figure 5 shows variation in G′ and G″ as a function of angular frequency ω for the ghee samples at the four selected temperatures. The figure shows that at 18 and 24 °C, the values of both G′ and G″ increased slightly with increase in the value of ω. Further, at these temperatures, the magnitude of G′ was higher than the value of G″ for the entire frequency sweep. The calculated values of G′ ranged from 1.12 × 103 to 2.33 × 103 and 7.69 × 101 to 2.8 × 102 Pa at 18 and 24 °C, respectively; whereas the values of G″ varied from 7.24 × 102 to 7.49 × 102 and 0.72 × 101 to 4.6 × 101 Pa at 18 and 24 °C. This inferred that in linear viscoelastic range, the elastic properties dominated over viscous properties for the ghee samples at 18 and 24 °C, and it remained phase stable in the entire LVR range. However, at 30 °C, the values of G′ and G″ ranged from 2.42 × 101 to 2.2 × 102 and 1.15 × 101 to 1.4 × 101 Pa, respectively with no zone of consistent difference. The values of G′ and G″ crossed over throughout LVR range with values of G″ > G′ in lower frequency range, indicating uncross-linked structure, phase inversion and frequency independent nature of the ghee samples at 36 °C. Unlike dense packed sample matrix, highly changeable elastic to viscous nature of ghee, indicative of soft, easy to work upon matrix makes it comparatively extractable for selective component extraction. Similar behavior of G′ and G″ under frequency sweep was reported for peanut butter (Citerne et al. 2001), butter and spreads (Borwankar et al. 1992) and plain butter (Rønholt et al. 2012).
As there was slight increase in G′ and G″ with frequency, indicating slight visco-elastic nature, plots of angular frequency ω versus G′ and G″ data at 18, 24, 30 and 36 °C were subjected to non-linear regression analysis with a power law function and the values of intercepts (K′, K″ and K*), slopes (n′, n″ and n*) and R2 are summarized in Online Resource-2 (ESM-2). From the values of K′, K″, K*, n′, n″ and n*, it can be concluded that the power law function is not applicable for predicting the value of G′ at 36 °C, where the parameters varied rather in linear manner with respect to each other. As observed by low R2 values, the power law did not fit well to predict the values of G″, except at 24 °C. The negative value of n″ for G″ at 18 °C might be due to slippage of sample particles during experimentation. High values of R2 for complex viscosity η* were estimated at 18, 24 and 30 °C, but it was not observed at 36 °C. It indicated that power-law type function was not adequate to describe the viscoelastic behaviour of ghee sample.
Verification of Cox–Merz rule
Cox–Merz rule states that the dynamic shear viscosity η* becomes nearly equal to the steady shear viscosity η when angular frequency equals the shear rate. In order to examine the applicability of the Cox–Merz rule (Eq. 13), the values of apparent viscosity η and complex viscosity η* of the ghee samples as obtained from the flow curve in controlled shear rotational test and frequency sweep oscillatory test were compared. It was observed that the magnitudes of η* were higher than those of η at all four temperatures studied, indicating that the Cox–Merz rule was not applicable to the ghee samples as shown in Online Resource 3 (ESM-3). The reason behind non-applicability of Cox-Merz rule might be due to the weak network and unstable nature of the ghee matrix at higher temperatures and shear rates. Similar behavior was reported by Oroian et al. (2013) for Spanish honey and Yilmaz et al. (2011) for commercial tomato ketchup samples.
Conclusion
Rheological characterization indicated that the ghee samples were non-Newtonian in nature with viscoelastic properties at the flour selected temperatures. It was shear-thinning fluid with exhibition of yield stress, indicative of structural network due to thermal properties of fat present, which declined with influence of temperature. The viscosity of the ghee samples was highly temperature dependent and decreased with increase in temperature. The amplitude sweep indicated spreadibility and absence of micro-crack formation phenomenon in deformation. The frequency sweep showed that the phase stability in terms of physical state was highly temperature dependent. With increase in temperature, the storage modulus G′ declined rapidly while the viscous nature tend to increase, as indicated by values of loss modulus G″.
References
Aneja RP, Mathur BN, Chandan RC, Banerjee AK (2015) Technology of Indian milk products: handbook on process technology modernization for professionals, entrepreneurs and scientists. Dairy India Yearbook, Delhi
Borwankar RP, Frye LA, Blaurock AE, Sasevich FJ (1992) Rheological characterization of melting of margarines and table spreads. J Food Eng 16:55–74
Bourne MC (2002) Physics and texture (Ch. 3). In: Bourne M (ed) Food texture and viscosity. Concept and measurement, 2nd edn. Academic Press, London, pp 59–106
Bruno M, Moresi M (2004) Viscoelastic properties of Bologna sausages by dynamic methods. J Food Eng 63:291–298
Citerne GP, Carreau PJ, Moan M (2001) Rheological properties of peanut butter. Rheo Acta 40:86–96
Codex Alimentarius (2011) Milk and milk products. In: Food and Agriculture Organization of the United Nations, 2nd edn. World Health Organization, Rome(ISBN:978-92-5-105837-4)
Das H (2005) Food processing operations analysis, 1st edn. Asian Books Private Ltd, New Delhi
De Roos KB (2000) Physicochemical models of flavour release from foods. In: Roberts DD, Taylor AJ (eds) Flavour release. American Chemical Society, Washington D.C., pp 126–141
Food Safety and Standards (Food Product Standards and Food Additives) Regulation (2011)
Franco Z, Nguyen QD (2011) Flow properties of vegetable oil–diesel fuel blends. Fuel 90:838–843
Ganguli NC, Jain MK (1973) Ghee: its chemistry, processing and technology. J Dairy Sci 56:19–25
Goodrum JW, Geller DP, Adams TT (2003) Rheological characterization of animal fats and their mixtures with fuel oil. Biomass Bioenergy 24:249–256
Gupta M, Singh D, Gularia P, Gupta S (2015) GC-MS analysis and identifications of chemical constituents of Syzygium aromaticum, Brassica compestris and cow ghee. J Chem Pharma Res 7(1):568–572
Huang J, Sathivel S (2008) Thermal and rheological properties and the effects of temperature on the viscosity and oxidation rate of unpurified salmon oil. J Food Eng 89:105–111
Hyun K, Kim SH, Ahn KH, Lee SJ (2002) Large amplitude oscillatory shear as a way to classify the complex fluids. J Non-Newtonian Fluid Mech 107:51–65
Juszczak L, Witczak M, Fortuna T, Banys A (2004) Rheological properties of commercial mustards. J Food Eng 63:209–217
Kim J, Kim DN, Lee SH, Yoo SH, Lee S (2010) Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem 118:398–402
Liu C, Yang M, Huang F (2012) Influence of extraction processing on rheological properties of Rapeseed Oils. J Am Oil Chem Soc 89:73–78
Lubbers S, Butler E (2010) Effects of texture and temperature on the kinetic of aroma release from model dairy custards. Food Chem 123:345–350
Mossaz S, Jay P, Magnin A, Panouillé M, Saint-Eve A, Déléris I, Juteau A, Souchon I (2010) Measuring and predicting the spreading of dairy products in the mouth: sensory, instrumental and modelling approaches. Food Hydro 24:681–688
Nik WBW, Ani FN, Masjuki HH, Giap SGE (2005) Rheology of bio-edible oils according to several rheological models and its potential as hydraulic fluid. Ind Crops Prod 22:249–255
O’Kuru REH, Carriere CJ (2002) Synthesis, rheological characterization, and constitutive modeling of polyhydroxy triglycerides derived from milkweed oil. J Agric Food Chem 50:3214–3221
Oroian M, Amariei S, Escriche I, Gutt G (2013) Rheological aspects of Spanish honeys. Food Bioprocess Technol 6:228–241
Ramesh B, Bindal MP (1987) Influence of fatty acid composition on softening point and melting point of cow, buffalo and goat ghee. Indian J Dairy Sci 40(1):52–54
Rønholt S, Kirkensgaard JJK, Pedersen TB, Mortensen K, Knudsen JC (2012) Polymorphism, microstructure and rheology of butter—effects of cream heat treatment. Food Chem 135:1730–1739
Santos JCO, Santos IMG, Souza AG (2005) Effect of heating and cooling on rheological parameters of edible vegetable oils. J Food Eng 67:401–405
Sathivel S, Huang J, Prinyawiwatkul W (2008) Thermal properties and applications of the Arrhenius equation for evaluating viscosity and oxidation rates of unrefined pollock oil. J Food Eng 84:187–193
Seuvre AM, Turci C, Voilley A (2008) Effect of the temperature on the release of aroma compounds and on the rheological behaviour of model dairy custard. Food Chem 108:1176–1182
Srimagal A, Tamanna R, Sahu JK (2016) Effect of light emitting diode on inactivation of Escherichia coli in milk. LWT J Food Sci Technol 71:378–385
Steffe JF (1996) Rheological methods in food process engineering, 2nd edn. Freeman Press, East Lansing
Suryakumari C, Anusha A, Anitha P, Ravindra D (2014) Formulation and evaluation of cow ghee as a base fast and sustained release chloroquine phosphate suppositiories. World J Pharm Pharm Sci 3(6):1493–1509
Taghizadeh M, Razavi SMA (2009) Modelling time-independent rheological behavior of pistachio butter. Int J Food Prop 12:331–340
Wiking L, De Graef V, Rasmussen M, Dewettinck K (2009) Relations between crystallisation mechanisms and microstructure of milk fat. Int Dairy J 19:424–430
Yılmaz M, Karaman S, Cankurt H, Kayacier A, Sagdic O (2011) Steady and dynamic oscillatory shear rheological properties of ketchup–processed cheese mixtures: effect of temperature and concentration. J Food Eng 103:197–210
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duhan, N., Sahu, J.K. & Naik, S.N. Temperature dependent steady and dynamic oscillatory shear rheological characteristics of Indian cow milk (Desi) ghee. J Food Sci Technol 55, 4059–4066 (2018). https://doi.org/10.1007/s13197-018-3332-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3332-6