Abstract
Rheological behavior of six crude rapeseed oils with different extraction methods including hot-pressing, solvent-extraction and cold-pressing were studied. Viscosities of the oils were measured with shear rates ranging from 0.1 to 200 s−1 at three different temperatures. The Casson model was used to fit the experimental data and the Arrhenius equation was applied to estimate the energy of activation for viscosity (E a). The extraction methods affected the total tocopherol, total phytosterols, total phenols, phosphorus and fatty acid composition. The hot-pressed medium-erucic rapeseed oil (HMRO) had the greatest viscosity, and the cold-pressed low-erucic rapeseed oil (CLRO) had the lowest viscosity among all the oils with shear rates >5 s−1 at 10 °C. The crude rapeseed oils exhibited Newtonian behavior at higher shear rates. The significant difference of viscosity of the six oils was reduced with increasing temperature, and there was no significant change (P > 0.01) among the oils with a shear rate of 100 s−1 at 50 °C. According to the values of E a, the following order of a change in viscosity was obtained as follows: CMRO > SMRO > HMRO > CLRO > SLRO > HLRO(C, cold pressed; S, solvent extracted; H, hot pressed; M, medium erucic; L, low erucic; RO, rapeseed oil). Minor components may be the contributing factors for the values of E a of rapeseed oils. The higher shear limiting viscosity (ηc) values calculated by the Casson model decreased as the temperature increased, but no significant change (P > 0.01) was observed for ηc by using different extraction methods at 50 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheology is the study of the deformation and flow of a material under a given stress. It has applications in foods and food ingredients for quality control during manufacture and processing. The rheological properties of food oils and fats are relevant to product quality and applications such as the stability of emulsions during storage, crystallization of oil blends and suitability for fuel additives or alternatives [1–3].
Rapeseed oil obtained from rapeseed has become the third leading source of vegetable oils. The edible oils industry's predominant methods for extracting rapeseed oil are primarily physical pressing and solvent extraction. The characteristics of rapeseed oils extracted by various approaches have been evaluated by previous research workers. Recently, research has been directed toward the rheological behavior of oils. It has been reported that the rheological properties of oils normally depend upon several factors including temperature, shear rate, concentration and pressure [4]. Nevertheless, the fatty acid composition, refining steps and extended frying times could lead to the changes in flow characteristics of food oils [5–9]. There are mathematical models for predicting the rheological behavior of oils, such as Newtonian, Bingham, Casson, Herschel-Bulkley, Cross and Carreau [4, 8]. Soybean, sunflower, olive, rapeseed, corn and rice oils all exhibit Newtonian behavior [10]. However, the oils can have non-Newtonian flow at shear rates less than 6 s−1, and exhibit Newtonian behavior above 10 s−1 shear rates [11–13].
However, little attention has been paid to the rheological properties of crude rapeseed oils obtained by different extraction methods. The objective of the present study is to evaluate the effects of different extraction processes on the rheological properties of crude rapeseed oils. Furthermore, the suitability of the Casson model for describing rheological behavior of crude rapeseed oils is also investigated.
Materials and Methods
Reagents
α-Tocopherol, γ-tocopherol and folin phenol were purchased from Sigma-Aldrich. Standard samples of phytosterols were supplied by Xi-an Lantian Biological Engineering CO., Ltd. (Xi-an, China). Acetonitrile and methanol of chromatographic grade were purchased from Merck (Darmstadt, Germany). Other chemicals and reagents were of analytical grade and were used without further purification.
Samples and Sample Preparation
Low-erucic rapeseed (LR), hot-pressed low-erucic rapeseed oil (HLRO) and solvent-extraction low-erucic rapeseed oil (SLRO) were supplied by Hulunbeier Jinjiao Bio-chem. Co., Ltd. Medium-erucic rapeseed (MR), hot-pressed medium-erucic rapeseed oil (HMRO) and solvent-extraction medium-erucic rapeseed oil (SMRO) were provided by Wuhan Zhongpai Oils and Grains Co., Ltd. The cold-pressed low-erucic rapeseed oil (CLRO) and cold-pressed medium-erucic rapeseed oil (CMRO) were obtained in our laboratory. The rapeseed was fed from the hopper to the single screw press (CA59G, Komet, Germany) at ambient temperature. The crude oil was separated from the cake particles by centrifuging at 6,000 rpm (15,900×g) for 15 min, and then the cold-pressed oil was filtered and collected until used.
Fatty Acid Composition
The fatty acid methyl esters (FAME) of the samples were obtained by vigorous mixing of a solution of oil in hexane (0.1 g in 2.5 mL) with 0.2 mL of 0.5 N methanolic potassium hydroxide at room temperature for 5 min. FAME were then injected into the GC system (Hewlett-Packard 6890 N, Agilent Technologies, USA) with a HP-88 capillary column (100 m × 0.25 mm i.d. × 0.2 μm film thickness). Helium was used as carrier gas, at a flux of 2 mL/min, and the split ratio was 1:50. The injector and flame ionization detector (FID) temperatures were set at 250 and 280 °C respectively. The temperature program was as follows: 120 °C for 1 min, heated to 175 °C at a rate of 10 °C/min and held for 10 min; then the temperature was increased to 210 °C at a rate of 5 °C/min and held for 5 min; then it was increased at a rate of 5 °C/min to 230 °C and held for 5 min. The results of the fatty acid composition are given in Table 1.
Determination of Phosphorus
Oil samples (5 g) added with zinc oxide (0.5 g) were weighted in the crucibles, and they were heated on an electric stove at 600 °C for 2 h until they tuned into white ashes. The total phosphorus content in the ashes was determined by applying AOCS Official Method Ca12-55 [14].
Determination of Tocopherols
Oil samples (5 g) were dissolved in 30 mL of anhydrous ethanol with 20 mL of a 1 g/L potassium hydroxide solution, and 5 mL of ascorbic acid (10%) was added. Then the mixture was heated at 100 °C for 30 min, and the saponification solution was transferred to a separatory funnel and extracted by 100 mL of diethyl ether. Afterwards, the ether layer was washed with deionized water and dehydrated using anhydrous sodium sulfate. The ether extract was evaporated with nitrogen at room temperature, and the residue was redissolved in 2 mL of ethanol. The ethanol solution was centrifuged at 5,000 rpm for 10 min. 2 μL of the supernatant was injected into a BEH C18 column (100 × 2.1 mm, 1.7 μm particle size, Acquity, Waters, USA) and analyzed by a UPLC system (Acquity, Waters) with an autosampler and a photodiode array (PDA) detector at a wavelength of 300 nm. The mobile phase was a mixture of methanol and high-purity water (95:5, vol/vol%) at a flow rate of 0.3 mL/min. The concentrations of tocopherols were acquired with Empower 2 software (Waters).
Determination of Total Phytosterols
Oil samples (5 g) were dissolved in 50 mL of potassium hydroxide-ethanol (1 mol/L) and heated at 100 °C for 1 h. Then the saponification solution was collected in a separatory funnel and extracted by 100 mL of diethyl ether. The ether extract was washed with 40 mL potassium hydroxide solution (0.5 mol/L) of and 40 mL of deionized water in turn for three replicates. The ether extract was transferred to a 100 mL volumetric flask and made up to volume with ether. 10 mL of the ether extract from the flask was evaporated with nitrogen at room temperature, and the residue was redissolved in 1 mL of ethanol for analysis.1 μL of the ethanol solution was injected into the GC (Hewlett-Packard 6890 N, Agilent Technologies) with a DB-5 capillary column (15 m × 0.32 mm i.d. × 0.1 μm film thickness). Nitrogen was used as carrier gas, at a flux of 1.5 mL/min, and the split ratio was 1:10. The injector and FID temperatures were both set at 350 °C. The temperature program was as follows: heated to 243 °C from180 °C at a rate of 3 °C/min and held for 0.5 min; then the temperature was increased to 340 °C at a rate of 50 °C/min and held for 0.5 min.
Determination of Total Phenols
Oil samples (2.5 g) were dissolved in 5 mL of hexane and extracted with 5 mL of methanol/water solution (80:20, vol/vol%). The mixture was centrifuged at 3,500 rpm for 10 min to collect the aqueous phase, which was dried under vacuum at room temperature. The dried sample was redissolved in 5 mL of methanol solution and transferred to a 50 mL volumetric flask. Then 2.5 mL of Folin-phenol reagent and 10 mL of saturated sodium carbonate solution were added, and made up to volume with deionized water. The absorbance was measured at 765 nm with a UV/VIS spectrometer (2802PCS, UNICO, China) after 30 min. The concentrations of total phenols (TP) were calibrated with tannic acid (TA).
Color Measurement
Color measurement of oil samples was carried out using a Lovibond colorimeter (WSL-2A, Shanghai Suoguang Light & Electricity Tech. co., Ltd., China). The color values were expressed as Y (yellow), R (red) and B (blue) with Lovibond tintometer of 25.4 mm.
Rheological Properties
The rheological characteristics of oils were determined by a rotator rheometer (AR2000, TA Instruments Ltd., USA) equipped with a 40 mm cone-plate sensor and 40 mm parallel sensor. The parallel sensor was for temperature ramps. Each sample was place on the temperature-controlled vessel and allowed to equilibrate to the required temperatures (10, 30 and 50 °C). Viscosity was measured at various shear rates from 0.1 to 200 s−1. The influence of temperature on viscosity (μ) for rapeseed oils can be expressed in an Arrhenius type of equation [5] involving absolute temperature (T), the universal gas constant (R), the equation constant (A) and the energy of activation for viscosity (E a):
Rheological Model
The Casson equation was used to describe the flow behavior of food oils because it works well at various shear rates by Eq. 2 [8]:
where τ xy = shear stress (Pa), τo = yield stress (Pa), ηc = higher shear limiting viscosity (Pa s) and dV x /D y is shear rate.
Statistical Analysis
The analyses were performed with three repetitions. Statistical analysis was conducted by the PASW Statistics, version 18.0. The significances of difference between the means were determined by ANOVA, using Tukey test (P < 0.01). The rheological data and mathematical model were carried out by Rheology Advantage Data Analysis, V5.7.0 (TA Instruments Ltd., USA). The software calculated the standard error (SE) of the model fit and a reasonable fit gave a value of less than about twenty.
Results and Discussion
The amounts of linoleic acid (LA) in the HLRO, SLRO and CLRO samples were statistically significantly different (P < 0.01). The LA content of the SMRO sample was greater than those of HMRO and CMRO, as demonstrated in Table 1. According to the contents of oleic acid and erucic acid, the results showed significant changes (P < 0.01) for the three medium-erucic rapeseed oils. But no significant differences (P > 0.01) were observed among the LROs. Although the extraction process seems to have a very small effect on the fatty acid composition, the fatty acid composition as a whole could indicate a stable trend. Similar results were reported by Zeng [15], in that the fatty acid composition of tea-seed oils varied according to the extraction methods, especially the amounts of LA.
The minor constituents and color of the rapeseed oils are listed in Table 2. The rapeseed oils were rich in γ-tocopherol and α-tocopherol, which also was described previously by Szydłowska-Czerniak et al. [16]. As can be seen, the concentrations of TTC, TPS, TP and phosphorus in the hot-pressed and solvent-extracted oils were significantly greater (P < 0.01) in comparison than in the cold-pressed oils. Table 2 also reports that the contents of TTC and phosphorus of SLRO and SMRO were significantly lower (P < 0.01) than those of the HLRO and HMRO samples, respectively. On the other hand, the color of HLRO was darker than that of SLRO and CLRO, and the MROs showed the similar results for color. It could be that the heat-treatment and solvents lead to a greater release of the minor components from rapeseed cells into the oil phase. Prior et al. [17] obtained the similar results that heat treatment increased the contents of non-triglyceride components in canola oils. Knuth and Homann [18] also reported that cold-pressed oils contained lower amounts of non-triglyceride constituents than oils prepared by conventional processes.
The flow behavior of the six rapeseed oils was measured at 10 °C showed in Fig. 1. As the shear rate increases, the shear stress is enhanced gradually. The similar trends were shown at 30 and 50 °C. A linear relation was observed between the shear stress and shear rate at higher shear rates, when the crude rapeseed oils extracted by different methods behaved as a Newtonian fluid. This result is shown in Fig. 2. Geller and Goodrum [11] reported, similar results that viscosity of triglyceride mixtures showed a transition from non-Newtonian to Newtonian behavior at shear rates >7 s−1.
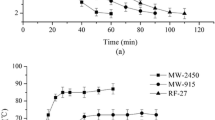
Figure 2 shows that HMRO had the highest viscosity at shear rates >5 s−1, and CLRO had the lowest viscosity among all the oils at 10 °C. Between MROs and LROs, the viscosities of HMRO and CMRO were significantly greater (P < 0.01) than those of HLRO and CLRO, respectively at shear rates >5 s−1. The differences in the oil viscosities may be due to the fatty acid composition, because MROs contain much more erucic acid than other oils. Noureddini et al. [19] measured the viscosities of eight fatty acids from C9 to C22, and observed that the chain length of fatty acids positively correlated with viscosity. That viscosity of vegetable oils was due to the fatty acid composition more than triacyglycerol species, which was suggested by Boyaci et al. [20].
However, a comparison of the viscosities of SMRO and SLRO listed in Fig. 2 indicates that they had no significant difference (P > 0.01) at shear rates greater than 100 s−1. The greater concentration of phosphatides in the SLRO shown in Table 2 could contribute to an increase in the viscosity even if SLRO contained lower amounts of erucic acid than SMRO. As shown in Fig. 3, viscosity decreased with increase in temperature at 100 s−1 for the six oils. The results are similar to those of Wang and Briggs [5]. The curves of Fig. 3 also suggest that the significant differences in viscosity between the oils could be reduced with increase in temperature, which means that the method of oil extraction could have little effect on the viscosity of oils at higher temperatures. Santos et al. [10] reported that the edible vegetable oils including rapeseed oil had Newtonian behavior during heating and cooling, and the viscosity of edible vegetable oils decreased as the temperature increased. It was explained that there were the greater molecular movement and a reduction in the intermolecular bond forces contributing to promoting flux increases and reduced viscosity. Ennouri et al. [12] suggested that the aggregates of crude oils were destroyed by increasing shearing and temperature and the oils could form a stable structural state at high shear stress.
The thermodynamic parameters calculated by Eq. 1 are listed in Table 3. It could be found that the values of E a of two kinds of hot-pressed and solvent-extraction rapeseed oils were lower than those of cold-pressed rapeseed oils, respectively. It seems to indicate that the amounts of minor constituents in oils could affect the E a values of the oils. Also, the value of E a of CMRO was significantly greater (P < 0.01) than those of the other five oils, which suggests that CMRO had a more rapid change in viscosity with temperature. In addition, the data of Table 3 illustrates that the values of Ea of MROs were greater than those of LROs. The results are consistent with those of Wang and Briggs [5]. They described that the longer the chain of the fatty acyl groups, the greater the value of E a.
According to the results in Table 4, the yield stresses of HMRO and HLRO were significantly greater (P < 0.01) than those of CMRO and CLRO at different temperatures, respectively. But the yield stresses of SMRO were lower (P < 0.01) that those of SLRO. Sathivela et al. [8] used four models to fit the crude and refined catfish oils, and suggested that the Casson model is best for predicting the rheological behavior of the catfish oils. Additionally, it can be found that the ηc values of the crude rapeseed oils decreased as the temperature increased. There was no significant difference (P > 0.01) in the ηc values of the oils at 50 °C based on the extraction methods.
References
Logaraj TV, Bhattachary S, Bhattachary S, Sankar KU, Venkateswaran G (2008) Rheological behaviour of emulsions of avocado and watermelon oils during storage. Food Chem 106:937–943
Reyes-Hernández J, Dibildox-Alvarado E, Charó-Alonso MA, Toro-Vazquez JF (2007) Physicochemical and rheological properties of crystallized blends containing trans-free and partially hydrogenated soybean oil. J Am Oil Chem Soc 84:1081–1093
Goodrum JW, Geller DP, Adams TT (2003) Rheological characterization of animal fats and their mixtures with #2 fuel oil. Biomass Bioenergy 24:249–256
Nik WBW, Ani FN, Masjuki HH, Giap SGE (2005) Rheology of bio-edible oils according to several rheological models and its potential as hydraulic fluid. Ind Crops Prod 22:249–255
Wang T, Briggs JL (2002) Rheological and thermal properties of soybean oils with modified fa compositions. J Am Oil Chem Soc 79:831–836
Kim J, Kim DN, Lee HS, Yoo SH, Lee S (2010) Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem 118:398–402
Davis JP, Sanders TH (2007) Liquid to semisolid rheological transitions of normal and high-oleic peanut oils upon cooling to refrigeration temperatures. J Am Oil Chem Soc 84:979–987
Sathivel S, Prinyawiwatkul W, Negulescu II, King JM, Basnayake BFA (2003) Effects of purification process on rheological properties of catfish oil. J Am Oil Chem Soc 80:829–832
Maskan M (2003) Change in colour and rheological behaviour of sunflower seed oil during frying and after adsorbent treatment of used oil. Eur Food Res Technol 218:20–25
Santos JCO, Santos IMG, Conceiçăo MM, Porto SL, Trindade MFS, Souza AG, Prasad S, Fernandes VJ Jr, Araújo AS (2004) Thermoanalytical, kinetic and rheological parameters of commercial edible vegetable oils. J Therm Anal Calorim 75:419–428
Geller DP, Goodrum JW (2000) Rheology of vegetable oil analogs and triglycerides. J Am Oil Chem Soc 77:111–114
Ennouri M, Evelyne B, Laurence M, Hamadi A (2005) Fatty acid composition and rheological behaviour of prickly pear seed oils. Food Chem 93:431–437
Santos JCO, Santos IMG, Souza AG (2005) Effect of heating and cooling on rheological parameters of edible vegetable oils. J Food Eng 67:401–405
Firestone D (1998) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS Press, Champaign
Zeng H-Y, Li C-Z, Jiang L-J (2005) GC-MS analysis of fatty acids from tea-seed oil extracted by different methods. J Tropical Subtropical Botany 13:271–274
Szydłowska-Czerniak A, Karlovits G, Dianoczki C, Recseg K, Szłyk E (2008) Comparison of two analytical methods for assessing antioxidant capacity of rapeseed and olive oils. J Am Oil Chem Soc 85:141–149
Prior EM, Vadke SV, Sosulski FW (1991) Effect of heat treatments on canola press oils. i. non-triglyceride components. J Am Oil Chem Soc 68:401–406
Knuth M, Homann T (1983) Method of recovering oil from oil-bearing vegetable matter. US Patent 4,467,713
Noureddini H, Teoh BC, Clements LD (1992) Viscosities of vegetable oils and fatty acids. J Am Oil Chem Soc 69:1189–1191
Boyaci IH, Tekin A, Çizmeci M, Javidipour I (2002) Viscosity estimation of vegetable oils based on their fatty acid composition. J food lipids 9:175–783
Acknowledgments
The authors are grateful to Hulunbeier Jinjiao Bio-chem. Co., Ltd. and Wuhan Zhongpai Oils and Grains Co., Ltd., for providing the rapeseed oils and rapeseed samples. This work was supported by the funding from Oil Crops Research Institute, Chinese Academy of Agricultural Sciences (Project No.1610172009002).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Liu, C., Yang, M. & Huang, F. Influence of Extraction Processing on Rheological Properties of Rapeseed Oils. J Am Oil Chem Soc 89, 73–78 (2012). https://doi.org/10.1007/s11746-011-1892-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1892-y