Abstract
The aim of the present study was to assess the drying kinetic of tucum fruits (epicarp and mesocarp) Astrocaryum aculeatum Meyer at three different temperatures (50, 60, and 70 °C). The physicochemical characterization, water activity, moisture content, including β-carotene and vitamin C content in-natura and dried fruits were analyzed. The fruit fractions presented high β-carotene, protein and lipid levels. Fatty acid profile showed oleic acid as the major fatty acid. Different mathematical models were computed to assess the drying process. The Page model was observed to be the best to describe the drying kinetic with the highest correlation coefficient (R2) 0.99 and the least Chi squared (χ2) close to 105 at the studied temperatures. The drying process reduced water activity to desirable levels in all trials and β-carotene retentions after drying remained at satisfactory levels, fact that resulted in minimum value of 63% and approximately 94% in some cases. Vitamin C retention was comparatively more around 20–40% compared to control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Amazonas State has a wide variety of fruit species of economic and nutritional potential, although they remain little explored. Tucum (Astrocaryum aculeatum Meyer) is a palm three native to Brazil, whose geographical distribution covers several Brazilian states such as Amazonas, Acre, Rondônia, Roraima and Pará. Moreover, they can be also found in other South American countries such as Peru, Colombia (Costa et al. 2011; Lorenzi et al. 2010), Guyana and Venezuela (Lorenzi et al. 2010).
The tucum fruit has oval or spherical shape with yellowish-green epicarp. The mesocarp presents a fibrous oily aspect with yellow-orange shade (FAO 1986). Mesocarp composition has been well described and characterized by high caloric density, and high lipid, protein, fiber and β-carotene contents (FAO 1986; Jobim et al. 2014; Sagrillo et al. 2015). Studies about its epicarp characteristics remain scarce, possibly because these residues are little explored in the food chain.
Overall, tucum fruits have good acceptance in their distribution areas and present increasing demand in Manaus market (Amazonas State, Brazil). This fruit is basically consumed in-natura or stuffing sandwiches, and in the form of creams and ice creams (Yuyama et al. 2008).
Tucum commercialization is carried out with little, or no technological processing, it actually consists of the fruit itself or, in some case, of its pulp (mesocarp). Its commercialization is also focused on popular markets and/or on strategic sales-points on the streets all year long (Costa et al. 2011).
According to Didonet (2012), tucum trading generates large amounts of residues since it is mainly focused on mesocarp exploitation. For example, the amount of wastes (epicarp and mesocarp) produced due to tucum commercialization in free fairs in Manaus area between 2011 and 2012 reached 268.5 tons. Besides the generated residues, the lack of adequate conditions for tucum commercialization also leads to high and recurrent losses (Didonet and Ferraz 2014). The shelf life of Astrocaryum aculetaum Meyer is short, 3–4 days; therefore, the specie is highly perishable (FAO 1986). Thus, new viable alternatives for tucum residue use become an important way to avoid waste and environmental issues (Manzato et al. 2017).
Thus, drying is one of the oldest methods applied to food products preservation after harvest, because it provides more stability to the product through undesirable-microorganism proliferation prevention (McMinn and Magee 1999) and consequently increases product shelf-life. However, since drying is known as a complex process of coupled heat and mass transfer, the drying kinetic studies and the application of mathematical models to describe drying experimental data achieves great practical importance to estimate relevant parameters such as: drying time, mass and thermal diffusivity coefficients, energy consumption, among others, that are required to develop new food processes including products and dryers. In this context, semitheoretical and empirical thin-layer drying models have been extensively applied to foods subjected to drying (Onwude et al. 2016) however they are limited to imposed drying conditions.
Accordingly, the aim of the present study was to assess the drying kinetics of tucum fruits as a strategy to increase their shelf life by preserving their physicochemical, vitamin C and carotenoid contents, as well as to consequently add value to their productive chain.
Materials and methods
Materials
The experiments were conducted with Tucum fruits (A. aculeatum Meyer) purchased at Manaus markets (Amazonas State, Brazil). The fruits were selected from batches presenting different ripeness degrees. All chemicals and standards were purchased at Sigma-Aldrich.
Fruit pre-processing
The fruits were grouped in three categories according to peel color and by taking into consideration each color as equivalent to the fruit’s ripening degree: group 1, G1 (lower yellow/red intensity) green fruits; group 2, G2 (intermediate yellow/red intensity) intermediate ripening stage; and group 3, G3 (greater yellow/red color intensity) ripe fruits. Each group comprised 20 fruits.
Epicarp and mesocarp samples were manually separated from the fruit and placed in metalized plastic bags labelled with the recorded sample mass; they were stored in a freezer until the analysis and the drying procedure were carried out.
Physicochemical features
Morphometric aspects and fruit pulp yield
The morphometric aspects were assessed with a digital caliper based on measurements applied to the longitudinal and transverse diameters of the fruits. The longitudinal diameter was measured from the apex at the basis of the fruit, whereas the transverse diameter was recorded from the point recording the largest diameter in the fruit.
Pulp mass and its fractions (epicarp, mesocarp and seed) were weighed on analytical scale after the Tucum fruits were peeled/pulped. Yield (%) was set according to the mass of each fruit fraction and to the mass of the whole fruit (Eq. 1).
Color analysis
Epicarp and mesocarp color analysis was performed using Hunter Lab Colorimeter, MiniScan Spectrophotometer XE Plus (Hunter Lab, Reston, VA). Color measurements were expressed through the Hunter values L*, a* and b* of the 10° standard observer and the D65 standard illuminant. The L* value, which indicated the lightness measurement, ranged from 0 (black) to 100 (white), the a* value ranged from − 100 (greenness) to + 100 (redness), the b* value ranged from − 100 (blueness) to +100 (yellowness) (Hutchings, 1999). The Hue angle was calculated through Eq. 2:
wherein, 0° or 360° correspond to red–purple, 90° to yellow, 180° to green and 270° to blue (Mcguire 1992).
Composition
Samples were analyzed in triplicate. The moisture content was determined through oven drying at 105 °C until reaching constant weight. The ash content was determined using muffle incineration at 550 °C. Proteins were calculated based on total nitrogen content according to Kjeldahl method (Kjeldahl 1883), considering a conversion by means of factor 5.75, which refers to proteins of vegetal origin. The lipid content was recorded through cold extraction by using chloroform, methanol and water as solvents, according to the ratio 1:2:0.8 v/v, respectively (Bligh and Dyer 1959). Carbohydrate contents were inferred by the 100%-difference in relation to other fractions.
Acidity and pH
The pH was measured using a previously calibrated Bel Engineering W3B digital potentiometer. Total acidity was determined through the AOAC Official Method 942.15 Acidity (Titratable) of Fruit Products by using 0.1 N NaOH (AOAC 1998).
Water activity
The water activity of the fruit samples was measured in triplicate, in Aqualab analyzer, model DEW, 4TEV, Decagon (USA).
Fatty acid profile
Lipids found through extraction carried out by Bligh and Dyer (1959) were converted into FAMEs according to the methodology described by the AOAC Official Method 996.06 (AOAC 2001). The esterified samples were injected into a Shimadzu GC/MS-QP 5050A gas chromatograph, which contained a DB-5MS capillary column (0.25 μm thick, diameter 0.25 mm) and FID. Helium was used as carrier gas. The GC operating conditions were: entrained gas flow into the column: 1.7 mL/min; Detector temperature: 280 °C, Injector temperature: 250 °C, Column temperature: 55 °C—1 min, 55–180 °C (15 °C/min), 180–205 °C (1 °C/min), 205–280 °C (20 °C/min) for 5 min; injected volume: 1.0 μL.
Vitamin C and carotenoid content
Vitamin C was determined through the AOAC Method 967.21 (AOAC 2002); whereas, the carotenoid content in the samples, before and after drying, was analyzed through the methodology proposed by Wilberg and Rodriguez-Amaya (1995), with modifications. Quantification of β-carotene content was set through the β-carotene standard-solution calibration curves, at 450 nm, generated in SHIMADZU spectrophotometer, model UV-mini 1240. Equation (3) suggested by Rodriguez-Amaya (2001) was used to calculate the retained carotenoid contents after drying:
The retained carotenoids (%) and Vitamin C contents of in-natura and dried fruits were determined at the end experiment.
Drying Procedure
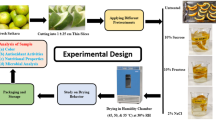
The drying stage in tucum samples was achieved in convective fixed bed dryer (Fig. 1) at different drying temperatures (50, 60 and 70 °C), by taking into consideration the groups previously defined as G1, G2 and G3.
Drying kinetic and mathematical modeling of the drying time
The drying kinetic was achieved through the gravimetric method. Samples were withdrawn from the drying oven at regular intervals (10 min) in order to determine their moisture content. The experiments were performed in triplicate and the moisture content of all samples was determined on a dry basis (db). The moisture ratio (XR) of samples during the drying experiments was calculated through Eq. (4):
wherein, XR is the adimensional moisture content ratio; and X0, Xe, Xi are the initial, equilibrium, and moisture contents at different drying times on a dry basis (db), respectively.
Three semi theoretical models (Newton, Page and Henderson/Pabis—Eqs. 5–7, respectively) were tested to fit the experimental data recorded during tucum epicarp/mesocarp drying. These models have been classically used in thin-layer drying.
wherein, t is drying time (h); and a, k and n are the constant parameters, respectively.
Statistical analysis
The morphometric results were subjected to analysis of variance (ANOVA) followed by Tukey test at 95% confidence level (p < 0.05). Centesimal composition, pH, acid index and carotenoid data were expressed through the mean of the triplicate and through their respective standard deviation values.
Regression analyses were carried out to adjust the kinetic models using the STATISTICA 8.0 software according to the Quasi-Newton estimation and least squares methodologies at convergence criterion 0.0001. The best model was selected according to the highest correlation coefficient (R2) recorded between the observed and predicted values (Eq. 8) and by minimizing the Chi squared (χ2) as defined by Eq. (9) (Akpinar 2008; Kirmaci et al. 2008; Kaleta and Górnicki 2010):
wherein, RSS and TSS are the residual and total sum of squares, or the sum of square error, respectively; XRexp and XRpre are the experimental and predicted dimensionless moisture ratios; n is the number of observations; and σ is the standard deviation.
Results and discussion
Fruit morphometric aspects
Morphometric measurement results are shown in Table 1. Fruits presented general mean 54 mm of longitudinal diameter (DL), 46 mm of transverse diameter (DT) and 61 g of fruit mass (MF). These parameters showed different mean values among the three analyzed groups, as well as higher DL, DT and MF values in G3 fruits, which were followed by the G2 and G1 fruits. Fruits had similar mean shape index regardless of the group; DL/DT between 1.17 and 1.19 indicates slightly oblong shape.
Fruit fractions used to measure total mass comprised 18.61% mesocarp, 11% epicarp and 70% seed. Approximately 5.5 kg of fruit was required to obtain 1 kg of pulp (mesocarp), or approximately 90 units. Moussa and Kahn (1997) also found similar mesocarp percentage (21.9% ± 4.1). This yield can also be compared to acai (Euterpe precatoria Mart.), which is another fruit of economic interest, whose epicarp and mesocarp record 33% of fruit mass (Yuyama et al. 2011).
The G1 fruits, although smaller, presented mesocarp mass and thickness similar to that of larger fruits, when yield was assessed per group. They recorded smaller seed percentage and it resulted in higher mean mesocarp yield (24%). The G1 fruits yield was followed by G3 (20%) and G2 (15%) fruits yield. Tucum production is almost exclusively extractive and, consequently, the fruits follow different size patterns that result in great pulp-yield variability. Moreover, different edaphoclimatic conditions, as well as season and harvest conditions, are preponderant factors to attain fruits of homogeneous quality features (Schroth et al. 2004).
Tucum fruit color analysis
The epicarp color analyses was carried out by measuring the exposed surfaces (ES) and the surfaces non-exposed to sun light (NES). Better homogeneity was observed in fruits in G1 through different measurements of parts exposed or not to sun light in all analyzed chromatic coordinates. Overall, fruits presented positive-value chromatic parameters in a* and b*, thus indicating red and yellow shades, respectively. The b* values were more expressive than the a* values, and it influenced the totality (Hue) of observed yellow-orange.
Chromatic parameter intensity varied in the groups, it presented more evident differences between G1 and G3 fruits. Regardless of the side exposed to sun light, G1 fruits recorded higher Hue values and lower L*, a* and b* values; in other words, they were less orange and darker than fruits in G3. Fruits in G2, in their turn, presented intermediate brightness (L*) and yellow shade (b*).
Although fruit peel color is a quite-relative criterion, some authors have correlated the color evolution aspects to fruit ripening parameters. Ferrer et al. (2005) assessed the color evolution in peaches and found increased linear a* according to ripening stage, and it is explained by chlorophyll loss. There was b* increase during the ripening period, as well as b* decrease during the climacteric period, fact that indicates the beginning of the senescence-like phenomena. According to these authors, although Hue is not a good ripening parameter, results have shown that it linearly diminished with time and was correlated to all the ripening parameters.
The experimentally determined color and morphological features presented significant differences at confidence level 95% (p < 0.05) and, consequently, it allowed validating the fruit categories G1, G2 and G3 adopted in the present study.
Physicochemical characteristics
Tucum fruit physicochemical properties are shown in Table 2. The nutritional value of these fruits was similar to, or even higher than, the reported levels of acknowledged food sources, when they were compared to other products. Protein content, for instance, was similar to that of quinoa (Chenopodium quinoa Willd.) (Nowak et al. 2016), although tucum (A. aculeatum Meyer) did not present all the essential amino acids, since it just comprised cysteine, tyrosine and histidine as limiting amino acids (Hall et al. 1980). In addition, the herein recorded carotenoid results were similar to those found in Moranga squash (Cucurbita pepo) and approximately two times higher than those recorded in raw carrot (Daucus carota) (Rodriguez-Amaya et al. 2008). Carotenoids are bioactive substances that have beneficial effects on human health (Rodriguez-Amaya 2001). β-carotene is the major vitamin A precursor among carotenoids. Moreover, its biological functions include prevention against certain cancer types and against cardiovascular diseases (Santos et al. 2013).
With regard to physicochemical composition, the lowest lipid content and highest carbohydrate content were identified in G1 group. This behavior may derive from the possible influence from fruit ripening stage, from the lesser-orange epicarp, or even from differences explained by the extractive character of tucum production, which leads to fruits presenting different patterns. In addition, the large number of distribution regions, as well as the inherent characteristics of its progeny, may explain the observed differences.
The fatty acid profile showed high oleic acid levels in oils extracted from the pulp and peel. The following fatty acids were found in the pulp oil: lauric (0.8%), myristic (1.0%), palmitic (13.8%), stearic (8.6%), oleic (62.0%) and linoleic (13.8%); whereas, the peel oil showed: myristic (0.9%), palmitic (4.6%), stearic (13.8%), oleic (76.0%) and linoleic (4.6%) fatty acids. Mambrim and Barrera-Arellano (1997) assessed the mesocarp oil from another species (Astrocaryum vulgare) and identified higher amount of fatty acids and lower percentage of unsaturated fatty acids (73.4%). In addition, the percentage of saturated fatty acids presenting atherogenic characteristics (22.9%) was higher in their study than in the present experiment (15.6%). These differences may also due to divergence between the studied species, to progeny characteristic or even to particularities of the analyzed epicarp fraction. Unsaturated fatty acid C18: 1 prevailed in the fatty acid profile of both studied fractions, mesocarp and epicarp. Furthermore, it is worth highlighting that the epicarp had lower atherogenic fatty acid composition than the mesocarp.
The fat consumption particularly performed by saturated fatty acids (SFA) is associated with cardiovascular risk factors (Santos et al. 2013); for example, lauric fatty acids (C12: 0), followed by myristic (C14: 0) and palmitic (C16: 0) acids, most significantly contribute to LDL-c increase and HDL-c decrease. On the other hand, stearic acids (C18: 0) are reported to have effect on the moderate LDL-c reduction and on the slight HDL-c increase. Diets rich in monounsaturated fatty acids (MUFA), in their turn, promote HDL-c increase and triglycerides decrease, albeit at small levels. At the same time, there is evidence that MUFA consumption leads to decreased LDL-c susceptibility to oxidation, and it inhibits the atherogenic process. Other evidence associated with MUFA includes insulin resistance and endothelial function enhancement, and reduced cardiovascular risk. The linoleic acid (C18: 2) reduces the incidence of cardiovascular diseases; some studies suggest its action in reducing the risk of Diabetes Mellitus (Santos et al. 2013).
Drying kinetic of tucum fruits
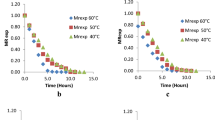
The purpose of studying tucum fruit pulp (mesocarp) and peel (epicarp) concerns preservation and shelf life increase. Simultaneously, the present study aimed at verifying tucum peel properties after drying, by taking into consideration the future applications that add value to the tucum production chain. Thus, changes in Tucum (mesocarp and epicarp) moisture content due to drying time at 50, 60 and 70 °C, respectively, are shown in Fig. 2a, c. The moisture content exponentially decreased due to drying time in a typical drying curve. These data were used to set logarithmic mathematical models that were fitted at the temperature level employed in these processes. It was observed that the equilibrium moisture content was faster achieved at 70 °C.
Tucum drying curves as a function of the drying temperature: a rate according dimensionless moisture content according to mesocarp drying time; b drying to dimensionless mesocarp moisture content; c dimensionless epicarp moisture content according to drying time; d Drying rate according to dimensionless epicarp moisture content. Symbols: (filled square) Group G3 at 50 °C, (open square) Group G3 at 70 °C, (filled circle) G1 at 50 °C, (open circle) Group G1 at 70 °C, (open triangle) Group G2 at 60 °C
Figure 2b, d illustrate the drying rate as a function of tucum mesocarp and epicarp moisture content ratio, respectively. Then, according to these results just the falling-rate drying period was observed for both cases. The shape of these drying curves depends on the structure of the material. Therefore, the drying rate is ruled by complex diffusion mechanisms of the moisture content (internal liquid or vapor flow), which control the water transport from the center up to the surface of tucum fruit mesocarp and epicarp, respectively. In fact, since the structure of these fruits varies, drying kinetic modeling is an important tool to determine the parameters and coefficients affecting the drying time and adopted to attain dried products of adequate quality.
Similar results were observed by Siqueira (2014), who studied tucum (A. vulgare Mart) drying. Indeed, a single falling-rate drying period has been often observed during the convective drying of foods from several sources, e.g., pilchard (Crataegus spp.) (Aral and Beşe 2016) and Physalis fruits (Vásquez-Parra et al. 2013).
Mathematical models assessed to describe the tucum fruit drying process
Fitted mathematical models applied to tucum drying process at 50, 60 and 70 °C, as well as their respective parameters, are shown in Table 3. The quality of the fitted models adopted to describe this drying process was based on the coefficient of determination R2 and χ2 and, consequently, the Page model allowed the best fit of the experimental data.
These models have been previously and satisfactory tested in food products. Sampaio et al. (2017) studied persimmon (Diospyros kaki Fuyu) mesocarp drying in fixed bed and found good drying-data adjustments to the Page model. Siqueira (2014) evaluated the epicarp and mesocarp drying in another tucum species (A. vulgare Mart) and recorded better adjustments through the Page, modified Page, Handerson and Pabis and Newton models.
Tucum fruit physicochemical characterization after drying
Table 4 shows the water activity (aw), moisture content, vitamin C and carotenoid content in tucum fruits epicarp and mesocarp before and after drying. The water activity results were higher than 0.916 in in-natura fruits; and it makes these fruits more prone to the proliferation of pathogenic microorganisms. Water activity reduced after drying, the recorded values were infeasible for the colonization of microorganisms such as bacteria, yeasts and molds, according to Barbosa-Canovas et al. (2003).
One of the aims for carrying out the present study was to evaluate carotenoids retention in tucum fruits after drying due to the important functional properties of these compounds. Carotenoids can be degraded due to the oxidation occurring during fruit processing and/or storage (Rodriguez-Amaya 2001). Results particularly evidenced carotenoid retention after dying, which ranged from 63 to 100% (Table 4). Therefore, the carotenoid content in dried tucum was preserved. Yuyama et al. (2008) observed discrete retention in β-carotene content (61%) after drying during tucum (A. aculeatum Meyer) mesocarp flour production. The observed differences can be explained by the different adopted experimental systems, i.e., their experiment was conducted at 60 °C for 72 h under hot air circulation; consequently, the fruits were subjected to a longer period of exposure to heat. However, the experiments performed in the present study were performed in convective fixed bed dryer (Fig. 1) for a much shorter drying time.
Similar to tucum carotenoid content, vitamin C content decreased under heat stress, since these compounds are highly unstable, thermolabile and tend to oxidize during the drying process. Mesocarp in-natura presents 19.29 mg of vitamin C/100 mL. This value is lower than in oranges from several species (around from 21 to 84 mg/100 mL), which are fruits known to have high vitamin C content (Couto and Canniatti-Brazaca 2010). However, tucum showed vitamin C content retention (Table 4) after drying comparable to that of in-natura fruits. Overall, the mesocarp showed little differences in vitamin C retention (around 30–40%) between different groups at the used temperatures (50, 60 and 70 °C) after drying. However, the epicarp did not show the same mesocarp pattern probably due to the heterogeneous pulp distribution in the peel resulting from the manual peeling procedure.
Conclusion
Tucum fruit processing through drying was attractive to increase the shelf life of this product, since the carotenoid and vitamin C contents were preserved at satisfactory levels.
The Page model showed the best fit to the experimental data among all the semi-theoretical mathematical models assessed to describe the tucum drying process. These results are important for tucum processing industry.
References
Akpinar EK (2008) Mathematical modelling and experimental investigation on sun and solar drying of white mulberry. J Mech Sci Technol 22(8):1544–1553
AOAC—Association of Official Analytical Chemists (1998) Official method 942.15 acidity (titratable) of fruit products, 16th edn, AOAC, Washington, DC
AOAC—Association of Official Analytical Chemists (2001) Official methods 996.06 fat (total, saturated and unsaturated in foods) hydrolytic extraction gas chromatographic method. First Action 1996. Revised 2001
AOAC—Association of Official Analytical Chemists (2002) Official methods 967.21 vitamin C (ascorbic acid) in vitamin preparations and juices: 2,6-dichloroindophenol titrimetric method final action, 17th edn, AOAC, Washington, DC
Aral S, Beşe AV (2016) Convective drying of hawthorn fruit (Crataegus spp.): effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chem 210:577–584
Barbosa-Canovas G, Fernandez-Molina JJ, Alzamora SM, Tapia MS, Lopez-Malo A, Welti Chanes J (2003) Handling and preservation of fruits and vegetables by combined methods for rural areas: technical manual. FAO, Roma
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Costa JR, da Leeuwen JV, Costa JA (2011) Tucumã of Amazonas Astrocaryum aculeatum G. Mey. In: Shanley P, Cymerys M, Serra M, Medina G (eds) Fruit trees and useful plants in Amazonian life. FAO, Rome, pp 205–2013
Couto MAL, Canniatti-Brazaca SG (2010) Quantification of vitamin C and antioxidant capacity of citrus varieties. Ciênc Technol Aliment 30(1):15–19
Didonet AA (2012) O mercado de um produto florestal não madeireiro e o resíduo sólido gerado pela sua comercialização: o caso do tucumã (Astrocaryum aculeatum G. Mey) nas feiras de Manaus. Dissertação (Mestrado em Ciências de Florestas Tropicais) Manaus–AM, Instituto Nacional de Pesquisas da Amazônia—INPA, pp 77
Didonet AA, Ferraz IDK (2014) Fruit trade of tucuma (Astrocaryum aculeatum g. Mey—arecaceae) at local market-places in Manaus (Amazonas, Brazil). Rev Bras Frutic 36(2):353–362
FAO (1986) Food and fruit bearing forest species, 3. Examples from Latin America, FAO forestry paper 44/3, Rome
Ferrer A, Remón S, Negueruela AI, Oria R (2005) Changes during the ripening of the very late season Spanish peach cultivar Calanda: feasibility of using CIELAB coordinates as maturity indices. Sci Hortic 105(4):435–446
Hall NT, Smoot JM, Knight RJ, Nagy S (1980) Protein and aminoacid compositions of ten tropical fruit by gas–liquid chromatography. J Agric Food Chem 28:1217–1221
Jobim ML, Santos RC, Alves CFS, Oliveira RM, Mostardeiro CP, Sagrillo MR, Souza Filho OC, Garcia LFM, Manica-Cattani MF, Ribeiro EE, Cruz IBM (2014) Antimicrobial activity of Amazon Astrocaryum aculeatum extracts and its association to oxidative metabolism. Microbiol Res 169:314–323
Kaleta A, Górnicki K (2010) Evaluation of drying models of apple (var. McIntosh) dried in a convective dryer. Int J Food Sci Technol 45(5):891–898
Kirmaci V, Usta H, Menlik T (2008) An experimental study on freeze-drying behavior of strawberries. Dry Technol 26:1570–1576
Kjeldahl JGCT (1883) Neue methode zur bestimmung des stickstoffs in organischen körpern. Fresenius J Anal Chem 22(1):366–382
Lorenzi H, Noblick L, Kahn F, Ferreira E (2010) Flora brasileira Lorenzi: Arecaceae (palmeiras). Instituto Plantarum, Nova Odessa, p 43
Mambrim MCT, Barrera-Arellano D (1997) Caracterización de aceites de frutos de palmeras de la región amazónica del Brasil. Grasas Aceites 48(3):154–158
Manzato L, Rabelo LCA, de Souza SM, da Silva CG, Sanches EA, Rabelo D, Mariuba LAM, Simonsen J (2017) New approach for extraction of cellulose from tucum’s endocarp and its structural characterization. J Mol Struct 1143:229–234
McGuire RG (1992) Reporting of objective color measurements. HortScience 27(12):1254–1255
McMinn WAM, Magee TRA (1999) Principles, methods and applications of the convective drying of foodstuffs. Food Bioprod Process 77(3):175–193
Moussa F, Kahn F (1997) Uso y potencial económico de dos palmas, Astrocaryum aculeatum Meyer y Astrocaryum vulgare Martius, em la Amazônia brasileña. Isntitut Français de Recherche Scientifique pour lê Développement em Coopération (ORSTOM). Brasília, DF, Brasil, pp 101–114
Nowak V, Du J, Charrondière UR (2016) Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem 193:47–54
Onwude DI, Hashim N, Janius RB, Nawi NM, Abdan K (2016) Modeling the thin-layer drying of fruits and vegetables: a review. Compr Rev Food Sci Food Saf Banner 15(3):599–618
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods. ILSI Press, Washington, p 65
Rodriguez-Amaya DB, Kimura M, Amaya-Farfan J (2008) Fontes brasileiras de carotenóides: Tabela brasileira de composição de carotenóides em alimentos. Ministério de Meio Ambiente (MMA). http://www.mma.gov.br/estruturas/sbf_agrobio/_publicacao/89_publicacao09032009113306.pdf. Accessed 3th Aug 2017
Sagrillo MR, Garcia LFM, de Souza Filho OC, Duarte MMMF, Ribeiro EE, Cadoná FC, da Cruz IBM (2015) Tucuma fruit extracts (Astrocaryum aculeatum Meyer) decrease cytotoxic effects of hydrogen peroxide on human lymphocytes. Food Chem 173:741–748
Sampaio RM, Neto Monteles JP, Perez VH, Marcos SK, Boizan MA, da Silva LR (2017) Mathematical modeling of drying kinetics of persimmon fruits (Diospyros kaki cv. Fuyu). J Food Process Pres 41:1–7
Santos RD, Gagliardi ACM, Xavier HT et al (2013) I Diretriz sobre o consumo de gorduras e saúde cardiovascular. Arq Bras Cardiol 100(1):1–40
Schroth G, da Mota MSS, Lopes R, de Freitas AF (2004) Extractive use, management and in situ domestication of a weedy palm, Astrocaryum tucum, in the central Amazon. For Ecol Manag 202(1):161–179
Siqueira FC (2014) Obtenção da polpa de tucumã (Astrocaryum vulgare Mart.) desidratada visando a preservação dos carotenoides. Dissertação (Mestrado em Ciência e Tecnologia de Alimentos). Belém, PA, Instituto de Tecnologia, Universidade Federal do Pará—UFPA, pp 79
Vásquez-Parra JE, Ochoa-Martínez CI, Bustos-Parra M (2013) Effect of chemical and physical pretreatments on the convective drying of cape gooseberry fruits (Physalis peruviana). J Food Eng 119(3):648–654
Wilberg VC, Rodriguez-Amaya DB (1995) HPLC quantitation of major carotenoids of fresh and processed guava, mango and papaya. LWT Food Sci Technol 28(5):474–480
Yuyama LKO, Maeda RN, Pantoja L, Aguiar JPL, Marinho HA (2008) Processamento e avaliação da vida-de-prateleira do tucumã (Astrocaryum aculeatum Meyer) desidratado e pulverizado. Ciênc Technol Aliment 28(2):408–441
Yuyama LKO, Aguiar JPL, Filho DFS, Yuyama K, Varejão MJ, Fávaro DIT, Vasconcellos MBA, Pimentel SA, Caruso MSF (2011) Caracterização físico-química do suco de açaí de Euterpe precatoria Mart. oriundo de diferentes ecossistemas amazônicos. Acta Amaz 41(4):545–552
Acknowledgements
The authors are grateful to the following Brazilian agencies: State University of the Northern Rio de Janeiro (UENF) and Research Foundation of Rio de Janeiro State (FAPERJ) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, M.B., Perez, V.H., Pereira, N.R. et al. Drying kinetic of tucum fruits (Astrocaryum aculeatum Meyer): physicochemical and functional properties characterization. J Food Sci Technol 55, 1656–1666 (2018). https://doi.org/10.1007/s13197-018-3077-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3077-2