Abstract
The bioactive compounds and “in vitro” antioxidant activity measured by three antioxidant assays of some traditional and non-traditional cold-pressed edible oils from Macedonia were object of this study. The fatty acid composition showed dominance of monounsaturated oleic acid in “sweet” and “bitter” apricot kernel oils with percentages of 66.7 ± 0.5 and 57.8 ± 0.3%, respectively. The most dominant fatty acid in paprika seed oil was polyunsaturated linoleic acid with abundance of 69.6 ± 2.3%. The most abundant tocopherol was γ-tocopherol with the highest quantity in sesame seed oil (57.6 ± 0.1 mg/100 g oil). Paprika seed oil, sesame seed oil and sweet apricot oil were the richest source of phytosterols. DPPH assay was the most appropriate for the determination of the antioxidant activity of cold-pressed sunflower oil due to high abundance of α-tocopherol with a level of 22.8 ± 1.1 mg/100 g of oil. TEAC assay is the best for the determination of the antioxidant activity of sesame seed oil and paprika seed oils as the richest sources of phenolic compounds. β-carotene assay was the most suitable assay for oils obtained from high pigmented plant material. Triacylglycerols and phytosterol profiles can be used as useful markers for the origin, variety and purity of the oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The need for consumption of high valuable edible oils with acceptable prices must be met by new resources or substitutes such apricot kernel oil or paprika seed oil (Kostadinović Veličkovska et al. 2015)
The oil from white apricot almond (Amygdalus communis L.) can improve the immune system and prevent many cardiovascular diseases, the level of LDL as well as many degenerative diseases (Tian et al. 2011, 2014; Matthäus and Özcan 2009a, b). Turan et al. (2007) determined the chemical composition of apricot kernel oil from “Malatya” apricots from Turkey in terms of fatty acids profile, triacylglycerols, tocopherols and phytosterols. The nutritional quality of apricot oil was tested in a period of 13 weeks with albino rats and the results did not indicate any toxic effect of amygdalin which on hydrolysis may release toxic hydrogen cyanide (Gandhi et al. 1997). The chemical composition of four apricot kernel samples provided from Malatya province were evaluated for moisture, ash, crude protein, crude oil, crude fiber, crude energy as well as contents of Na, P, K, Ca, Mg, Fe, Zn, Mn and Cu (Özgan 2000).
In the study of Silva et al., the chemical determination of the composition and antioxidant activity of the oil from the seeds from paprika (Capsicum annuum L.) was studied (Silva et al. 2013). According to their findings, the seeds from “sweet Italian” and “Reus long pairal” varieties of C. annuum were potential sources of bioactive compounds. Silva et al. quantified three phytosterols in both varieties campesterol, stigmasterol and β-sitosterol with domination of campesterol and β-sitosterol (Silva et al. 2013). Jarret et al. (2013) published fatty acid composition from nine Capsicum species. Results from the study of Jarret et al. indicated a very high level of linoleic acid in all varieties of Capsicum annuum. Chemical composition of some paprika seed oils from different locations in Turkey studied by Matthäus and Özcan (2009a, b) indicated valuable products with high levels of linoleic acid in the range of 69.5–74.7 g/100 g of oil, appreciable amounts of γ-tocopherol from 306.6 to 602.6 mg/kg oil and total abundance of phytosterols in the range from 3134.0 to 7233.7 mg/kg oil.
Different radical scavenging tests such as DPPH, ABTS and β-carotene assay and their relationships with phenolic compounds have been studied on extra virgin olive oils as well as other seed oils such as sunflower oils after frying, olive pomace oil from Italy and flaxseed oil (Krinsky and Johnson, 2005; El-Adawy and Taha 2001)
In this study, identification and quantification of the most important major and minor components in the oils such as fatty acid composition, vitamin-E-active compounds, phytosterols, and total phenolic compounds was performed in order to determine the classes of compounds with the highest impact on antioxidant activity of cold-pressed sunflower, flaxseed, sesame seed, bitter and sweet apricot kernel and paprika seed oils.
Furthermore, the modified procedures for DPPH, TEAC and ß-carotene assays was applied and the results from different antioxidant assays on the six oils was discussed. The last objective of this work was to find a relationship between major and minor bioactive components responsible for the “in vitro” antioxidant activity obtained by three different antioxidant assays.
Materials and methods
Harvesting and selection of plant material
Seeds from sunflower (Helianthus annuus, L.) were collected in September 2012 from the fields of the Ovče Pole and Štip valleys. Flaxseed (Linum usitatissimum, L.) was collected at the beginning of October 2012 from the fields of Povardarje valley. Seeds from sesame (Sesamum indicum, L.) were collected in October 2012 from the fields of Štip valley. Seeds from red paprika (Capsicum annuum, L. ssp. Macrocarpum) were collected in October 2012 from the sown fields of Strumica valley. The kernels from apricots (Prunus armeniaca L.) were collected in June 2012 from the region of Prespa lake.
Purification and cold pressing
The samples of six cold pressed oils were produced in Macedonian company for production of cold pressed edible oils “Filla”.
Fatty acid composition
In brief, 2 drops of oil were dissolved in 1 ml of heptane. Furthermore, 50 µL of sodium methylate (2 mol/L) was added and the samples were vigorously mixed for 1 min. Afterwards 100 µL of distilled water was added to each sample. After centrifugation of the samples, the lower phase was removed while the upper phase was mixed with 50 µL of 1 M HCl (with methyl orange for acidification control. After centrifugation at 4500 g for 10 min, the n-heptane phase was transferred to a new vial and the fatty acid methyl esters were analyzed using an Agilent 6890 GC-chromatograph (Agilent Technologies, Santa Clara, CA) equipped with a CP7420 Select FAME column (Agilent Technologies, Santa Clara, CA) (100 m × 0.25 mm i.d. with 0.25 μm film thickness) and FID detector following the ISO standard ISO 5509:2000 (ISO 2000). The oven temperature was programmed to increase from 150 to 240 °C with rate of 1.5 °C/min and maintained isotherm at 240 °C for 20 min. Pentadecanoic acid was used as an internal standard for quantitative analysis. The injector and detector temperature were both 260 °C. Hydrogen was used as the carrier gas at an average velocity of 2 ml/min.
Vitamin-E-active compounds
For determination of vitamin-E-active compounds, a solution of 250 mg of oil in 25 mL of n-heptane was directly used for the HPLC. The HPLC analysis was conducted using a Merck-Hitachi low-pressure gradient system, fitted with a L-6000 pump (Merck-Hitachi, Darmstadt, Germany), a Merck-Hitachi F-1000 fluorescence spectrophotometer (Darmstadt, Germany; detector wavelengths for excitation 295 nm, for emission 330 nm), and a ChemStation integration system (Agilent Technologies Deutschland GmbH, Böblingen, Germany). Sample volumes of 20 μL were injected by a Merck 655-A40 autosampler (Merck-Hitachi, Darmstadt, Germany) onto a Diol phase HPLC column 25 cm × 4.6 mm ID (Merck, Darmstadt, Germany) used with a flow rate of 1.3 mL/min. The mobile phase consisted of 99 mL n-heptane + 1 mL tert-butyl methyl ether. 5,7-dimethyltocol was used as internal standard as the most suitable compound for samples of oils which did not contain significant amount of α-tocotrienol.
Phytosterols
The phytosterol composition of the oils was determined following (ISO 12228, 1999). In brief, 250 mg of oil was saponified with a solution of ethanolic potassium hydroxide by boiling under reflux. The unsaponifiable matter was isolated by solid-phase extraction on an aluminium oxide column (Merck, Darmstadt, Germany) on which fatty acid anions were retained and sterols passed through. The sterol fraction was separated from other unsaponifiable matter by thin-layer chromatography (Merck, Darmstadt, Germany), re-extracted from the TLC material, and afterwards, the composition of the sterol fraction was determined by GLC using betulin as internal standard. The compounds were separated on a SE 54 CB (50 m long, 0.25 mm ID, 0.25 μm film thickness) (Macherey–Nagel, Düren, Germany). Further parameters were as follows: hydrogen as carrier gas, split ratio 1:20, injection and detection temperature adjusted to 320 °C, temperature program, 245–260 °C at 5 °C/min.
Triacylglycerides
Analyses were carried out according to the German official methods for fats and oils DGF C-VI 13a. In brief, oils were solved in acetone (50 mg/ml) and 20 µl of this solution was injected into a HPLC system consisting of a pump (LaChrom L-7100, Merck, Darmstadt, Germany), two RP18 columns in series (25 mm × 4 mm, packed with spherical material 5 µm, Lichrocart, Merck, Darmstadt, Germany) at 25 °C in a column oven (LaChrom L-7360, Merck, Darmstadt, Germany) and a refractive detector (LaChrom L-7490, Merck, Darmstadt, Germany). The eluent was pure propionitrile at 0.7 mL/min.
Total phenolic content
Each extract from the oil was prepared by three times extraction of the oil with a mixture of methanol–water (80:20, v/v). 2 mL of each extract was mixed with Folin-Ciocalteu reagent (1.0 mL) and distilled water (10.0 mL) and diluted to 25.0 mL with a 290 g/L solution of sodium carbonate. The samples were incubated in the dark for 30 min. The absorbance was measured at 760 nm. Gallic acid was used as standard for the calibration curve (Table 1), prepared in methanol–water (80:20, v/v) in the range from 30 to 300 mg/L. TPC values were determined using an equation obtained from the calibration curve of gallic acid (Table 1).
Antioxidant assays
Extraction of oil samples
A liquid–liquid extraction (LLE) system was used to extract the phenolic compounds present in the oils. According to Carrasco-Pancorbo et al. (2005), 9 g of each oil was dissolved in 6 mL of hexane, and the solution was extracted successively with four portions from 3 mL of methanol/water (60:40, v/v) solution. The combined extracts of the hydrophilic layer were brought to dryness in a rotary evaporator under reduced pressure and temperature of 40 °C. At the end, the residue was redissolved in 0.5 mL of methanol/water (60:40, v/v). All the measurements were done using five times diluted extract with methanol.
DPPH assay
The calculations of antioxidant activities of the samples under study were expressed as percentage of decolorization of a solution of the stable radical DPPH (2,2-diphenyl-1-picrylhydrazyl radical) at 517 nm. DPPH reagent was dissolved in hexane to obtain a solution with a concentration of 0.5 M. For the calibration curve, a standard of α-tocopherol with concentrations in the range from 100 to 500 mg/L was used (Table 1). After incubation time of 15 min, the samples were measured by mixing of 10 μL of sample to 490 μL of DPPH radical.
Trolox equivalent antioxidant capacity (TEAC) assay
The Trolox equivalent antioxidant assay applied on methanolic extracts of six oils determined the degree of decolorization of green/blue ABTS radical. For this purpose, 10 mL of ABTS solution was prepared from 35.13 mg of ABTS and 6.51 mg of K2S2O8 dissolved in Nanopure water to volume. For the calibration curve 12.52 mg of Trolox standard were diluted in 5 mL ethanol (97%). Four standard solutions were prepared for calibration curve in the range from 10 to 100 mg/L (Table 1) and were measured spectrophotometrically at 734 nm.
ß-carotene assay
For this purpose, a stock solution of β-carotene/linoleic acid mixture was prepared as follows: 0.5 mg of β-carotene was dissolved in 1 ml chloroform, and 25 μL of linoleic acid and 200 mg of Tween-40 were added as emulsifier since β-carotene is not water soluble.
Chloroform was completely evaporated using a vacuum evaporator. Then, 100 mL of oxygen-saturated distilled water was added with vigorous shaking at a rate of 100 U/min for 30 min; 2500 μL of this reaction mixture was transferred into test tubes, and 350 μL volumes of cold pressed sunflower oil, sesame oil and flaxseed oil were added. The emulsions were incubated for up to 90 min at room temperature. The same procedure was repeated with a positive control of Trolox (as standard) and a blank sample.
The absorbance of the mixture was measured at 490 nm after incubation time of 90 min. Antioxidant activities of the cold-pressed oils were compared with that of Trolox as calibration standard and the blank sample.
Statistical analyses
The statistical analysis one-way ANOVA was applied in order to see the level of every particular minor and major compound by consideration of the type of oil with the significance level of 0.05. The level of significance of differences between the percentages of fatty acids, level of tocopherols, level of phytosterols, total phenolic content and values of antioxidant activity measured by DPPH, TEAC and β-carotene assay mean values was determined at 5% by a one-way ANOVA using Tukey’s test. This treatment was performed by SPSS v.16.0 software (IBM Corporation, USA).
The ANOVA results were classified using letters (different letters mean significant differences among results). The letters are a, b and c according to the decrease of the result values.
Results and discussion
Bioactive compounds of cold pressed oils
Fatty acid composition
The fatty acid composition of the oils under study are presented in Table 2. The most dominant fatty acids in sunflower oil obtained from unconventional high-linoleic sunflower seeds were linoleic acid and oleic acid with the abundance of 58.3 ± 0.03 g/100 g and 29.9 ± 0.02 g/100 g, respectively. Flaxseed oil was the richest source of α-linolenic acid (ALA) with 56.9 ± 0.01 g/100 g.
Oleic acid as monounsaturated fatty acid was the most dominant in both varieties of apricot kernel oils, 66.7 ± 0.5 g/100 g in “bitter” and 57. 8 ± 0.3 g/100 g in “sweet” apricot oil. However, the working group of Özcan published higher percentage of oleic acid in the oils from five varieties of almond kernels. According to their findings, the oil from “Nonpareil” variety had the lowest amount of oleic acid (72.5%) and the highest amount was determined in “Cristomoroto” variety (79.9%) (Özcan et al. 2011). Similarly, this fatty acid was the most abundant in oils from seven varieties of hazelnut with maximum percentage in “Palas” variety (Kanbur et al. 2013). On the other hand, the most dominant fatty acid in oils from different varieties of walnuts was linoleic acid with the highest level in “Kaman-2” variety (Özcan et al. 2010). Furthermore, the most dominant fatty acid in cold-pressed paprika seed oil was linoleic acid with percentage of 69.6 ± 2.3%. Pérez-Gálvez et al. (1999) published over 77% of linoleic acid in fresh and dried seeds from two varieties “Jaranda” and “Jariza” of Capsicum annuum L. The same fatty acid in both apricot kernel oils was lower than 30%. Comparing the results from the fatty acid composition of apricot kernel oil with those published in the work of Tian et al. (2011) showed very different results for the two main fatty acids: oleic and linoleic acid. On the other hand, apricot kernel oil which was examined by Tian et al. had 40.9% oleic and 49.3% linoleic acid which can induce lower oxidative stability in comparison to Macedonian apricot kernel oil. The fatty acid profile of Macedonian apricot kernel oils were very similar to the results published for Turkish oil from Prunus spp. in the work of Matthäus and Özcan. Kostadinoviċ Veličkovska et al. (2015) published over 70% of oleic acid in apricot kernel oil which is similar to “bitter” variety of the oil published in this study. The major fatty acid in flaxseed oil was α-linolenic acid (56.88%) and its amount belongs to the range (39.90-60.42) published by working group of Goyal (2014).
Oxidative stability of oils
The results from the fatty acid composition and the oxidative stability of the same oils in Table 2 showed a very good relationship. As we can see from Table 2, cold-pressed “bitter” and “sweet” apricot kernel oils had a very good oxidative stability of 15.6 ± 0.7 and 11.8 ± 0.9 h respectively. The relatively high oxidative stability of these oils can be explained not only by the high content of monounsaturated oleic acid but also phenolic compounds and flavonoids can participate in oxidative stability of the oils (Martínez Nieto et al. 2010).
Vitamin-E-active compounds
The vitamin-E-active compounds as the minor compounds in the oils are presented in Table 3. The results showed that α-tocopherol is the most dominant vitamin-E-active compound in sunflower oil with a level of 22.8 ± 1.1 mg/100 g. γ-tocopherol was the most dominant vitamin-E-active in the rest five oils with level in sesame seed oil (57.6 ± 0.1 mg/100 g oil) and a significant amount of plastochromanol-8 was detected in flaxseed oil (16.8 ± 2.1 mg/100 g oil). Paprika seed oil was a rich source of γ-tocopherol with abundance of 25.7 ± 1.3 mg/100 g of oil. γ-tocopherol represent 96.6% of the total vitamin-E-active compounds in paprika seed oil. According to the findings of Matthäus and Özcan (2009a, b), results from Macedonian variety is similar to Turkish varieties “Italy yellow” and “Italy sweet”, since all other Turkish varieties had higher amounts of γ-tocopherol. Vitamin-E-active compounds retarded primary stage of oxidation, but its antioxidant activity is significantly lower toward secondary oxidation products (Alizadeh et al. 2016). We quantified almost identical quantities of vitamin-E-active compounds in sesame seed oil from Macedonia and Karnataka region from India (57.9 ± 0.1 and 57.5 ± 1.6 mg/kg respectively). However, the level of the same compounds in sunflower oil (30.3 ± 1.6 mg/kg) was significantly lower than result published by working group of Sunil (49.7 ± 1.1 mg/kg) (Sunil et al. 2015).
Phytosterols
Apart from vitamin-E-active compounds, phytosterols are another group of minor components with structure, similar to that of cholesterol making them to valuable nutritional compounds responsible for the reduction of serum LDL cholesterol and atherosclerotic risk. The levels of phytosterols in the six oils are presented in Table 4. The lowest level of total phytosterols was detected in “bitter” apricot oil and flaxseed oil (2816 ± 210.7 mg/kg and 2922 ± 101.5 mg/kg, respectively) and the highest level of total phytosterols was observed for “sweet” apricot oil and paprika seed oil (over 5500 mg/kg).
Table 4 shows that the main phytosterol in all six oils was ß-sitosterol with over 50% of the total amount of phytosterols. The total amount of phytosterols in flaxseed oil was lower than the results presented by Ciftci et al. (2012), but campesterol was also the next dominant phytosterol after β-sitosterol. The amount of β-sitosterol in sunflower oil (2004 ± 79.2 mg/kg) was very similar with the amount of the same phystosterol in sunflower oil (2209 ± 0.3 μg/g) reported in the work of Ramadan (Ramadan, 2015)
As we can notice from the results in Table 4, there is significant difference between the total amount of phytosterols in “bitter” and “sweet” apricot oil. “Sweet” apricot kernel oil had almost double of the quantity of phytosterols (5684 ± 312.7 mg/kg) in comparison to “bitter” apricot oil (2816 ± 210.7 mg/kg). Campesterol in “sweet” variety was with level of 278.0 ± 29.1 mg/kg in comparison to 67.5 ± 12.5 mg/kg of the same phytosterol in “bitter” variety. Stigmasterol was abundant six times more in “sweet” apricot kernel oil in comparison to “bitter” apricot kernel oil. Δ7-campesterol and chlerosterol were almost double in sweet variety in comparison to the same phytosterols in bitter variety.
The most indicative phytosterol as a marker for paprika seed oil was Δ5-avenasterol with the level of 1141 ± 8.1 mg/kg which was 19.9% from the total amount of phytosterols in paprika seed oil. The results for pytosterols for Macedonian paprika seed oil were in good agreement with the level of total phytosterols of “Anamur table (bitter)” Turkish variety with 6643.5 ± 19.9 mg/kg oil (Matthäus and Özcan 2009a, b). However, Silva et al. (2013) quantified only three phytosterols in both varieties campesterol, stigmasterol and β-sitosterol with domination of campesterol and β-sitosterol.
TAG composition
The TAG composition presented in Table 5 was in excellent relationship with fatty acid composition presented in Table 2. The LOL and LLL were the most dominant TAGs in sunflower oil (28.0 ± 4.6 and 22.0 ± 0.3%). The TAG profile of sunflower oil can be an indication of high linoleic sunflower oil which is in good correlation with the fatty acid composition. Since the amount of linolenic acid in flaxseed oil was over 55%, the most dominant TAG was LnLnLn with abundance of 19.6 ± 1.5%.
The proportion of OOO TAG in the “bitter” apricot oil was 39.9 ± 9.9%. The most dominant TAGs in “sweet” variety of apricot oil were OOO and LOO with levels of 27.3 ± 3.1 and 25.3 ± 12.1%, respectively. LLL was the most dominant in paprika seed oil with 40.0 ± 9.3%.
It seems that each oil (even varieties of apricot kernel oils) exhibits very characteristic TAG pattern that can differentiate the variety of the oils. Thus, “sweet” apricot oil exhibits lower level of OOO in comparison to “bitter” apricot oil. On the other hand, the level of LLL in “sweet” apricot kernel oil is almost double in comparison to the same triacylglycerol in “bitter” apricot kernel oil. Results presented in Table 5 indicated that abundance of each TAG can be used as fingerprint for the origin of the oil. Even oils from different varieties have different TAG profiles which can be used as valuable markers for determination of adulteration of expensive oils with cheaper oils.
Total phenolic content (TPC)
The results obtained for the total content of phenolic compounds of the six oils are presented in Table 6. It can be seen that the highest level of total phenolic compounds from methanol extracts was obtained for sesame seed oil (214.1 ± 9.1 mg gallic acid/L oil). Paprika seed oil was the second valuable source of phenolic compounds (117.4 ± 4.9 mg gallic acid/L oil). For flaxseed and sunflower oil, similar levels of total phenolic compounds were quantified below 60 mg gallic acid/L oil. Žilić et al. (2010) stated that the main compound in both oils was chlorogenic acid with traces of caffeic acid, ferulic acid, rosmarinic acid, myrcetin and rutin. Tuberoso et al. (2007) published only traces of syringic acid and vanillic acid in an extract of sunflower oil and vanillin in an extract of flaxseed oil with levels of 2.5 mg/kg of oil. The lower level of phenolic components published by Konsoula and Liakopoulou-Kyriakides (2010) was related to sesame seed oils obtained by extraction of the oils from the seeds with hexane when most of the phenolic acids will remain in the resulting sesame seed cake.
The impact of bioactive compounds of the oils on the antioxidant activities determined by three different assays
The results obtained for the total content of phenolic compounds of oils were in strong relationship with the TEAC assay presented in Table 6. The extract from sesame seed oil indicated the highest total content of phenolic compounds equivalent to 214.1 mg of gallic acid/L oil which correspond to the highest antioxidant activity determined by the TEAC assay (87.3 mg Trolox/L oil). Furthermore, sesame lignans such sesamin, sesamolin, sesamol as well as flavonoids, phenylpropanoids and tannins are potential antioxidant compounds which attribute significantly to the total antioxidant activity of sesame seed oil (Tuberoso et al. 2007; Wan et al. 2014; Bhatnagar et al. 2015; Abdelazim et al. 2013). Antioxidant activity of the oils can be significantly influenced by the high level of unsaturated fatty acids, in particular flaxseed oil. In addition, freshly produced flaxseed oil contains cyclolinopeptides, which are able to improve the oxidation stability (Sharav et al. 2014).
Our results obtained from TPC and TEAC assay indicated the importance of individual phenolic compounds and their contribution to the total antioxidant potential of methanolic extracts from oils (Kostadinović Veličkovska and Mitrev 2013; Taghvaei and Jafari 2015). Although sunflower oil and flaxseed oil had very similar levels of total phenolic compounds (50.5 and 59.5 mg of gallic acid equivalents/L of oil, respectively), the antioxidant activity of flaxseed oil was almost five times higher. Flaxseed oil also contains p-coumaric acid, sinapic acid, syringic and gallic acids which can enhance the antioxidant activity of this oil against ABTS radicals (Mridula et al. 2015; Goyal et al. 2014). However, tannins, caffeic acid and other phenolic acids presented in trace amounts in sunflower oil had different levels of antioxidant activity depending on the applied test system and had significant in vitro lipid peroxidation inhibitory property and reducing power close to the standard antioxidants, BHT and BHA, but much lower potential against free radicals such DPPH (Kasote et al. 2011).
Comparing the total content of vitamin-E-active compounds (30.3 ± 1.6 mg/100 g of oil) and the value for the antioxidant activity of sunflower oil obtained by the DPPH assay (348.7 ± 46.4 mg of α-tocopherol/L oil), it can be conclude that the DPPH assay is the most appropriate method for the determination of the antioxidant activity as a function of the total vitamin-E-active compounds in sunflower oil. On the other hand it has been taken into consideration that direct injection of oil into a heptane solution of DPPH can result in the absorption of light at 515 nm by pigments such as carotinoids that can strongly influence the results of the DPPH assay (Chen et al. 1996). Our results were in perfect agreement with order of effectiveness of oils in inhibiting of DPPH radical in the work of Ramadan and Moersel, since sunflower oil had greater antioxidant potential than flaxseed oil (Ramadan and Moersal, 2006)
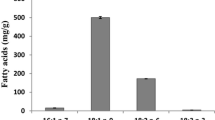
Comparing the results in Table 6 obtained from different assays, it can be noticed that β-carotene assay indicated paprika seed oil as oil with highest antioxidant capacity (48.6 ± 5.9% inhibition of α-linolenic acid oxidation). This can be related to the fact that paprika seed oil was the richest source of carotenoids and other pigments which are the most sensitive to β-carotene assay (Arimboor et al. 2015). Significant antioxidant potential of sunflower oil (32.6 ± 7.4%) in comparison to all other oils with dominance of γ-tocopherol is remarkable. It might be that α-tocopherol had higher protective effect during interruption of the conjugated double bond system in β-carotene when this molecule was exposed to radicals or oxidized species even in lower concentrations as γ-tocopherol (Kostadinović Veličkovska et al. 2016).
Conclusion
Antioxidant activity of cold-pressed edible oils depends on the origin of the seeds from which the oil is obtained, their chemical composition and the applied antioxidant assay.
This study showed that flaxseed oil had remarkable quality since this oil was the richest source of unsaturated fatty acids and with sesame seed oil were the richest sources of vitamin-E-active compounds. Furthermore, sesame seed oil, “sweet” apricot kernel oil and paprika seed oil are valuable due to the high levels of phytosterols (over 5000 mg/kg oil).
The results of the three different assays showed that oils which are consisted of very low levels of phenolic compounds and higher levels of vitamin-E-active compounds, respectively, such as sunflower oil, the most appropriate assay for the determination of the antioxidant activity should base on liposoluble radicals such DPPH. β-carotene assay was the most appropriate assay for oils obtained from high pigmented plant material as seeds from red paprika. On the other hand, for oils which consist of significant levels of phenolic compounds and lignans, the most appropriate assay is TEAC assay.
Significant amount of carotenoids, γ-tocopherol and phytosterols makes paprika seed oil interesting for further evaluation and incorporation as functional and dietary food.
References
Abdelazim AA, Mahmoud A, Ramadan-Hassanien FM (2013) Oxidative stability of vegetable oils as affected by sesame extracts during accelerated oxidative storage. J Food Sci Technol 50:868–878
Alizadeh L, Nayebzadeh K, Mohammadi A (2016) A comparative study on the in vitro antioxidant activity of tocopherol and extracts from rosemary and Ferulago angulata on oil oxidation during deep frying of potato slices. J Food Sci Technol 53:611–620
Arimboor R, Natarajan RB, Menon KR, Chandrasekhar LP, Moorkoth V (2015) Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: analysis and stability—a review. J Food Sci Technol 52:1258–1271
Bhatnagar AS, Hemavathy J, Gopala Krishna AG (2015) Development of a rapid method for determination of lignans content in sesame oil. J Food Sci Technol 52:521–527
Carrasco-Pancorbo A, Cerretani L, Bendini A, Segura-Carretero A, Gallina-Toschi T, Fernandez-Gutierrez A (2005) Analytical determination of polyphenols in olive oils. J Sep Sci 28:837–858
Chen ZY, Chan PT, Ho KY, Fung KP, Wang J (1996) Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem Phys Lipids 79:157–163
Ciftci ON, Przybylski R, Rudzińska M (2012) Lipid components of flax, perilla, and chia seeds. Eur J Lipid Sci Technol 114:794–800
El-Adawy TA, Taha KM (2001) Characteristics and composition of different seed oils and flowers. Food Chem 74:47–54
Gandhi VM, Mulki MJ, Mukerji B, Iyer VJ, Cherian KM (1997) Safety evaluation of wild apricot oil. Food Chem Toxicol 35:583–587
Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M (2014) Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol 51:1633–1653
Jarret RL, Levy IJ, Potter TL, Cermak SC (2013) Seed oil and fatty acid composition in Capsicum spp. J Food Compost Anal 30:102–108
Kanbur G, Arslan D, Özcan MM (2013) Some compositional and physical characteristics of some Turkish hazelnut (Corylus avellanna L.) variety fruits and their corresponding oils. Int Food Res J 20(5):2161–2165
Kasote DM, Hegde MV, Deshmukh KK (2011) Antioxidant activity of phenolic components from n-butanol fraction (PC-BF) of defatted flaxseed meal. Am J Food Technol 6:604–612
Konsoula Z, Liakopoulou-Kyriakides M (2010) Effect of endogenous antioxidants of sesame seeds and sesame oil to the thermal stability of edible vegetable oils. LWT Food Sci Technol 43:1379–1386
Kostadinović Veličkovska S, Mitrev S (2013) Characterization of fatty acid profile, polyphenolic content and antioxidant activity of cold pressed and refined edible oils from Macedonia. J Food Chem Nutr 1:16–21
Kostadinović Veličkovska S, Brühl L, Mitrev S, Mirhosseini H, Matthäus B (2015) Quality evaluation of cold pressed edible oils from Macedonia. Eur J Lipid Sci Technol 117:2023–2035
Kostadinović Veličkovska S, Mitrev S, Mihajlov LJ (2016) Physicochemical characterization and quality of cold-pressed peanut oil obtained from organically produced peanuts from Macedonian “Virginia” variety. Grasas Aceites 67:118
Krinsky NI, Johnson EJ (2005) Carotenoid actions and their relation to health and disease. Mol Aspects Med 26:459–516
Martínez Nieto L, Hodaifa G, Lozano Peña JL (2010) Changes in phenolic compounds and Rancimat stability of olive oils from varieties of olives at different stages of ripeness. J Sci Food Agric 90:2393–2398
Matthäus B, Özcan MM (2009a) Chemical evaluation of some paprika (Capsicum annuum L.) seeds oil. Eur J Lipid Sci Technol 111:1249–1254
Matthäus B, Özcan MM (2009b) Fatty acids and tocopherol contents of some Prunus spp. kernel oils. J Food Lipids 16:187–199
Mridula D, Barnwal P, Singh KK (2015) Screw pressing performance of whole and dehulled flaxseed and some physico-chemical characteristics of flaxseed oil. J Food Sci Technol 52:1498–1506
Özcan MM, Iman C, Arslan D (2010) Pysico-chemical properties, fatty acids and mineral content of some walnuts (Juglans regia L.) types. Agric Sci 1(2):62–67
Özcan MM, Ünver A, Erkan E, Arslan D (2011) Characteristics of some almond kernel and oils. Sci Hortic 127:330–333
Özgan MM (2000) Composition of some apricot Prunus armeniaca kernels growing in Turkey. Acta Aliment 29(3):289–293
Pérez-Gálvez A, Garrido-Fernández J, Mínguez-Mosquera MI, Lozano-Ruiz M, Montero-de-Espinosa V (1999) Fatty acid composition of two new pepper varieties (Capsicum annuum L. cv. Jaranda and Jariza). Effect of drying process and nutritional aspects. J Am Oil Chem Soc 76:205–208
Ramadan MF (2015) Oxidation of β-sitosterol and campesterol in sunflower oil upon deep- and pan-frying of French fries. J Food Sci Technol 52(10):6301–6311
Ramadan MF, Moersal JT (2006) Screening of the antiradical action of vegetable oils. J Food Compost Anal 19:838–842
Sharav O, Shim YY, Okinyo-Owiti DP, Sammynaiken R, Reaney MJT (2014) Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J Agric Food Chem 62:88–96
Silva LR, Azevedo J, Pereira MJ, Valentao P, Andrade PB (2013) Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem Toxicol 53:240–248
Sunil L, Vanitha Reddy PR, Gopala Krishna AG, Urooj Asna (2015) Retention of natural antioxidants of blends of groundnut and sunflower oils with minor oils during storage and frying. J Food Sci Technol 52(2):849–857
Taghvaei M, Jafari SM (2015) Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol 52:1272–1282
Tian H, Zhang H, Zhan P, Tian F (2011) Composition and antioxidant and antimicrobial activities of white apricot almond (Amygdalus communis L.) oil. Eur J Lipid Sci Technol 113:1138–1144
Tian H, Zhan P, Zhang H (2014) Development of fatty acid fingerprint of white apricot almond oil by gas chromatography-mass spectrometry. Eur J Lipid Sci Technol 116:126–133
Tuberoso IGC, Kowalczyk A, Sarritzu E, Cabras P (2007) Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem 103:1494–1501
Turan S, Topcu A, Karabulut I, Vural H, Hayaloglu AA (2007) Fatty acid, triacylglycerol, phytosterol and tocopherol variations in kernel oil of Malataya Apricots from Turkey. J Agric Food Chem 55:10787–10794
Wan Y, Li H, Fu G, Chen X, Chen F, Xiea M (2014) The relationship of antioxidant components and antioxidant activity of sesame seed oil. J Sci Food Agric 95:2571–2578
Žilić S, Maksimović Dragišić J, Maksimović V, Maksimović M, Basić Z, Crevar M, Stanković G (2010) The content of antioxidants in sunflower seed and kernel. Helia 33:75–84
Acknowledgement
Financial support from Deutscher Akademischer Austausch Dienst (DAAD) for Sanja Kostadinović Veličkovska as a participant in the program “Academic Reconstruction of South Easter Europe” is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kostadinović Veličkovska, S., Catalin Moţ, A., Mitrev, S. et al. Bioactive compounds and “in vitro” antioxidant activity of some traditional and non-traditional cold-pressed edible oils from Macedonia. J Food Sci Technol 55, 1614–1623 (2018). https://doi.org/10.1007/s13197-018-3050-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3050-0