Abstract
Fried foods, both deep-fried and pan-fried, are enjoyed by people worldwide. Frying is one of the main factors leading to formation of phytosterols (PS) oxidation products (POP) in vegetable oils. The aim of this study was to measure the oxidation of β-sitosterol (24α-ethyl-5-cholesten-3β-ol) and campesterol (24α-methyl-5-cholesten-3β-ol) in commercial sunflower oil (SFO) during deep- and pan-frying of French fries for different periods (30, 60, 120 and 240 min). The total amount of PS in SFO was 4732 μg/g, wherein the major PS were β-sitosterol and campesterol. The results of POP were confirmed by the GC-MS analysis that monitored the formation of oxides during frying. Upon frying, total PS content decreased whereas the highest decrease was measured after 240 min of frying. The oxidative stability (OS) of different sitosterol and campesterol during both frying methods was evaluated. In general, pan frying resulted in more PS oxidation than deep frying. β-Sitosterol oxides predominated while campesterol oxides were formed to a lesser extent. 7-Ketositosterol, followed by 7β-hydroxysitosterol, 5,6-epoxy derivatives and 7α-hydroxysitosterol were the main POP induced during frying. The proportion of 7-keto derivatives decreased during frying while the proportion of 7β-hydroxy derivatives increased. The formation of POP might be a limiting factor for frying in SFO for long periods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytosterols (PS) are bioactive compounds located in the plant cell membranes, where they play functional roles (Lehtonen et al. 2011). Edible oils are the main sources of PS followed by grains, fruits and vegetables (Piironen et al. 2000). The human diet contains around 200–300 mg PS/day. PS are regarded to have health-promoting properties in preventing cardiovascular diseases by inhibiting the intestinal absorption of cholesterol, accordingly, the consumption of 2 g/day of PS could reduce the risk of heart diseases by about 25 % (Kritchevsky and Chen 2005). Therefore, PS have been recently incorporated into novel foods (Koschutnig et al. 2010; Menéndez-Carreño et al. 2010; Lehtonen et al. 2011).
Oxidative stability (OS) of PS could be defined as their resistance to oxidation and the resulting deterioration that affect food quality. Phytosterols oxidation products (POP, Fig. 1 ) could have toxic effects on human organisms similar to those of cholesterol oxidation products (COP) (Garcia-Cruset et al. 2002). POP were shown to be accumulated in the serum and liver of mice (Tomoyori et al. 2004), and to have cytotoxic effects on mammalian cells (Maguire et al. 2003). POP were identified in the plasma of human subjects in amounts ranging from 4.80 to 57.2 ng/mL (Grandgirard et al. 2004). However, several aspects of the possible toxic effects of POP are still to be elucidated (Tomoyori et al. 2004; Lea et al. 2004). Maguire et al. (2003) reported that β-sitosterol oxides exhibit less severe but similar toxicity patterns to those found for COP. On the other hand, Lea et al. (2004) concluded that POP do not exhibit a genotoxic potential. Hiroko et al. (2004) mentioned that POP are absorbed but they do not promote the development of atherosclerosis in apo E deficient mice. Ryan et al. (2005) compared the biological effects of COP and POP wherein they concluded that POP have qualitatively similar toxic effects to COP. Recent study (Alemany et al. 2013) evaluated the bioaccessibility of PS and their POP after simulated gastrointestinal digestion in fruit, milk and fruit-based milk beverages. Accessibility of PS ranged between 2.62 and 6.48 %. Only oxides of β-sitosterol were detected in beverages wherein the bioaccessibility of total POP ranged between 19.08 and 49.29 %. Bioaccessibility of POP was higher than that of PS, suggesting different patterns of solubility for these compounds.
Food processing conditions such as high temperatures and exposure to oxygen, light, water or metals (pro-oxidants), may enhance PS oxidation. Oxidation may begin enzymatically or by attack of reactive oxygen species (Lütjohann 2004). In addition, the OS of PS is affected by molecular structure, lipid matrix composition and interactions between these variables (Soupas et al. 2005; Lehtonen et al. 2011).
Deep- and pan-frying are popular methods in home- cooking and restaurants. Despite the negative perception of fried foods in the Western diet, frying is considered to have almost the same or even less effect on nutrient losses as compared to other cooking methods. Moreover, the nutritive value of food increases due to the absorption of frying vegetable oils, which are rich in bioactive lipids including essential fatty acids and tocols (Chiou et al. 2009). However, during frying the oil undergoes a series of reactions, including hydrolysis, oxidation and thermal decomposition. The quality of the frying medium is important since, through absorption, it contributes to the quality of the final product. In PS-contained frying media, in addition to changes occurring in the oil matrix, the formation of POP during frying is of interest because of potential adverse effects of POP on health (Guardiola et al. 2004; Garcia-Llatas and Rodriguez-Estrada 2011).
Several studies were undertaken to determine the effects of different cooking methods on the fatty acids of vegetable oils, in particular deep-frying; and only a few studies have been conducted on PS oxidation during frying. In the oils used to make French fries, the POP contents were 40–47 mg/kg of lipids before frying and 56–59 mg/kg of lipids after 48 h of frying (Dutta 1997). Yet, less information on the effect of pan-frying can be found. Lampi et al. (2004) reported that less than 2 % of PS was oxidized during pan frying of rapeseed oil. The major POP were 7α- and 7β-hydroxysterols, 7-ketosterols and epoxysterols. Recently, Garcia-Llatas and Rodriguez-Estrada (2011) and Vanmierlo et al. (2013) published reviews on the knowledge and future perspectives of PS-enriched food, particularly focused on occurrence of POP and their biological effects.
Each vegetable oil is characterized by typical stabilities against oxidation, dependent on the fatty acids composition as well as the content and composition of minor compounds including tocopherols, certain sterols, hydrocarbons, carotenoids, phenolics, and trace metals. To the best of knowledge, not much is known about the effects of the frying process on the OS of PS in commercial sunflower oil (SFO) used for frying. Thus, there is a need to know the levels and distribution of main POP in vegetable oils, produced on a pilot or industrial scale, especially during thermal-processing. The goal of this study was to measure and compare the oxidation of endogenous PS in SFO during deep- and pan- frying of French fries for different periods (30, 60, 120 and 240 min) by analyzing the formation of major secondary POP and the amount of unoxidized PS. The results will be of importance for achieving a better understanding of PS oxidation in edible oils upon thermal processing.
Material and methods
Materials
Commercial sunflower oil (SFO) was purchased from local market (Zagazig, Egypt). N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA; >98 %; E. Merck, Darmstadt, Germany) and trimethylcholorosilane (TMCS; 99 %; Fluka Chemie, Buchs, Switzerland) were used as a 99:1 mixture for silylation. Analytical grade anhydrous pyridine, anhydrous Na2SO4 (E. Merck), diethyl ether (J.T. Baker, Holland) and KOH (Eka Nobel, Surte, Sweden), HPLC grade heptane and acetone (Rathburn Chemicals, Walkerburn, Scotland), 99.5 % ethanol and water (purified by Milli-Q Plus, Molsheim, France) were used. Bond Elut SiOH solid-phase extraction (SiOH-SPE) cartridges (500 mg, Varian, Harbor City, CA, USA) were used in purification of sterol oxides. 5-Cholesten-3β,19-ol (19-hydroxycholesterol) and 3β-hydroxy-5α-cholestane (dihydrocholesterol) were used as internal standards (ISTD) and were purchased from Steraloids (Newport, RI, USA) and Sigma (St. Louis, MO, USA), respectively.
Characterization of fatty acids and tocols profile of SFO
SFO was analyzed for its fatty acids and tocols contents. Fatty acids were determined as methyl ester (FAME) derivatives by GC-flame ionization detection (GC-FID) according to Metcalfe et al. (1966). For tocols analysis, the SFO samples were dissolved in heptane and analyzed by HPLC according to Schwartz et al. (2008).
Frying experiments and sample preparation
Deep frying experiment
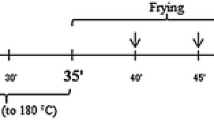
The deep frying experiments were conducted in a possibly similar manner as the actual household cooking process. An electric fryer (Tefal classis 650, S.A.S. Seb Selongey Cedex, RC. Dijon, France) was used for frying. The fryer was equipped with a thermostat and supplied with an inert cross-linked steel wire-mesh which allowed the food to be dipped into the oil, without coming in contact with the fryer’s inner surface. The oil temperature was monitored with a digital thermometer attached to a steel probe (Comark N1092 Starter Kit Thermometer, Comark Limited, Hertfordshire, UK). In every frying session, 100 ± 5 g French fries (commercial brand) were deep fried for 6 min in 1.0 L oil, without replenishment. The oil was first heated at 175 °C ± 5 °C and allowed to equilibrate at this temperature for 10 min. In total, 16 batches of the French fries, 100 gm per batch, were fried for 6 min at intervals of 9 min for 4 h (240 min). Excess oil in French fries was allowed to drain on a cross-linked steel wire-mesh and was added to the oil remaining in the fryer. The fryers were left uncovered during the frying period. The fryer was turned off after 120 min frying (after 8 frying sessions) and the oil was left for 2 h and allowed to cool to room temperature. About 20 g of frying oil from the fryer was withdrawn using Pasteur pipette and sampled into bottles after 30, 60, 120 and 240 min. The oil samples were analyzed directly for sterols composition and sterols oxides content.
Pan frying experiment
The pan frying experiments were conducted in the manner possibly similar to the actual household cooking process. Pan frying was performed in an uncovered stainless steel pan fryer (7 cm high, diameter 22 cm) using an electric plate of a conventional electric kitchen cooker equipped with a thermostat. The oil temperature was monitored during frying with a digital thermometer attached to a steel probe (Comark N1092 Starter Kit Thermometer, Comark Limited, Hertfordshire, UK). In each frying session, 100 ± 5 g French fries (commercial brand) were pan fried for 6 min in 1.0 L oil, without replenishment. The oil was first heated at 185 °C ± 5 °C and allowed to equilibrate at this temperature for 10 min. In total, 16 batches of the French fries, 100 gm per batch, were fried for 6 min at intervals of 9 min for 4 h (240 min). The fryers were left uncovered during the frying period. The fryer was turned off after 120 min frying (after 8 frying sessions) and the oil was left for 2 h and allowed to cool to room temperature. About 20 g of frying oil from the fryer was withdrawn using Pasteur pipette and sampled into bottles after 30, 60, 120 and 240 min. The oil samples were analyzed directly for sterols composition and sterols oxides content.
Sterol analysis (GC–FID)
The PS contents of SFO samples were analyzed before and during frying, using direct hot saponification method (Soupas et al. 2004, 2005). Dihydrocholesterol (0.2 mg/mL) used as an ISTD was added to 0.25 g of native and fried SFO before hot saponification. The unsaponifiable lipids were extracted by diethyl ether-heptane (1:1, v/v). An aliquot of the extract was silylated and the trimethylsilyl ether (TMS ether) derivatives were determined by an Agilent Technologies 6890 N GC-FID system equipped with an Rtx-5 w/Integra Guard capillary column (crossbond 5 % diphenyl-95 % dimethyl polysiloxane; film thickness 0.10 μm, 60 m × 0.32 mm i.d.; Restek, Bellefonte, PA, USA), an autosampler, an on-column injection system and ChemStation 3.1 software. Helium was used as the carrier gas at a constant flow (110 kPa at 200 °C). The initial temperature was 70 °C (1 min), then programmed with 60 °C/min to 245 °C (1 min) and then 3 °C/min to 275 °C (41 min). The detector temperature was 300 °C. A reference sample (rapeseed oil) was analyzed in each sample batch to check the daily repeatability of the method and a sterol standard mixture (cholesterol, dihydrocholesterol and stigmasterol) was analyzed to evaluate the GC performance.
POP analysis (GC–MS)
POP were determined according to the method described by Soupas et al. (2004). Artifact formation and losses were avoided by working in the dark and at room temperature. Native and fried SFO samples (0.5 g) were gently cold saponified overnight after the addition of 19-hydroxycholesterol (0.9–1.8 μg) used as an ISTD. The unsaponifiable lipids were extracted by diethyl ether. Sterol oxides were purified from the extract by silica SPE (SiOH-SPE). A secondary ISTD (dihydrocholesterol) was added to the samples after SPE elution to calculate the recovery of the ISTD. Oxides were silylated and the TMS ether derivatives were injected into a GC-MS system composed of a Hewlett Packard 6890 Series GC coupled to an Agilent 5973 MS (Palo Alto, CA, USA). On-column injection technique and an Rtx-5MS w/Integra Guard capillary column, 60 m × 0.25 mm i.d. (crossbond 5 % diphenyl-95 % dimethyl polysiloxane; Restek), and film thickness 0.10 μm, were used. A sterol standard mixture was also analyzed to evaluate the GC performance. β-Sitosterol oxides were identified by GC-MS in full scan mode (m/z 100–600) and quantified in SIM mode. As commercial standards of POP were not available, the calibration curves for POP were constructed indirectly via GC-FID, as described by Soupas et al. (2005). The main POP formed, 7α- and 7β-hydroxysterols, 5,6α- and 5,6β-epoxysterols and 7-ketosterols, were used as markers of PS oxidation. The main sitosterol oxide TMS ether derivatives were quantified by SIM acquisition of the following target and qualifier ions: m/z 353.3 and 366.4 for 19-hydroxycholesterol (ISTD), m/z 484.5 and 485.5 for 7α- and 7β-hydroxysitosterol, m/z 412.4 and 502.5 for 5,6α- and 5,6β-epoxysitosterol, and m/z 500.5 and 395.3 for 7-ketositosterol.
Statistical analysis
All experimental procedures were performed in duplicate and their mean values (± standard deviation) were given. Data collected were analyzed statistically and mean differences were analyzed for significance by employing a two-sample t-test using the proprietary software SAS version 9.3 (SAS Institute, Inc.) at p <0.05.
Results and discussion
Composition of SFO
The fatty acid composition of edible oil is a key factor influencing oil stability. Frying oil should have a long frying life and good organoleptic attributes, and it should be low in saturated and trans fatty acid and relatively low in polyunsaturated fatty acids (PUFA) (Mehta and Swinburn 2001). SFO contained high level of PUFA (61.4 %), followed by monounsaturated fatty acids (MUFA, 26.9 %) and saturated fatty acids (SFA, 11.7 %). The amount of total tocols in SFO was 715 μg/g. α-Tocopherol was the main compound (633 μg/g) which comprised more than 80 % of total tocols, while β- and γ-tocopherol found in lower levels (27.9 and 24.3 μg/g, respectively). The determined level of TBHQ was about 20 μg/g in SFO. The amount of PS was 4732 μg/g oil, thus, its effect on oxidation of PS during frying was considered important. β-sitosterol was the most abundant PS (2209 μg/g) which accounts for more than 46.7 % of total PS followed by campesterol (296 μg/g) and stigmasterol (266 μg/g). Other PS such as campestanol, sitostanol, ∆5-avenasterol, stigma-5,24 (25)-dienol, gramisterol, cycloartenol, ∆7-stigmastenol, ∆7-avenasterol, 24-methylencycloartanol and citrostadienol were detected in lower amounts or in traces (data not shown).
Impact of frying on the oxidative stability of SFO
Impact of frying on the total amount of PS
The main PS in SFO were β-sitosterol and campesterol. Therefore, only β-sitosterol and campesterol oxides were detected in quantifiable amounts. Table 1 shows the influence of deep- and pan-frying on the total PS content at different intervals of the frying experiments. At the beginning the total PS content was 4732 μg/g while after 240 min of deep- and pan-frying, the total PS contents were 3547 and 3096 μg/g, respectively. Figure 2 presents the percentage of deterioration of PS during frying. In general, the total PS content decreased upon frying, wherein the highest degree of deterioration was found after 240 min of pan frying (47 %). After 240 min of frying, the PS losses were ca. 31 % in deep fried SFO. It could be seen that pan frying of SFO had greater impact on oxidation than deep frying.
Oxidative stability of β-sitosterol and campesterol in SFO during frying
The initial total sitosterol and campesterol contents in SFO before frying were 2505 μg/g (Table 1). Generally, frying resulted in deterioration and/or decrease in the total sitosterol and campesterol total levels, wherein the highest decrease was recorded again after 240 min of pan frying (ca. 43 % of initial total sitosterol and campesterol). After 240 min of deep frying the percentage of decrease in the total sitosterol and campesterol levels was ca. 26 % of initial total sitosterol and campesterol. The results concerning β-sitosterol and campesterol oxides were confirmed by the GC-MS analysis which monitored the formation of the secondary POP during frying (Table 2). Oxidation products were identified by their elution order and mass spectral properties (Lampi et al. 2002; Grandgirard et al. 2004). All results were calculated as “oxidized PS μg/g oil”, and also as “percentages of PS oxides of unoxidized PS at that time point”.
Figure 3 summarizes percentage of the total amounts of five β-sitosterol oxides and five campesterol oxides formed before and during frying. Initially, 29.3 μg/g of β-sitosterol and campesterol oxidation products were found in the native SFO (Table 2). The longer the frying time, the more PS were oxidized. With frying, the amount of oxides formed in SFO increased slowly during the first 60 min reaching 40.8 μg/g and 44.9 μg/g oxides for deep- and pan-frying, respectively. After 60 and 120 min of frying, it could be noted that pan frying increased the oxidation of PS in SFO than deep frying process (Fig. 3). After 240 min of deep frying, the levels of total oxides increased quickly with a total level of 72.5 μg/g oxides. In pan frying, the levels of total oxides increased significantly with a total level of 164.7 μg/g oxides after 4 h of frying.
Because previous PS oxidation studies have been performed in various model systems, the comparison of the results is difficult. The results suggest that the oxidation of lipid matrix and PS is even more complex. Regarding the OS of PS in vegetable oils, some aspects should be considered, such as the degree of unsaturation of the lipid fraction and the occurrence and type of antioxidants. Studies on the effects of co-oxidizing matrix lipids are controversial because some indicate that PS oxidation is enhanced by unsaturated lipids (Osada et al. 1993), while others have shown that oxidation is more pronounced in saturated than in unsaturated lipid matrix (Lampi et al. 2002). The high unsaturation level and tocopherols content of the lipid matrix, in SFO, might have a protective effect on PS oxidation. Unfortunately, no exact comparisons can be made between the results of this study and the literature, since the different physicochemical states of the PS and the presence of the matrix have significant influence on the rate of PS oxidation.
Distribution of β-sitosterol and campesterol oxides during frying
Frying accelerates PS oxidation because of its high temperature and large surface-to-volume ratio, which allows oxygen adsorption by frying oil. In general, deep and pan frying of SFO induced PS oxidation but in the present study both frying methods had no significant effect during the first 60 min of frying.
Thermo-oxidation of PS can give rise to a number of products including ketones, alcohols, epoxides and dienes. In this investigation, main sitosterol and campesterol oxides (epimers of 7-hydroxysterols, the epimers of 5,6-epoxysterols and 7-ketosterols) were detected. The major PS oxides formed during thermo-oxidation were identified by their elution order and mass spectrometric data (Lampi et al. 2002) and were then used for markers of oxidation. β-Sitosterol and campesterol oxides in the SFO were determined both by the GC-FID and GC-MS techniques. As results derived from GC-FID were found comparable to those from GC-MS, only GC-MS data are presented. The concentration of sitosterol and campesterol total oxides in SFO at different frying times is shown in Table 2. As sitosterol was the main PS in SFO, its oxides predominated among POP. Oxides from campesterol were also formed but their contents were much lower. Between sitosterol and campesterol, larger differences were, however, observed in their susceptibility to oxidation. Campesterol seemed to be more stable than sitosterol. The percentage of total sitosterol and campesterol oxides in native SFO were 81.8 and 18.2 %, respectively. After 240 min of deep frying the percentage of total sitosterol and campesterol oxides reached 87.5 and 12.5 % of total oxides, respectively. In pan fried SFO, the percentage of total sitosterol and campesterol oxides after 4 h reached 83.5 and 16.5 % of total oxides, respectively.
In addition to the total amounts of sitosterol and campesterol oxides, the distribution of the individual oxides was studied. The trends in changes in sitosterol oxides’ profiles during deep- and pan-frying are presented in Fig. 4. The changes in campesterol oxides’ profiles during deep- and pan-frying are presented in Fig. 5. It was obviously noted that frying method and frying time mainly affect the distribution of sitosterol and campesterol oxidation products. 7-keto-, 7β-hydroxy-, and 7α-hydroxysitoterol were the main POP in the native SFO (Fig. 4). Of the quantifiable oxides in the native SFO, sitosterol oxides comprised the major POP (Table 2) wherein 7-ketositosterol was the main oxide (57.4 % of total oxides) followed by 7β-hydroxysitoterol (10.7 %), 7α-hydroxysitosterol (6.2 %) and 5,6α-epoxysitosterol (5.5 %). Campesterol oxides comprised the minor POP in native SFO, wherein 7-ketocampesterol was the main campesterol oxide (accounted for only 7.3 % of total oxides) followed by 5,6α-epoxycampesterol (5.2 %) and 7β-hydroxycampesterol (2.7 %). Frying process, frying time and structure of the PS compound, seemed to affect distribution. However, comparisons between PS structures were difficult since the product profiles were quite different in SFO. In the case of refined oils, Dutta (1997) reported total POP contents of 41.0, 39.9, and 46.7 ppm in a palm/rapeseed oil blend, a sunflower oil, and a high-oleic sunflower oil, respectively. These values included also dihydroxy derivatives. The same author found 7-keto derivative of β-sitosterol only, in the range of 1.6–14.1 μg/g, whereas the 7-hydroxy derivatives ranges were between 1.3 and 7.7 μg/g for β-sitosterol. This is an interesting finding indicating that the structural difference between the two PS, i.e., one methyl group, has no great effect on their thermo-oxidation. There were some minor deviations in the ratios of some individual sitosterol and campesterol oxidation products, but they could be accounted for by coelution in the GC analyses.
The trends in changes in sitosterol oxides during both deep- and pan-frying are presented in Fig. 4. It was obviously noted that frying type and frying time mainly affect the distribution of sitosterol oxides. 7-Ketositosterol, followed by 7β-hydroxysitosterol, 7α-hydroxysitosterol and 5,6-epoxysitosterol were the main sitosterol oxides found in the SFO during frying. This oxidative behaviour agrees with what has already been observed in previous studies of COP (Zunin et al. 1998), where the 7-keto derivative was pointed out as a tracer of the oxidation process. In addition, 7-keto derivative was the major POP in emulsified spreads (Conchillo et al. 2005). Thus, it was concluded that 7-keto derivatives represent a simple, reliable marker of the extent of PS oxidation in food (Cercaci et al. 2007). Although the distribution of POP may depend on the oxidation phase/status as well (Kemmo et al. 2005), the 7-keto derivatives were the most abundant oxides.
During both deep- and pan-frying the individual POP increased in oil samples. The main observations concerning sitosterol oxides distribution were that the proportion of 7-keto derivatives decreased during frying and the proportion of 7-hydroxy derivatives increased. Figure 4 clearly shows the proportional decrease of 7-ketositosterol during frying and the simultaneous increase of 7β-hydroxysitosterol during pan frying. Those levels of proportional changes in sitosterol oxides were more in pan frying than in deep frying. The longer the frying time, the faster did these changes appear to happen. Concerning campesterol oxides distribution (Fig. 5), the proportion of 7-keto derivatives and 5,6α-epoxycampesterol decreased during deep frying, while the proportion of 7-hydroxy derivatives and 5,6β-epoxycampesterol increased (Fig. 5a). The same trend of changes in campesterol oxides was also observed during pan frying with exception of increasing the proportion of 5,6α-epoxycampesterol during pan frying (Fig. 5b).
These results revealed that 7-keto derivatives (of sitosterol and campesterol) content were not greatly affected by frying as observed for the other oxides. 5,6-Epoxides are formed by the interaction of a hydroperoxide radical and an unoxidized sterol (Giuffrida et al. 2004). Epoxides was observed at the first time point in SFO. Therefore, it could be presumed that enough peroxides for epoxide formation was found during the whole frying period. Interestingly, at the beginning of frying, the amount of 5,6α-epoxysitosterol was higher than that of 5,6β-epoxysitosterol. However, during frying, the proportion of 5,6β-epoxysitosterol rapidly increased, while the proportion of 5,6α-epoxysitosterol slightly decreased. The same observation was also reported during frying of vegetable oils when α- and β-epimers of 5,6-epoxysitosterols behaved as mentioned earlier (Zhang et al. 2005). Smith (1987) introduced a mechanism for 6αβ-OOH-3-ketocholesterol formation; cholesterol 3β-alcohol dehydrogenates to cholest-5-en-3-one, which in turn rearranges to cholest-4-en-3-one and then oxygenates to epimeric 6αβ-OOH-3-ketocholesterol.
The distribution of hydroxy, epoxy and keto compounds, and the changes in the proportions of keto compounds in particular, seemed to be associated with the phase of oxidation (Soupas et al. 2004). 7-Ketosterols accumulated when oxidation had not yet reached the dynamic state. Once oxidation reached a dynamic state, the major products were 5,6-epoxysterols and 7-hydroxysterols (Fig. 4). Interestingly, a study of Giuffrida et al. (2004) revealed that the formation of epoxidized lipids proceeded readily in contact with TAG hydroperoxides, in the absence of molecular oxygen. It might be that the increase in the proportions of 5,6-epoxysterols during frying, in present study, was the result of the high temperature in which the oxygen availability is lower and can become limiting.
The formation of uncharacterized POP
To understand the overall deterioration of PS compounds in SFO, both the secondary oxides formation and the losses in the original PS content were studied. During frying experiments the loss of original PS was also measured (Figs. 6 and 7). In accordance with previous studies (Soupas et al. 2005; D’Evoli et al. 2006), the secondary oxidation products, which were measured in PS oxidation studies, did not account for all the PS losses during frying. Figure 6 demonstrates the relationships between the total amounts of quantified secondary β-sitosterol oxidation products and the losses of original sitosterol contents during 240 min of deep frying (Fig. 6a) and pan frying (Fig. 6b). Figure 7 shows the relationships between the total amounts of quantified secondary campesterol oxides and the losses of original campesterol contents during deep frying (Fig. 7a) and pan frying (Fig. 7b). POP measured do not account for all the PS losses and there may be a significant “gap” between them. After frying for 60 min a clear gap was found, wherein the greatest “gap” observed after 240 min of frying. Under the conditions applied, the “gap” was the largest when SFO was pan fried for 240 min, being 35.8 % of unknown sitosterol oxidation products (Fig. 6b) and 40.1 % of unknown campesterol oxidation products (Fig. 7b). Figure 7 compares the sums of the total amounts of the quantified campesterol oxides and the unaltered campesterol with the initial campesterol contents at the four time points of deep- and pan-frying. As can be noted, the gap increased with increasing the frying time for both frying applications.
Oehrl et al. (2001) noticed a large “gap” in their PS oxidation study, in which drastic heating conditions were applied. In the case of PS compounds, it is also possible that the “gap” partly originates from the formation of steradienes and -trienes, i.e., steroidal hydrocarbons with two or three double bonds in the ring structure. These structures are formed at high temperatures, e.g., through the dehydration of native sterols or 7-ketosterols, with a subsequent subtraction of the OH group from position 3, or through the elimination of water molecules from 7-hydroxysterols (Bortolomeazzi et al. 2003; Soupas et al. 2005). Moreover, it was mentioned that dimers and polymers are formed under thermal conditions. Changes in the lipid matrix at high temperatures may lead to structures that bind sterols, making them analytically less available (Soupas et al. 2005). This study revealed that even though the formation of secondary oxidation products is low after 60 min of frying, losses in the original PS content occur.
The data showed that the extent of oxidative reactions of PS in SFO during frying may differ from each other in terms of the secondary oxide contents, product profiles, and the gaps. It can be stated that the analyzed SFO, with high levels of PS, PUFA and antioxidants, might had relatively low levels of induced POP during frying, which indicates that the potential oxidation of PS is controlled, to a certain extent, by the presence of antioxidants. However, longer frying time and/or pan-frying might be a limiting factor for frying in SFO for longer times.
Conclusions
In conclusion, the results showed that pan frying induces PS oxidation more than deep frying. In general, the longer the heating time, the more did PS oxidize. Higher temperature of pan frying (ca. 185 °C) than the deep frying temperature (ca. 175 °C) should be considered. Unlike electric fryers, under domestic pan frying conditions, it is hard to control the temperature of frying (may reach 200–220 °C) which may significantly accelerates PS oxidation. Thus, using electric fryers with controlled and stabled temperature is recommended for long frying processes.
References
Alemany L, Cilla A, Garcia-Llatas G, Rodriguez-Estrada MT, Cardenia V, Alegría A (2013) Effect of simulated gastrointestinal digestion on plant sterols and their oxides in enriched beverages. Food Res Inter 52:1–7
Bortolomeazzi R, Cordaro F, Pizzale L, Conte LS (2003) Presence of phytosterol oxides in crude vegetable oils and their fate during refining. J Agric Food Chem 51:2394–2401
Cercaci L, Rodriguez-Estrada MT, Lercker G, Decker EA (2007) Phytosterol oxidation in oil-in-water emulsions and bulk oil. Food Chem 102:161–167
Chiou A, Kalogeropoulos N, Salta FN, Efstathiou P, Andrikopoulos NK (2009) Pan-frying of French fries in three different edible oils enriched with olive leaf extract: Oxidative stability and fate of microconstituents. LWT-Food Sci Technol 42:1090–1097
Conchillo A, Cercacl L, Ansorena D, Rodriguez-Estrada MT, Lercker G, Astiasaran I (2005) Levels of phytosterol oxides in enriched and nonenriched spreads: application of a thin-layer chromatography-gas chromatography methodology. J Agric Food Chem 53:7844–7850
D’Evoli L, Huikko L, Lampi A-M, Lucarini M, Lombardi-Boccia G, Stefano Nicoli S, Piironen V (2006) Influence of rosemary (Rosmarinus officinalis L.) on plant sterol oxidation in extra virgin olive oil. Mol Nutr Food Res 50(818):823
Dutta PC (1997) Studies on phytosterol oxides. II. Content in some vegetable oils and in French fries prepared in these oils. J Am Oil Chem Soc 74:659–666
Garcia-Cruset S, Carpenter KLH, Codony R, Guardiola F (2002) Cholesterol oxidation products and atherosclerosis. In: Guardiola F, Dutta PC, Codony R, Savage GP (eds) Cholesterol and phytosterol oxidation products: analysis, occurrence and biological effects. AOCS Press, Champaign, pp 241–277
Garcia-Llatas G, Rodriguez-Estrada M (2011) Current and new insights on phytosterol oxides in plant sterol-enriched food. Chem Phys Lipids 164:607–624
Giuffrida F, Destaillats F, Robert F, Skibsted LH, Dionisi F (2004) Formation and hydrolysis of triacylglycerol and sterol epoxides: role of unsaturated triacylglycerol peroxyl radicals. Free Rad Biol Med 37:104–114
Grandgirard A, Martine L, Demaison L, Cordelet C, Joffre C, Berdeaux O, Semon O (2004) Oxyphytosterols are present in plasma of healthy human subjects. Br J Nutr 91:101–106
Guardiola F, Bou R, Boatella J, Codony R (2004) Analysis of sterol oxidation products in foods. J AOAC Inter 87:441–466
Hiroko T, Yayoi K, Tomoko H, Ikuyo I, Hiroyoshi S, Masao S, Ikuo I, Katsumi I (2004) Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. J Nutr 134:1690–1969
Kemmo S, Soupas L, Lampi A-M, Piironen V (2005) Formation and decomposition of stigmasterol hydroperoxydes and secondary oxidation products during thermo-oxidation. Eur J Lipid Sci Technol 107:805–814
Koschutnig K, Kemmo S, Lampi A-M, Piironen V, Fritz-Ton C, Wagner K-H (2010) Separation and isolation of β-sitosterol oxides and their non-mutagenic potential in the Salmonella microsome assay. Food Chem 118:133–140
Kritchevsky D, Chen SC (2005) Phytosterols - health benefits and potential concerns: a review. Nutr Res 25:413–428
Lampi A-M, Juntunen L, Toivo J, Piironen V (2002) Determination of thermo-oxidation products of plant sterols. J Chrom B 777:83–92
Lampi A-M, Soupas L, Juntunen L, Piironen V (2004) COST 927 Thermally Processed Food: Possible Health Implications. Publishing European Commission, Prague
Lea LJ, Hepburn PA, Wolfreys AM, Baldrick P (2004) Safety evaluation of phytosterol esters. Part 8. Lack of genotoxicity and subchronic toxicity with phytosterol oxides. Food Chem Toxicol 42:771–783
Lehtonen M, Lampi A-M, Ollilainen V, Struijs K, Piironen V (2011) The role of acyl moiety in the formation and reactions of steryl ester hydroperoxides. Eur Food Res Technol 233:51–61
Lütjohann D (2004) Sterol autoxidation: from phytosterols to oxyphytosterols. Br J Nutr 91:3–4
Maguire L, Konoplyannikov M, Ford A, Maguire AR, O’Brien NM (2003) Comparison of the cytotoxic effects of β-sitosterol oxides and a cholesterol oxide, 7β-hydroxycholesterol, in cultured mammalian cells. Br J Nutr 90:767–775
Mehta U, Swinburn A (2001) Review of factors affecting fat absorption in hot chips. Crit Rev Food Sci Nutr 41:133–154
Menéndez-Carreño M, Ansorena D, Astiasarán I, Piironen V, Lampi A-M (2010) Determination of non-polar and mid-polar monomeric oxidation products of stigmasterol during thermo-oxidation. Food Chem 122:277–284
Metcalfe LD, Schmitz AA, Pelka JR (1966) Rapid preparation of fatty acid esters from lipids for Gas chromatographic analysis. Anal Chem 38:514–515
Oehrl LL, Hansen AP, Rohrer CA, Fenner GP, Boyd LC (2001) Oxidation of phytosterols in a test food system. J Am Oil Chem Soc 78:1073–1078
Osada K, Kodama T, Yamada K, Sugano M (1993) Oxidation of cholesterol by heating. J Agric Food Chem 41:1198–1202
Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi AM (2000) Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric 80:939–966
Ryan E, Chopra J, McCarthy F, Maguire AR, O’Brien NM (2005) Qualitative and quantitative comparison of the cytotoxic and apoptotic potential of phytosterol oxidation products with their corresponding cholesterol oxidation products. Br J Nutr 94:443–451
Schwartz H, Ollilainen V, Piironen V, Lampi A-M (2008) Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J Food Comp Anal 21:152–161
Smith LL (1987) Cholesterol autoxidation. Chem Phys Lipids 44:87–125
Soupas L, Juntunen L, Lampi A-M, Piironen V (2004) Effects of sterol structure, temperature, and lipid medium on phytosterol oxidation. J Agric Food Chem 52:6485–6491
Soupas L, Huikko L, Lampi A-M, Piironen V (2005) Esterification affects phytosterol oxidation. Eur J Lipid Sci Technol 107:107–118
Tomoyori H, Kawata Y, Higuchi T, Ichi I, Sato H, Sato M (2004) Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. J Nutr 134:1690–1696
Vanmierlo T, Husche C, Schött HF, Pettersson H, Lütjohann D (2013) Plant sterol oxidation products-Analogs to cholesterol oxidation products from plant origin? Biochimie 95:464–472
Zhang X, Julien-David D, Miesch M, Geoffroy P, Raul F, Roussi S, Aoude-Werner D, Marchioni E (2005) Identification and quantitative analysis of β-sitosterol oxides in vegetable oils by capillary gas chromatography–mass spectrometry. Steroids 70:896–906
Zunin CP, Calcagno C, Evangelisti F (1998) Sterol oxidation in infant milk formulas and milk cereals. J Dairy Res 65:591–598
Acknowledgments
The author would like to thank Egyptian Science and Technology Development Fund (STDF Project NO 21) for the financial support. The author also thanks Prof. Vieno Piironen and Dr. Anna-Maja Lampi from University of Helsinki for scientific cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramadan, M.F. Oxidation of β-sitosterol and campesterol in sunflower oil upon deep- and pan-frying of French fries. J Food Sci Technol 52, 6301–6311 (2015). https://doi.org/10.1007/s13197-015-1738-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1738-y