Abstract
The interest in application of biocatalysis during natural milk fat flavours development has increased rapidly and lipases have become the most studied group in the development of bovine milk fat flavours. Lipozyme-435, Novozyme-435 and Thermomyces lanuginosus Immobilized (TL-IM) lipases were used to hydrolyze anhydrous milk fat (AMF) and anhydrous buffalo milk fat (ABF) and their volatile flavouring compounds were identified by solid-phase micro-extraction gas chromatography/mass spectrometry (SPME-GC/MS) and then compared at three hydrolysis intervals. Both AMF and ABF after lipolysis produced high amount of butanoic and hexanoic acids and other flavouring compounds; however, highest amount were produced by Lipozyme-435 and Novozyme-435 followed by TL-IM. The hydrolyzed products were assessed by Rancimat-743 for oxidative stability and found both that, for AMF and ABF treated butter oil, Lipozyme-435 and TL-IM were generally more stable compared to Novozyme-435. For both AMF and ABF treated butter oil, Lipozyme-435 was observed to cause no further oxidation consequences which indicates Lipozyme-435 was stable during hydrolysis at 55 °C for 24 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk fat is extensively used as a food and is a unique ingredient in the food industry as a raw material in having natural milk fat flavours. Recently, there has been a trend towards producing natural milk fat flavours to replace the synthetic milk fat flavours due to consumer demands for natural foods which is associated with increasing knowledge and understanding about health concerns and hence, the manufacturing of dairy flavours (Ha and Lindsay 1993) has been of great interest to the flavour industry (Wang and Xu 2009). The International Organization of the Flavour Industry defines natural flavouring ingredients as “those obtained by appropriate physical, enzymatic or microbiological processes from material of vegetable or animal origin, either in the raw state or after processing for human consumption” (Gandhi 1997). Milk fat is an important raw material in the milk industry and related confectionery industries because of its unique fatty acids (FA) composition compared to other fats those results in a mixture of triacylglycerols with a wide range of molecular weight and degree of unsaturation. Milk fat is one of the few natural sources containing a significant amount of short and medium chain fatty acids (C4-C12), those would be around 25 % as estimated on a molar basis (Lubary et al. 2009).
Production of desirable flavours for dairy products commonly employs lipases (Gandhi 1997) due to their unique specificities for fatty acids and controlled hydrolysis of milk fat triglycerides (Ha and Lindsay 1993; Kurtovic et al. 2011). These dairy flavours are generally known as volatile free fatty acids (FFA) which impart organoleptic properties (Horii et al. 2010) of richness and creaminess in dairy products (Balcao and Malcata 1998; Kurtovic et al. 2011). The short chain fatty acids (FA) such as butanoic, hexanoic and octanoic acid are important contributors to the desired “buttery” flavours (Saerens et al. 2008) and thus much focus is paid on lipases which have high specificities for these short chain FA (Horii et al. 2010; Kurtovic et al. 2011; Martínez-Monteagudo, et al. 2014) in milk fats. In contrast, long chain fatty acids (e.g., C10–C18) have either soapy or bitter flavors (Ghosh et al. 1996), which are generally not suitable for food production (Horii et al. 2010). Therefore, efforts should be made to decide which lipases may be used in order to minimize the level of long chain fatty acids during milk fat hydrolysis.
Microbial enzymes are often more preferred in comparison to enzymes derived from plants or animals due to; the great variety of catalytic activities they present, possible high yields, the regular supply, more convenient and safer production process (Hasan et al. 2006). In particular, both academic and industrial viewpoints on microbial lipases research have substantially progressed mainly due to increased availability and stability of the commercial available enzymes (Wu et al. 1996).
In the food industry, lipases are extensively used for the production of dairy flavours via lipolysis of the triacylglycerides present in milk fat (Garcia et al. 1992) giving rise to free fatty acids which provide characteristic flavours to many dairy productss, particularly to cheese and dairy flavour concentrates (Horii et al. 2010). There are many potential applications for these flavours which include - additive to bakery products (bread, cake and cookie mixes) (Ghosh et al. 1996), cereal products (flakes), candies (chocolate products and toffees), dairy products (coffee whiteners, confectionary creams, cheese and butter spreads) and a variety of other products (popcorn seasoning, sauces, salad dressings and snack foods) (Regado et al. 2007). However the products mentioned above require high processing temperature, there should be no compromise on the oxidation stability of the lipolysed milk flavour product, in order to avoid its oxidation during processing of the products to which it is added unless otherwise render it unfit for human consumption. Another important consideration of the lipolysed milk flavor product is its stability during storage, which is another consequence of lipid oxidation.
The solid-phase micro-extraction (SPME) is a relatively simple preparation technique which is based on the partition of the analyte between the extraction phase on the outside of a small fused-silica fibre, and the matrix (Vítová et al. 2006). SPME is an attractive alternative to other conventional extraction sampling techniques because it is fast, sensitive, solvent-free and economical (Van Aardt et al. 2005). SPME has gained a lot of interest in a broad field of food analysis (Štoudková and Zemanová 2007) and the technique is also highly sensitive towards volatile milk fat products (Vítová et al. 2006).
Recent studies have focused mainly on analyzing different kinds of milk fat flavour compounds produced when milk fat is treated with lipases. To the best of our knowledge, none of these studies relates the effect of high processing temperatures on stability of hydrolyzed milk fat produced. Therefore, the objective of this study was to monitor the profile changes of volatile flavour compounds at three different hydrolyzing intervals within 24 h from the lipase treated butter oil by using SPME and assess the stability of the hydrolyzed milk fat at higher temperatures by measuring induction time using Rancimat 743.
Materials and methods
Materials
Fresh unsalted buffalo butter (83.48 % fat, solids not fat 2.91 %, moisture 13.61 % and peroxide value 0.145 meq/kg) was collected from the Department of Dairy Science, Faculty of Agriculture, Suez Canal University (Ismailia, Egypt). Anhydrous milk fat (AMF) with moisture content 0.14 % (determined by DUHAUS MB45 moisture analyzer) was a gift from Kerry Oil & Grains Industries Shanghai Company. Three immobilized lipases (Lipozyme-435 (≥20,000 U/g) obtained from Rhizomucor miehei, Novozyme-435 (≥5000 U/g) obtained from Candida antarctica and Lipozmye TL-IM (≥50,000 U/g) obtained from Thermomyces lanuginosus) were gifts from Novozyme (Shandong) Innovation & Business Center, China.
Preparation of anhydrous buffalo milk fat
Water from fresh melted butter was removed by a separating funnel at 60 °C. Oil was then filtered out using normal filter paper (grade No. 1) and dried under vacuum at 0.14 MPa for 30 min in a hot water bath. Thereafter, anhydrous nitrogen was bubbled in the melted buffalo butter oil for 5 min (to help in removing residual oxygen and water if any). The dried buffalo butter oil with (0.13 % moisture). The obtained anhydrous buffalo milk fat (ABF) was stored under nitrogen at −20 °C until experimental use.
Performance of milk fat hydrolysis reactions
Hydrolysis of both AMF and ABF were carried out at 55 °C in a 125 mL Erlenmeyer flask (fitted with a silicon rubber on top). Ten grams of milk fat were used in every experimental set up. Minimum possible amounts of each lipase were used but taking into consideration that their optimum level of FFA production was achieved (optimization procedure was also done at different lipase concentration with milk fat). For Novozyme-435 and TL-IM both of 250 U/g were used while for Lipozyme-435, 500 U/g milk fat was applied. Also phosphate buffers at different pH levels and buffer concentrations were tried; however, pH 7.5 with 0.2 M concentrations were found optimum in our experimental set up for providing high level of FFA for all three lipases used in this study. Therefore, pH 7.5 with 0.2 M concentrations was used for the rest of all experiments. The ratio between milk fat and buffer concentration was kept constant at 2:1 throughout the experiment. The flasks were shaken at 200 rpm in a thermostat steam bath vibrator (SHZ-82). Before lipase was added in the reaction vessel, both AMF and ABF were warmed for 15 min while shaking at 200 rpm. The hydrolysis was done at 12, 18 and 24 h of intervals. After each hydrolysis interval, the hydrolyzed milk fat was separated from the lipase by filter paper (grade No. 1) and immediately centrifuged at 2147×g for 10 min. The top layer was separated and stored at −20 °C until further analysis. The lipase was re-activated by washing several times with hexane and then dried at room temperature while keeping into account of their number of cycles and their activities. Each hydrolysis was done in triplicates.
Determination of FFA content
The percentage of FFA was determined according to Leitgeb and Knez (1990) and expressed in terms of oleic acid (w/w). The analysis was carried out in triplicates immediately after the end of every hydrolysis.
Oil stability index
The oxidation induction time (OIT) of the AMF, ABF and lipase-treated butter oil was determined using automated Metrohm Rancimat A 743 apparatus (Metrohm AG, Herison, Switzerland) following the method of Omar et al. (2010) with some modifications. All samples were first melted at 50 °C and then 3 g of each sample was weighed into the reaction vessels. Distilled water (50 mL) was added to the measuring vessels which were maintained at room temperature (25 °C). Electrodes were attached for measuring changes in conductivity and the samples were heated at 120 °C under a purified air flow rate of 20 L/h. The experiments were carried out in triplicates.
SPME-GC/MS analysis
Volatile organic compounds in the AMF, ABF and lipase-treated butter oil were analyzed by SPME-GC/MS, based on Van Aardt et al. (2005) method but with some modifications. Two grams sample of AMF, ABF or lipase treated butter oil at different hydrolysis periods were taken at the end of the hydrolysis and placed in a 15 ml glass vial fitted with a self-sealing septum. The vial was incubated at 45 °C for 20 min with a magnetic stirrer in order to reach adsorption and desorption equilibrium for organic volatile compounds in CAR/PDMS, prior to volatiles adsorption for another 20 min, using a 75 μm carboxen poly(dimethyl siloxane)-coated SPME fibre (Supelco, Bellefonte, PA, USA). The adsorbed volatiles were then analyzed by Thermo Scientific ISQ GC-MS (Trace GC Ultra gas chromatograph combined with ISQ, Thermo Scientific, USA) under the following conditions: injection temperature 280 °C; splitless mode; 5 min desorption time; Rtx-5Sil DB-5 MS capillary column 30 m × 0.25 mm i.d. × 0.25 μm film thickness (Restek, Bellefonte, PA, USA); oven temperature programmed: 35 °C start, 15 °C /min to 180 °C, then 20 °C /min to 260 °C, and held at 260 °C for 30 s; He carrier gas, flow rate 1.2 ml/min; detector temperature 280 °C. The peaks were identified by comparison with the mass spectral database (NIS). The mass spectrometer operated in the electron impact (EI) ionization mode at 70 eV, and mass spectral data were acquired in the mass range of 33–300 amu at 0.7 scans/s.
Statistical analysis
SPSS statistical software (v. 19.0, IBM SPSS, Chicago, IL, USA) was used in data analysis. One-way analysis of variance (ANOVA) was applied to find significant differences in percent FFA production, induction time and volatile organic compounds of the AMF, ABF and lipase-treated butter oil. Duncan multiple range test was used (P < 0.05).
Results and discussion
Kinetics of FFA release from milk fat
Both the AMF and ABF were hydrolyzed using three different commercial lipases as shown in Table 1. Using the AMF, all the three different commercial lipases used in this study were able to produce more than 50 % of FFA within 12 h of hydrolysis. However, Lipozyme-435 hydrolyzed AMF into highest amount of FFA when compared with Novozyme-435 and TL-IM and was found to be significantly different (P < 0.05). This showed that the initial kinetics of hydrolysis by Lipozyme-435 was higher when compared with other lipases used in this study. However, at 18 h of hydrolysis, the increase in percent release of FFA for the Lipozyme-435 was not higher than that of at the 12 h and no significant difference was observed by all three lipases in this period of hydrolysis. After 24 h of hydrolysis the percentage of FFA was above 70 % in case of Lipozyme-435 which was significantly different when compared with that of other lipases. The initial release of high FFA for Lipozyme-435 was also observed by Bourlieu et al. (2012) who reported hydrolysis catalyzed by M. miehei presented an exponential profile with an initial stage of high release rate of FFA (24.8 ± 1.3 μmol min−1) followed by an acute slowing down after the release of around 300 μmol FFA. The lowest amount of FFA was produced by TL-IM in this study.
As for ABF, Lipozyme-435 was the best in producing high percentage FFA when compared to that of the other two lipases (Table 1). This suggests that Lipozyme-435 is the best in hydrolyzing the AMF and ABF than other lipases, however, only a little amount of FFA was released by this lipase at 18 h of hydrolysis. This can be explained by the kinetic release of FFA for Novozyme-435 and TL-IM which were slow at 12 h of hydrolysis and then the kinetic release increased within 18 h of hydrolysis before slowing down again at 24 h of hydrolysis. After 24 h of hydrolysis, Lipozyme-435 was also producing higher amount of FFA than other lipases. The lowest yield in percent FFA was found also in TL-IM as shown in Table 1. High amount of FFA production was also reported by Kurtovic et al. (2011) who used palatase in their study on fresh cream.
Oxidative stability
Oxidative stability is an important parameter for the quality assessment of animal and vegetable fats and oils (Omar et al. 2010). The mechanism of the Rancimat method is based on measuring changes of the electrical conductivity of water caused by the formation of short-chain compounds when fats and oils are oxidized under elevated temperature and at the same time accelerated aeration (an air flow of 20 L/h). Oxidation of unsaturated fatty acid residues leads to rancidity, off-flavors, and musty odours (Aguedo et al. 2008).
The Rancimat method was used to assess the OIT (Table 2) which is an indication of the oxidative stability of a fat. The OIT values of the control samples (AMF and ABF without hydrolysis) and lipase treated butter oil were compared for oxidative stability. For the AMF, the values of IT of the control and lipase treated samples were significantly different (P < 0.05) except for Lipozyme-435. In assessing Lipozyme-435, both control and lipase treated samples at the three different hydrolysis intervals showed no significant difference. This showed that Lipozyme-435 is able to hydrolyze the AMF without causing any further oxidation consequences towards the product at 55 °C for the three different hydrolysis intervals used. This is in agreement with Aguedo et al. (2008) who reported that the IT for the interesterification products was not significantly different from that of the initial blends. However, for Novozyme-435 the IT values were lower throughout the hydrolysis periods when compared with that of the control. TL-IM showed tremendously higher than the control sample at hydrolysis periods of 12 and 18 h. The IT values at hydrolysis periods of 12 and 18 h were 4.68 ± 0.08 and 4.52 ± 0.19 respectively; which are almost 1 h higher in comparison to the control sample which had IT values of 3.66 ± 0.09 h. However, further increase in hydrolysis time beyond the 18 h resulted in lower IT by TL-IM (2.26 ± 0.27) than the control sample as observed after 24 h hydrolysis time period. This showed that increasing more than 18 h of hydrolysis periods can lower oxidative stability of the product when TL-IM lipase is used in the AMF lipolysis process.
When the ABF lipase treated butter oil was analyzed for oxidative stability; the three lipases used in this study were found to perform well towards oxidation when compared to AMF lipase treated butter oil. For Lipozyme-435, the values of IT were significantly different at the three different hydrolysis periods when compared with the control sample. This confirms the oxidative stability of the hydrolyzed product of this enzyme at all the three hydrolysis periods analyzed. Surprisingly, when the ABF was used with Novozyme-435; the IT values were higher and significantly different in the first two hydrolysis periods when compared with the control sample. It was also noted that in ABF lipase-treated butter oil for TL-IM, the IT values were also significantly different (P < 0.05) when compared with the control sample. In general, the performance towards oxidative stability for both Lipozyme-435 and TL-IM of the product at 12 and 18 h hydrolysis periods was higher when compared to Novozyme-435 for AMF lipase treated butter oil. The same observation was also reported by Kimoto et al. (1994) who studied the oxidative stability of enzymatically interesterified fish oil and found it to be more stable than the control.
SPME-GC/MS analysis of volatile compounds
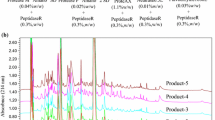
In this study, the volatile compounds were determined at three different hydrolysis periods (12, 18 and 24 h). The chromatograms in Fig. 1a–c and d–f show the profile of AMF and ABF lipase treated butter oil produced by three lipases at 18 h of hydrolysis respectively. Tables 3 and 4 show the volatile compounds identified in AMF and ABF respectively when three commercial lipases were used. Short chain FAs (butanoic and hexanoic acid) were the dominant volatile compounds in both AMF and ABF lipase treated butter oil and these are responsible for the cheesy (sharp, acrid and piquant) odours (Zellner et al. 2008; Kurtovic et al. 2011).
In principle SPME technique is quantitative (Kurtovic et al. 2011), therefore; the actual peak areas were compared among the lipase treated butter oil. In this study, 24 volatile flavouring compounds were identified in AMF sample (Table 3). The highest amounts of volatile flavouring compounds were in the ranges of 18.03–2.50 % peak area and were butanoic, phenol, o-(methylthio), propane, 1,3-di(octadecyloxy), 2-heptanone, docosanoic acid docosyl, benzaldehyde, acetaldehyde and n-hexadecanoic acid. The other volatile flavouring compounds were in the region below 2.50 % peak area. Within 24 h of hydrolysis at three different intervals of hydrolysis; Lipozyme-435, TL-IM and Novozyme-435 were able to produce 14–20, 14–17 and 13–14 different volatile flavouring compounds respectively. Therefore, Lipozyme-435 was an important lipase in producing many different volatile flavouring compounds in AMF sample. Some volatile flavouring compounds found in AMF sample were not identified once after hydrolysis within 24 h. This may be due to the higher flavour note produced after hydrolysis tended to cease another lower flavour note or may be due to complete hydrolysis by the lipase. However, Ha and Lindsay (1990) reported that certain branching of the carbon chain can significantly lower the flavor thresholds of FFA by citing an example of the aroma threshold of decanoic acid (C10) in water being at 16 ppm, and that for 4-ethyloctanoic acid (also C10) has been found to be 1.8 ppb. This phenomenan was also observed within three different hydrolysis periods of those three lipases used in this study. The peak areas of major volatile flavouring compounds derived from AMF are summarized in Fig. 2a, b and c.
In Table 3, Novozyme-435 was found to produce more butanoic and hexanoic acids in all the three hydrolysis periods than the rest of the enzymes used in this study. This can be explained by the fact that Novozyme-435 is a non-specific enzyme, being able to hydrolyze all ester bonds in triacylglycerol regardless of their positions or types of fatty acids and because butanoic and hexanoic acids are found nearly entirely in the sn-3 positions in bovine milk fats (Ha and Lindsay 1993). Lipozyme-435 was able to maintain the level of butanoic acid in all three different intervals of hydrolysis; while, a higher level of butanoic acid was produced by TL-IM within 12 h of hydrolysis and then slowly increased at 18 and 24 h of hydrolysis. For the case of hexanoic acid, all three lipases were found to compete with each other from one hydrolysis interval to another. However, Novozyme-435 was found to produce more hexanoic acid. TL-IM was characteristically differentiated from others by producing higher level of octanoic acid. The odour importance of this compound is well known as “creamy”, “whey”. Twelve and eighteen hours of hydrolysis periods were found to effectively produce higher amount of volatile flavouring compound compared to 24 h of hydrolysis. However, for the other two lipases; there was no significant difference within the first two hydrolysis periods except at 24 h whereby Lipozyme-435 was able to produce more octanoic acid than Novozyme-435. Another compound that TL-IM was found to produce significantly in higher amount was n-decanoic acid (P < 0.05) and the odour description of this is “sweety-waxy”. Within 24 h of hydrolysis, decanoic acid and decanoic acid decyl were highly produced by Lipozyme-435 than other lipases. Therefore, Lipozyme-435 has its own special characteristic behavior of producing these compounds unlike other enzymes in this study. TL-IM and Lipozyme-435 produced more decanoic and dodecanoic acids compared to the amounts produced by Novozyme-435. Decanoic and dodecanoic acids are well known for their contribution to characteristic aroma (Peterson, and Reineccius 2003). Ha and Lindsay (1993) found that pregastric lipase had an ability to release high concentrations of octanoic and decanoic acids from caprine milk; hence both TL-IM and Lipozyme-435 are comparable with pregastric lipase in producing these two flavouring compounds. It was also noted in this study that after 24 h of hydrolysis period; 2-heptanone, bicyclopentylidene, 2-nonanone, docosanoic acid docosyl, 4-methylhexanoic acid, octadecane, 2-methyl docosane, stearic acid allyl, eicosanoic acid, (Z)-7-hexadecenal and oleic acid which were in AMF sample were not detected. This shows that all the three lipases were able to completely hydrolyze those volatile flavouring compounds or were shielded by other flavouring compounds. Lipozyme-435 and Novozyme-435 were greatly able to hydrolyze 1,3-di(octadecyloxy)- to the lowest level and for the phenol, o-(methylthio); Novozyme-435 was better in terms of hydrolysis compared to Lipozyme-435.
Unlike in the AMF sample, 34 volatile flavouring compounds were identified in ABF sample (Table 4), which is an increment of 10 more volatile flavouring compounds when compared to the AMF sample. This shows that the ABF sample had more volatile flavouring compounds than the AMF sample. The highest amounts of volatile flavouring compounds were in the region 18.15–2.50 % peak area and included 6-undecylamine, phenol, o-(methylthio), propane, 1,3-di(octadecyloxy), decanoic acid decyl, butanoic acid, hexanoic acid, tetradecanoic acid, hexadecanoic acid methyl ester, acetaldehyde, dimethylaminoethanol acetate and benzaldehyde. The other 23 volatile flavouring compounds were below 2.50 % peak area. After 24 h of hydrolysis; TL-IM produced 13–18 different volatile flavouring compounds, Lipozyme-435 was able to produce 11–16 different volatile flavouring compounds and Novozyme-435 produced 10–13 different volatile flavouring compounds. Therefore, TL-IM and Lipozyme-435 are important lipases in producing many different volatile flavouring compounds in the ABF. A summary of the major volatile flavouring compounds peak areas are shown in Fig. 3a, b and c.
Table 4 shows that Lipozyme-435 produced more butanoic acid in the first 12 h of hydrolysis and then reduced in the 18 and 24 h periods, while Novozyme-435 produced lower at 12 h but the amount was increased at 18 and 24 h intervals of hydrolysis. However, TL-IM was able to maintain the level of butanoic acid in all three different intervals of hydrolysis though it was lower in amount when compared to the other lipases (P < 0.05). Significant differences were found for the production of hexanoic acid at 12, 18 and 24 h periods of hydrolysis for the case of Lipozyme-435 and Novozyme-435. However, at 18 h no significant difference was observed between Novozyme-435 and TL-IM in the production of hexanoic acid; while at 12 and 24 h of hydrolysis, the amounts were comparable with the other two lipases (Table 4). According to O’Connor et al. (2001) the short chain FAs in milk fat are predominantly at the sn-3 position, hence there is no doubt that the three lipases used in this study had an ability to hydrolyze short chain FAs at sn-3 position.
Again for the ABF, TL-IM was characteristically differentiated from other lipases in this study by producing higher level of octanoic acid in all three different hydrolysis intervals while decanoic acid decyl was highly hydrolyzed by Lipozyme-435 and Novozyme-435 than TL-IM. Therefore, Lipozyme-435 and Novozyme-435 have their own special characteristic behavior in hydrolyzing this compound when compared to TL-IM. In both the AMF and ABF treated lipases, TL-IM was found to produce highest amount of acetaldehyde which is characterized by its sweet and pungent smell (Kondyli et al. 2003). Besides the high amount present in ABF sample of 6-undecylamine, all lipases were able to highly hydrolyze after 24 h of hydrolysis. Both phenol, o-(methylthio) and propane, 1, 3-di(octadecyloxy)- were also highly hydrolyzed by Novozyme-435 after 24 h when compared to the other two lipases. However, Lipozyme-435 was able to hydrolyze to the lowest amount after 12 h of hydrolysis but when hydrolysis intervals were extended to 18 and 24 h the amounts of those two compounds also increased.
Conclusion
According to our analysis of volatile compounds, both the AMF and ABF samples contain a higher number of different volatiles compounds. It was also found that all the three microbial lipases used in this study were able to produce short chain fatty acids (especially butanoic and hexanoic acids) and other different important short and medium chain fatty acids (flavouring compounds) which can be used in many dairy and dairy related products. However, Lipozyme-435 and Novozyme-435 were able to produce higher peak area intensities compared to TL-IM. In addition, this study found that the hydrolyzed products of both Lipozyme-435 and TL-IM exhibited higher oxidative stability at 12 and 18 h of hydrolysis intervals compared to those of Novozyme-435. Since the oxidative stability is very important for the milk fat flavoring products at high temperature during mixing with other processing foods, the present study may be useful at food industrial level to test for their suitability.
References
Aguedo M, Hanon E, Danthine S, Paquot M, Lognay G, Thomas A, Vandenbol M, Thonart P, Wathelet JP, Blecker C (2008) Enrichment of anhydrous milk fat in polyunsaturated fatty acid residues from linseed and rapeseed oils through enzymatic interesterification. J Agric Food Chem 56:1757–1765
Balcao VM, Malcata FX (1998) Lipase catalyzed modification of milk fat. Biotechnol Adv 16:309–341
Bourlieu C, Rousseau F, Briard-Bion V, Madec MN, Bouhallab S (2012) Hydrolysis of native milk fat globules by microbial lipases: Mechanisms and modulation of interfacial quality. Food Res Int 49:533–544
Gandhi NN (1997) Applications of lipase. J Am Oil Chem Soc 74:621–634
Garcia HS, Malcata FX, Hill CG, Amundson CH (1992) Use of Candida rugosa lipase immobilized in a spiral wound membrane reactor for the hydrolysis of milk fat. Enzym Microb Technol 14:535–545
Ghosh PK, Saxena RK, Gupta R, Yadav RP, Davidson S (1996) Microbial lipases: production and applications. Sci Prog 79:119–158
Ha JK, Lindsay RC (1990) Method for the quantitative analysis of volatile free and total branched-chain fatty acids in cheese and milk fat. J Dairy Sci 73:1988–1999
Ha JK, Lindsay RC (1993) Release of volatile branched-chain and other fatty acids from ruminant milk fats by various lipases. J Dairy Sci 76:677–690
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzym Microb Technol 39:235–251
Horii K, Adachi T, Tanino T, Tanaka T, Kotaka A, Sahara H, Hashimoto T, Kuratani N, Shibasaki S, Ogino C (2010) Fatty acid production from butter using novel cutinase-displaying yeast. Enzym Microb Technol 46:194–199
Kimoto H, Endo Y, Fujimoto K (1994) Influence of interesterification on the oxidative stability of marine oil triacylglycerols. J Am Oil Chem Soc 71:469–473
Kondyli E, Massouras T, Katsiari MC, Voutsinas LP (2003) Free fatty acids and volatile compounds in low-fat Kefalograviera-type cheese made with commercial adjunct cultures. Int Dairy J 13:47–54
Kurtovic I, Marshall SN, Miller MR, Zhao X (2011) Flavour development in dairy cream using fish digestive lipases from Chinook salmon (Oncorhynchustshawytscha) and New Zealand hoki (Macruronusnovaezealandiae). Food Chem 127:1562–1568
Leitgeb M, Knez Ž (1990) The influence of water on the synthesis of n-butyl oleate by immobilized Mucor miehei lipase. J Am Oil Chem Soc 67:775–778
Lubary M, Ter Horst JH, Hofland GW, Jansens PJ (2009) Lipase-catalyzed ethanolysis of milk fat with a focus on short-chain fatty acid selectivity. J Agric Food Chem 57:116–121
Martínez-Monteagudo SI, Khan M, Temelli F, Saldaña MD (2014) Obtaining a hydrolyzed milk fat fraction enriched in conjugated linoleic acid and trans-vaccenic acid. Int Dairy J 36:29–37
O’Connor CJ, Bang K-A, Taylor CM, Brimble MA (2001) Determining the regio-and typo-selectivity of calf pregastric lipase. J Mol Catal B Enzym 16:147–157
Omar KA, Shan L, Wang YL, Wang X (2010) Stabilizing flaxseed oil with individual antioxidants and their mixtures. Eur J Lipid Sci Technol 112:1003–1011
Peterson DG, Reineccius GA (2003) Determination of the aroma impact compounds in heated sweet cream butter. Flavour Frag J 18:320–324
Regado MA, Cristóvao BM, Moutinho CG, Balcao VM, Aires-Barros R, Ferreira JPM, Xavier MF (2007) Flavour development via lipolysis of milkfats: changes in free fatty acid pool. Int J Food Sci Technol 42:961–968
Saerens K, Descamps D, Dewettinck K (2008) Release of short chain fatty acids from cream lipids by commercial lipases and esterases. Biotechnol Lett 30:311–315
Štoudková EVBLH, Zemanová J (2007) Application of SPME-GC method for analysis of the aroma of white surface mould cheeses. J Food Nutr Res 46:84–90
Van Aardt M, Duncan SE, Marcy JE, Long TE, O’Keefe SF, Nielsen-Sims SR (2005) Aroma analysis of light-exposed milk stored with and without natural and synthetic antioxidants. J Dairy Sci 88:881–890
Vítová E, Loupancová B, Zemanová J, Stoudkova H, Brezina P, Babák L (2006) Solid-phase micro-extraction for analysis of mould cheese aroma. Czech J Food Sci 24:268–274
Wang B, Xu S (2009) Effects of different commercial lipases on the volatile profile of lipolysed milk fat. Flavour Frag J 24:335–340
Wu XY, JÄÄskelÄinen S, Linko WY (1996) Purification and partial characterization of Rhizomucor miehei lipase for ester synthesis. Appl Biochem Biotechnol 59:145–158
Zellner BA, Dugo P, Dugo G, Mondello L (2008) Gas chromatography–olfactometry in food flavour analysis. J Chromatogr A 1186:123–143
Acknowledgments
The authors gratefully thank the secretary and all members of the group of State Key Laboratory of Food Science and Technology, Synergetic Innovation Center of Food Safety and Nutrition, School of Food Science and Technology, Jiangnan University for their invaluable contributions to the present work. We are also thank Shanghai Kerry Oils & Grains Industries and Novozyme (Shandong) Innovation & Business Center, China for their provision of related raw materials. This work has received funding from the National Natural Science Foundation of China (Grant No. 131401525).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omar, K.A., Gounga, M.E., Liu, R. et al. Effects of microbial lipases on hydrolyzed milk fat at different time intervals in flavour development and oxidative stability. J Food Sci Technol 53, 1035–1046 (2016). https://doi.org/10.1007/s13197-015-2158-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-2158-8