Abstract
There is increased interest in berry fruits due to health benefits, and maintenance of fruit quality for longer periods of time has been a priority. We previously found that starch based coatings applied on raspberries was associated to volatile compounds production due to anoxic conditions. The objective of this work was to design more hydrophobic coatings with reduced thickness. A starch-beeswax dispersion containing 2 % (w/v) modified tapioca starch added with either 0.5 or 1.0 % (w/v) beeswax microparticles was produced, and used for spray coating freshly harvested blackberries (Rubus spp.). Coatings were air dried, packed in plastic trays and stored up to 16 days at 4 °C and 88 % relative humidity. Storage quality parameters such as hardness, respiration rate, anthocyanins content, total phenols, color changes and weight loss were evaluated. We did not find Interactions among coating ingredients, and incorporation of beeswax reduced moisture transfer rate. Coatings did not occlude the stomata and apparently did not over-hydrate the cuticle. This characteristic allowed appropriate gas exchange (O2 and CO2), and reduced accumulation of volatile compounds associated to fermentative metabolism. Respiration rates were 4.207 ± 0.157, 4.557 ± 0.220 and 4.780 ± 0.050 mmol CO2 kg−1 h−1 for control, 0.5 and 1 % of wax content in coatings, respectively. However, ethylene production increased throughout storage time along with beeswax concentration, indicating stressful conditions for the fruit. This trend appears to be related with changes in total phenols and anthocyanins during storage. Edible coatings based on starch and hydrophobic particles should be reformulated to maintain quality of stored berry fruits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide blackberries market grew 44 % from 1995 to 2005, and this trend is expected to continue until 2015 in countries such as United States, Mexico, Chile, China, Romania and Poland (Strik et al. 2007). Besides economic aspects, there is an increased interest in berry fruits due to their high content of antioxidant compounds (Krüger et al. 2011). Thus, any attempt to maintain the quality of fresh fruits for longer periods of time is a priority for both, producers and consumers (Ribeiro et al. 2007).
One alternative to preserve berries quality is the application of starch based edible coatings. However, a drawback of this approach is that starch coatings may trigger the decay of berry like fruits due to excessive hydration of the cuticles and occlusion of stomata (Han et al. 2004; Pérez-Gallardo et al. 2012b). Coating efficiency depends on the tolerance of the vegetable tissue to internal atmosphere modification and induced metabolic changes (Vargas et al. 2006; Pérez-Gallardo et al. 2012b). Thus, we tested the hypothesis that using reduced coatings thickness and increased hydrophobicity, berry fruits freshness may be preserved for longer time.
Gelatinized starch film-forming dispersions may produce a continuous matrix able to support beeswax micro-particles useful for coating fruit surfaces (Phan The et al. 2005; Pérez-Gallardo et al. 2012a). Reduced thickness coatings are produced using dispersions at low starch concentration that may help viscosity control and structural recovery of the starch dispersion. This is necessary to optimize coating application and dry coating thickness (Peressini et al. 2003).
Thin coatings may also decrease surface hydration by decreasing resistance to water vapor diffusion (Cisneros-Zevallos and Krochta 2003). Additionally, an increase in beeswax micro-particles may produce higher hydrophobicity of coating dispersion (Paunov et al. 2007; Dickinson 2010), leading to lower water vapor permeability of coatings (Peressini et al. 2003; Pérez-Gago and Krochta 2001).
The aim of this work was to study the effect of thin coatings made of oxidized tapioca starch added with different concentrations of beeswax micro-particles on the quality parameters of coated fresh blackberries during cold storage.
Materials and methods
Tapioca modified starch (Textra starch, National starch, Bridgewater, NJ, USA) was used as carrier material for beeswax microparticles. Sorbitol, beeswax, stearic acid, morpholine and Tween 20 were purchased from Sigma (Sigma, St. Louis, MO, USA).
Preparation of coatings
A gelatinized dispersion of starch was prepared by heating 2 % (w/v) starch slurry at 90 °C for 15 min, followed by rapid cooling to room temperature on ice bath. Coatings were prepared by mixing a beeswax suspension of known solids content with the starch dispersion added with 1.2 % (w/v) sorbitol. Final beeswax concentrations of 0.5 and 1 % (w/v) were assayed and formulations were designated as WS0.5 and WS1, respectively. From WS1 suspension films were obtained by the casting method using 8 mL of WS1 into leveled square glass plates (10 × 10 cm) and allowed to dry for 48 h at 50 % RH and 25 °C. The characteristic infrared spectra of starch, lipids and other film components were used to evaluate structural interactions among ingredients by means of Fourier transform infrared spectroscopy (FTIR) using the attenuated total reflectance (ATR) technique. This information helps to gain fundamental understanding of how chemical functional components (for example that of beeswax) are distributed and interact at the micro scale in the films produced through the emulsification method (Muscat et al. 2014). A Vector 33 (Bruker Corporation, Massachusetts, EUA) equipment was used to scan the samples from 4000 to 650 cm−1.
Preparation of beeswax microparticles suspension
Beeswax suspension was prepared by emulsifying a mixture of stearic acid-beeswax-morpholine (Hagenmaier and Baker 1994) at 21,500 rpm (Ultraturrax, Staufen, Germany) in hot water for 5 min. The emulsion was then rapidly cooled at room temperature using an ice bath and filtered through cheese cloth. Tween 20 was slowly added to a final concentration of 0.05 % (w/v) for better emulsification and to increase the coating wettability. Particle size diameter was determined using a Zetasizer ZSP (Malvern Instruments, Malvern, U.K.) using dual angle measurement and a refractive index of 1.450 (n = 30).
Rheological characterization of coatings
Recovery tests were performed at 25 ± 0.1 °C using a Physica-MCR302 rheometer (Anton Paar, Germany) in rotational mode using CC27 concentric cylinders geometry. Three-step measurements of low-high-low shear conditions at 5, 1000 and 5 s−1 were conducted. Viscosity curves were performed using a shear range of 0.1–1000 s−1, and before each measurement samples were rested for 10 min. Results are reported as the average of three independent preparations. Data analysis was conducted using the rheometer software (Rheoplus, Anton Paar, Germany).
Fruit treatments and storage
Blackberries at commercial ripening stage of uniform size, visually free of physical damage and fungal infection were purchased from a local producer. Fruits were placed over a Teflon coated grid, sprayed with coating solution (0.1 L/kg of fruit) and dried at 20 °C using air flowing at 2 m/s, for 2 h. Uncoated blackberries were used as control. After coating the fruits were packaged in ventilated clamshell containers of 170 g in capacity and stored in a refrigerated chamber at 4 °C and 88 % relative humidity. All surfaces in contact with blackberries were previously sanitized (200 ppm NaOCl) to avoid possible contamination.
Physicochemical analysis of blackberries
Respiration rate
About 200 g of fruit were placed into closed chambers and the carbon dioxide (CO2) and ethylene (C2H4) concentrations from each chamber were automatically measured every 8 h, using a HP 5890A gas chromatograph (GC, Agilent, Avondale, PA, USA). The sampling system was previously described by Patterson and Apel (1984). The GC was equipped with a thermal conductivity detector, a 0.53 mm × 30 m GS-Q-PLOT column (Agilent Technologies) for measuring CO2, and a FID connected to a 0.53 mm × 15 m GS-Q-PLOT column (Agilent Tech.) for measuring ethylene. The system uses two electronic switching valves to read from one flow path. The oven, injector, and TCD were held at 30, 200, and 90 °C, respectively, while the FID was held at 200 °C. The CO2 and ethylene columns had flow rates of 8 and 10 mL/min, respectively. The fruits were kept in a chamber at 15 °C, and measurements were done in triplicate.
Analysis of volatiles
Samples of blackberries were prepared by blending 50 g of fruit with 100 mL distilled water and filtered. Twenty mL of filtrate was poured into a plastic vial and stored at −20 °C until analysis. Aroma related compounds were quantified using the solid phase micro extraction (SPME) technique described by Yang and Peppard (1994), followed by GC coupled to mass spectrometry (GC/MS) as described by Birla et al. (2005).
Total phenols
The total phenolic content of the extracts were determined according to the Folin-Ciocalteu colorimetric method (Singleton and Rossi 1965). Briefly, 100 μL of the extracts were oxidized with 250 μL of 1 N Folin-Ciocalteu reagent. After 5 min, 1250 μL of a 20 % Na2CO3 solution was added to stop the reaction and the sample was stored for 2 h. The absorbance was measured against a prepared blank at 760 nm. Results are expressed as mg of gallic acid equivalents (GAE) per g of fresh fruit.
Total anthocyanins
The total anthocyanin content was determined using the pH differential method (Giusti and Wrolstad 2001). Samples were diluted using pH 1.0 and pH 4.5 buffers, and absorbance measurements were made at 510 and 700 nm on a Shimadzu UV-2550 UV/Vis spectrophotometer. Total anthocyanin content was calculated and expressed as cyanidin-3-glucoside (Cyd-3-glu)/100 g of fresh weight (FW), using an extinction coefficient (ε) of 26,900 L/(cm mol) and a molecular weight of 449 g/mol.
Weight loss
Approximately 200 g of fruit was weighed at days 0, 3, 6, 9 and 16. Weight loss was expressed as percentage loss of the initial weight, in triplicate.
Surface color development
Blackberry color was evaluated using a Minolta spectrophotometer CM2002 (Minolta, Japan), calibrated and attached to an appropriated device to reduce sampling area. L* (lightness), a*(redness) and b* (yellowness) values were registered after 0, 3, 6, 9 and 16 days. For each fruit three different sites were measured. Chroma and hue angle were used to indicate changes in color according to McGuire (McGuire 1992).
Hardness
Hardness was measured by nondestructive compression of the blackberries and the force per unit deformation was taken as a measure of hardness (Tetteh et al. 2004). Measurements were performed using a TA-TX Texture analyzer (Stable Micro Systems, Surrey, England) equipped with a compression cell of 5 kg and a cylindrical probe of 5 mm in diameter moving at 1 mm/s, until 3 % sample compression since at that deformation no bruising or leaking were observed. A total of 15 blackberries of each lot were used for each storage time of 0, 3, 6, 9 and 16 days. Tests were conducted in triplicate.
Visualization of fruit coating by scanning electron microscopy (SEM)
Blackberry surface was observed using a Quanta 200F environmental electron microscope (FEI, Hillsboro, Oregon, USA), using low vacuum mode with the sample in its native state.
Statistical analysis
All tests were conducted in triplicate and means ± standard deviation are reported. The means were analyzed for statistical significance (p < 0.05) using Tukey’s test from JMP statistical software, version 5.0.1.
Results
Microstructure and rheological properties of coatings
Both coating suspensions formed a continuous matrix of tapioca starch embedded with beeswax micro particles (Fig. 1). The average size of the particles within the beeswax suspension was 260.1 ± 2.5 nm, and the broadness of the size distribution as evaluated by the polydispersity index (PDI) was 0.292 ± 0.005, indicating a mixture with uniform particle size. The coating thickness was ~5–7 μm and ~7–12 μm for the WS0.5 and WS1, respectively. The micrographs also showed a continuous and smooth surface without cracks or pores. The coating was distributed over the entire scanning area, and as the beeswax concentration increased more surface area was occupied by a thicker coating (Fig. 1a and c). The small gaps shown would probably be made up with more beeswax added to the coating suspension, leading to a more efficient water vapor barrier property. This effect was observed for a beeswax-chitosan emulsion coated paper (Zhanga et al. 2014).
The flow curves showed a shear-thinning behavior, and data were fitted to the Carreau-Yasuda model, with a correlation coefficient (R) of 0.99, because of its continuity from low to high shear rates. According to Lawal et al. (2011) this model describes the fluid rheology better than the power law relationship. A Zero shear viscosity of 97.11 ± 2.84 and 89.38 ± 0.11 mPa·s as well as an infinite viscosity of 14.54 ± 0.46 and 13.36 ± 0.18 mPa·s were obtained for WS1 and WS0.5, respectively. A recovery test indicated an instantaneous viscosity recovery (Fig. 2a–b). Infinite shear viscosity and recovery tests are a good indication of the behavior of film forming suspensions (FFS) during application of spray coating and draining. Proper draining, sagging, leveling and finally coating appearance are parameters significantly affected by the flow properties (Peressini et al. 2003).
Analysis of molecular interactions of coating ingredients
The FTIR/ATR spectra of each ingredient of WS1 suspension and its resulting film were compared, and the significant peaks assigned to any one of the ingredients corresponded to those observed in the films (Table 1). This table indicates no apparent interactions of the ingredients in the cast film, as evaluated by this technique. However, Bourbon et al. (2011) analyzed by FTIR chitosan films added with different materials and reported peaks displacements higher than 20 cm−1, indicating ingredients interactions. Coating surface morphology and beeswax distribution were quite uniform (Fig. 1) indicating increased surface hydrophobicity. Muscat et al. (2014) utilized chemical maps and CH2 absorption values from synchrotron-based FTIR, contact angles values and SEM images to establish a reasonable link among the distribution of wax, the surface hydrophobicity and surface morphology (topography) of high amylose starch-glycerol-beeswax-Tween 80 films.
Effect of blackberry coatings on respiration rate and ethylene production
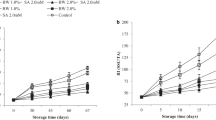
After initial equilibration, coated blackberries showed an increased respiration rate and ethylene production when compared with the uncoated counterparts (Fig. 3a–b). Average values of blackberries respiration rate were 4.207 ± 0.157, 4.557 ± 0.220 and 4.780 ± 0.050 [mmol CO2/(kg h)] for uncoated, WS0.5 and WS1, respectively. On the other hand, ethylene fluxes were 0.012 ± 0.004, 0.041 ± 0.007 and 0.058 ± 0.011 [μmol C2H4/(kg h)] for uncoated, WS1 and WS0.5 coatings. The increased respiration rate indicates that fruits were stressed by the applied coating. Ethylene production probably indicates that accelerated ripening was also triggered. This behavior has been associated with stress factors (Aguayo et al. 2006) and related to a decrease of blackberries shelf life (Perkins-Veazie et al. 2000).
Effect of coatings on volatile compounds
Tapioca starch based coatings added with beeswax microparticles apparently did not affect terpenes synthesis in blackberries which reflects the benefit of avoiding stomata occlusion (Figs. 1 and 4). Even so, both coatings showed a significant increase in volatiles such as 1-octanol, and ethanol and aldehydes for WS0.5 after 9 days (Table 2, Fig. 4), which have been related to fermentative metabolism. This behavior may be due to an accelerated ripening process triggered by coatings, leading to higher ethylene and CO2 production and some volatiles accumulation in the intracellular tissue (Amarante and Banks 2010). Berry like fruits stored under controlled atmosphere at high CO2 content are affected on their usual volatile compounds production suggesting an enhanced fermentation process (Almenar et al. 2005; Pérez-Gallardo et al. 2012b). Besides, terpenes production may continue in fruits stored at high CO2 levels, until precursors are depleted (Harb et al. 2008); however, no significant changes were found in this work (Table 2, Fig. 4). Fermentative metabolism found in coated blackberries suggests a possible negative effect on flavor.
Changes in weight and hardness of cold stored blackberries
Hardness of fruits tended to decrease with storage time but it was not significant between treatments. However, coated fruits lost more weight than their uncoated counterparts (Fig. 3d), despite the cuticle of coated blackberries was not affected by hydration as shown by SEM micrographs (Fig. 1). Weight loss of coated fruits decreased when beeswax particles content increased from 0.5 to 1 %. Lipid particles prolong the transfer distance of water molecules, because moisture migrates much faster in hydrophilic matrix than in lipid phase, and the increase in beeswax enhances the “apparent tortuosity” of the formulated coating leading to its increased water vapor barrier property (Navarro-Tarazaga et al. 2011). The process of water vapor transfer takes place initially from the fruit cuticle and then through the coating structure (Fisk et al. 2008). Here this process was facilitated by the thin starch based coating, which resulted in a decreased moisture accumulation on the fruit surface and therefore lower water vapor transfer resistance (McHugh et al. 1993), that helped to avoid cuticle hydration. The lack of interactions among ingredients of the coating material (Analysis of molecular interactions of coating ingredients) may have resulted in a little water transfer barrier, besides a probable shortage of hydrophobic material within the coating structure, allowing a reduced water transfer resistance.
The total weight loss represented 7.6 ± 0.13 % for uncoated blackberries while for coatings using WS0.5 and WS1 showed 11.55 ± 0.71 and 9.72 ± 0.42 % respectively, at the end of the experiment. This indicates that despite the lack of structural interactions of beeswax with the film matrix, an increased concentration reduces water vapor transfer, but this effect was overcome by coating induced stress. The incorporation of 5 % beeswax in starch-glycerol films led to increased hydrophobicity and randomly distributed wax within these films, and would probably decrease moisture migration when applied to fresh foods (Muscat et al. 2014). Therefore, higher proportion of beeswax in the coating formulation may improve the water barrier properties of the coating used in this study. A report on coated cherry tomatoes using hydroxypropyl methylcellulose (HPMC) containing 40 % beeswax (dry basis) resulted in either 30 % weight loss or gain, depending on the type of food preservatives added to the formulation (Fagundes et al. 2014). In another report, it is concluded that HPMC-beeswax (20 % w/w) coatings have the potential to extend the shelf life of plums, depending on coating composition (Navarro-Tarazaga et al. 2008).
Anthocyanins and total phenol changes throughout storage
Uncoated blackberries showed significantly higher anthocyanins content (p > 0.05) than coated counterparts, at any storage time, except for coated WS1 at days 6 and 9 (Fig. 5). Later, WS0.5 coated blackberries showed low but similar anthocyanins content, while those coated with WS1 showed higher values and a slight but significant decrease at the end of the storage conditions. At the end of storage time total phenols content was significantly higher in uncoated blackberries that in coated counterparts (Fig. 5).
Coatings may cause decreased oxygen levels which negatively affected anthocyanins synthesis, similarly to high CO2 fruit treatments (Romero et al. 2008). A decrease in available oxygen may also restrict phenolic compounds accumulation (Bodelón et al. 2010). Moreover, CO2 accumulation may damage internal tissues promoting oxidation of phenolic compounds by enzymatic reactions involving polyphenoloxidase and peroxidase (Duan et al. 2011). On the other hand, modification of the fruit internal atmosphere may slow down the biochemical reactions leading to anthocyanins synthesis (Tzoumaki et al. 2009).
Reduction in the anthocyanins and total phenols of coated blackberries may be attributed to the stress induced by coatings rather than hypoxia conditions due to stomata occlusion (Imahori et al. 2008). Reduction of phenolic compounds content has been found in chitosan coated strawberries (Vargas et al. 2006) and cassava starch coated blackberries (Oliveira et al. 2012) but the mechanism proposed is the modification of the internal atmosphere of the fruit.
Color changes during storage
Hue angle of uncoated blackberries increased at 3 days of storage and then it was fairly constant while in the uncoated blackberries changes in Hue° slightly decreased for all treatments (Fig. 5 a–c). These changes indicate the appearance of a reddish color. On the other hand, chroma values were constant for the storage period. Color is genetically determined and subject to changes throughout fruit ripening like variation in anthocyanins content (Han et al. 2004; Krüger et al. 2011). Hue angle reduction combined with increased anthocyanins content suggests a reduction in fruit acidity (Oliveira et al. 2012; Perkins-Veazie and Collins 2002). Acidity reduction appears in tissues where the available oxygen has decreased, which leads to consumption of the more accessible organic acids (Marsh et al. 2000). Color quantification is important because acceptance of blackberries relies on their black color which may be affected if an increase in redness appears (Gonçalves et al. 2007; Krüger et al. 2011).
Conclusions
The low percentage of starch and the increase of beeswax particles content which despite the lack of structural interactions within the coating matrix, allowed the production of thin starch based coatings exhibiting different barrier properties which did not occlude the stomata of blackberries. Besides, coatings tested preserved the integrity of the fruit cuticle and maintained their hardness.
However, coating application appears to stress the blackberry fruits evidenced by an increase in respiration rate and ethylene production. This helped to avoid significant accumulation of volatile compounds related to anaerobic metabolism but triggered the ripening processes which, in turn, increased water loss, reduced anthocyanins and phenol synthesis and caused a significant decrease of hue value.
References
Aguayo E, Jansasithorn R, Kader AA (2006) Combined effects of 1-methylcyclopropene, calcium chloride dip, and/or atmospheric modification on quality changes in fresh-cut strawberries. Postharvest Biol Technol 40:269–278
Almeida MR, Alves RS, Nascimbem LBLR, Stephani R, Poppi RJ, Oliveira LFC (2010) Determination of amylose content in starch using Raman spectroscopy and multivariate calibration analysis. Anal Bioanal Chem 397:2693–2701
Almenar E, Hernández-Muñoz P, Lagarón JM, Catalá R, Gavara R (2005) Controlled atmosphere storage of wild strawberry fruit (Fragaria vesca L.). J Agric Food Chem 54:86–91
Amarante C, Banks NH (2010) Postharvest physiology and quality of coated fruits and vegetables. Hortic Rev 26:161–238
Birla SL, Wang S, Tang J, Fellman JK, Mattinson DS, Lurie S (2005) Quality of oranges as influenced by potential radio frequency heat treatments against Mediterranean fruit flies. Postharvest Biol Technol 38:66–79
Bodelón OG, Blanch M, Sanchez-Ballesta MT, Escribano MI, Merodio C (2010) The effects of high CO2 levels on anthocyanin composition, antioxidant activity and soluble sugar content of strawberries stored at low non-freezing temperature. Food Chem 122:673–678
Bourbon AI, Pinnheiro AC, Cerqueira MA, Rocha CMR, Avides MC, Quintas MAC, Vicente AA (2011) Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J Food Eng 106:111–118
Chen J, Liu C, Chen Y, Chen Y, Chang P (2008) Structural characterization and properties of starch/konjac glucomannan. Carbohydr Polym 74:946–962
Cisneros-Zevallos L, Krochta JM (2003) Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J Food Sci 68:503–510
Dickinson E (2010) Food emulsions and foams: stabilization by particles. Curr Opin Colloid Interface Sci 15:40–49
Duan J, Wu R, Strik BC, Zhao Y (2011) Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol Technol 59:71–79
Fagundes C, Palou L, Monteiro AR, Pérez-Gago MB (2014) Effect of antifungal hydroxypropyl methylcellulose-beeswax edible coatings on gray mold development and quality attributes of cold-stored cherry tomato fruit. Postharvest Biol Technol 92:1–8
Fisk CL, Silver AM, Strik BC, Zhao Y (2008) Postharvest quality of hardy kiwifruit (Actinidia arguta ‘Ananasnaya’) associated with packaging and storage conditions. Postharvest Biol Technol 47:338–345
Galat A (1980) Study of the Raman scattering and infrared absorption spectra of branched polysaccharides. Acta Biochim Pol 27:135–142
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. John Wiley & Sons, New York, pp F1.2.1–F1.2.13
Gonçalves B, Silva AP, Moutinho-Pereira J, Bacelar E, Rosa E, Meyer AS (2007) Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem 103:976–984
Hagenmaier RD, Baker RA (1994) Wax microemulsions and emulsions as citrus coatings. J Agric Food Chem 42:899–902
Han C, Zhao Y, Leonard SW, Traber MG (2004) Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria x ananassa) and raspberries (Rubus ideaus). Postharvest Biol Technol 33:67–78
Harb J, Bisharat R, Streif J (2008) Changes in volatile constituents of blackcurrants (Ribes nigrum L. cv. ‘Titania’) following controlled atmosphere storage. Postharvest Biol Technol 47:271–279
Imahori Y, Takemura M, Bai J (2008) Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biol Technol 49:54–60
Kizil R, Irudayaraj J, Seetharaman (2002) Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J Agric Food Chem 50:3912–3918
Krüger E, Dietrich H, Schöpplein E, Rasim S, Kürbel P (2011) Cultivar, storage conditions and ripening effects on physical and chemical qualities of red raspberry fruit. Postharvest Biol Technol 60:31–37
Lawal OS, Lapasin R, Bellich B, Olajiwola TO, Cesaro A, Yoshimura M, Nishinari K (2011) Rheology and functional properties of starches isolated from five improved rice varieties from West Africa. Food Hydrocoll 25:1785–1792
Marsh K, Gonzalez P, Echeverría E (2000) PPi Formation by reversal of the tonoplast-bound H+-pyrophosphatase from ‘Valencia’ orange juice cells. J Am Soc Hortic Sci 125:420–424
McGuire RG (1992) Reporting of objective color measurements. Hortic Sci 27:1254–1255
McHugh TH, Avena-Bustillos R, Krochta JM (1993) Hydrophilic edible films: modified procedure for water vapor permeability and explanation of thickness effects. J Food Sci 58:899–903
Muscat D, Tobin MJ, Guo Q, Adhikar B (2014) Understanding the distribution of natural wax in starch–wax films using synchrotron-based FTIR (S-FTIR). Carbohydr Polym 102:125–135
Navarro-Tarazaga ML, Sothornvit R, Pérez-Gago MB (2008) Effect of plasticizer type and amount on hydroxypropyl methylcellulose-beeswax edible film properties and postharvest quality of coated plums (cv. Angeleno). J Agric Food Chem 56:9502–9509
Navarro-Tarazaga ML, Massa A, Pérez-Gago MB (2011) Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (cv. Angeleno). Food Sci Technol-LEB 44:2328–2334
Oliveira D, Kwiatkowski A, Rosa C, Clemente E (2012). Refrigeration and edible coatings in blackberry (Rubus spp.) conservation. J Food Sci Tech 1–7. doi:10.1007/s13197-012-0702-3
Patterson ME, Apel GW (1984) A computer operated controlled atmosphere research facility. HortSci 19:551
Paunov VN, Cayre OJ, Noble PF, Stoyanov SD, Velikov KP, Golding M (2007) Emulsions stabilised by food colloid particles: role of particle adsorption and wettability at the liquid interface. J Colloid Interface Sci 312:381–389
Peressini D, Bravin B, Lapasin R, Rizzotti C, Sensidoni A (2003) Starch-methylcellulose based edible films: rheological properties of film-forming dispersions. J Food Eng 59:25–32
Pérez-Gago MB, Krochta JM (2001) Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J Agric Food Chem 49:996–1002
Pérez-Gallardo A, Bello-Pérez LA, García-Almendárez B, Montejano-Gaitán G, Barbosa-Cánovas G, Regalado C (2012a) Effect of structural characteristics of modified waxy corn starches on rheological properties, film-forming solutions, and on water vapor permeability, solubility, and opacity of films. Starch-Starke 64:27–36
Pérez-Gallardo A, Mattinson SD, Lazcano-Peralta A, Fellman JK, Barbosa-Cánovas G, García-Almendárez B, Regalado C (2012b) Effect of native and acetylated-crosslinked waxy corn starch-beeswax coatings on quality attributes of raspberries during storage. Starch-Starke 64:665–673
Perkins-Veazie P, Collins JK (2002) Quality of erect-type blackberry fruit after short intervals of controlled atmosphere storage. Postharvest Biol Technol 25:235–239
Perkins-Veazie P, Clark JR, Huber DJ, Baldwin EA (2000) Ripening physiology in ‘Navaho’ thornless blackberries: color, respiration, ethylene production, softening, and compositional changes. J Am Soc Hortic Sci 125:357–363
Phan The D, Debeaufort F, Luu D, Voilley A (2005) Functional properties of edible agar-based and starch-based films for food quality preservation. J Agric Food Chem 53:973–981
Piermaria J, Bosch A, Pinotti A, Yantorno O, Garcia MA, Abraham AG (2011) Kefiran films plasticized with sugars and polyols: water vapor barrier and mechanical properties in relation to their microstructure analyzed by ATR/FT-IR spectroscopy. Food Hydrocoll 25:1261–1269
Ribeiro C, Vicente AA, Teixeira JA, Miranda C (2007) Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol Technol 44:63–70
Romero I, Sanchez-Ballesta MT, Maldonado R, Escribano MI, Merodio C (2008) Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at low temperature. J Plant Physiol 165:522–530
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Strik BC, Clark JR, Finn CE, Bañados MP (2007) Worldwide blackberry production. HortTechnol 17:205–213
Tetteh MK, Prussia SE, NeSmith DS, Verma BP, Aggarwal D (2004) Modeling blueberry firmness and mass loss during cooling delays and storage. T ASAE 47:1121–1127
Tzoumaki MV, Biliaderis CG, Vasilakakis M (2009) Impact of edible coatings and packaging on quality of white asparagus (Asparagus officinalis, L.) during cold storage. Food Chem 117:55–63
Vargas M, Albors A, Chiralt A, González-Martínez C (2006) Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol Technol 41:164–171
Yang X, Peppard T (1994) Solid-phase microextraction for flavor analysis. J Agric Food Chem 42:1925–1930
Yang H, Irudayarai J, Paradkar MM (2005) Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem 93:25–32
Zhanga W, Xiaoa H, Qianba L (2014) Beeswax–chitosan emulsion coated paper with enhanced water vapor barrier efficiency. Appl Surf Sci 300:80–85
Acknowledgments
We thank Valerie Lynch of the Franceshi Microscopy & Image center of WSU and Scott Mattinson of the Department of Horticulture and Landscape Architecture. We are also grateful to Consejo Nacional de Ciencia y Tecnología for PhD grant No. 219424 to APG, and financial support for project No.166751
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Gallardo, A., García-Almendárez, B., Barbosa-Cánovas, G. et al. Effect of starch-beeswax coatings on quality parameters of blackberries (Rubus spp.). J Food Sci Technol 52, 5601–5610 (2015). https://doi.org/10.1007/s13197-014-1665-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1665-3