Abstract
D2 gastrectomy is the globally accepted standard surgical procedure for operable gastric cancer, and lymph node (LN) dissection is considered as the critical part of radical surgery and closely related to the prognosis. The splenic hilar LN (SHLN) or level 10 are to be removed during standard D2 total gastrectomy. In situ and ex situ spleen-preserving lymphadenectomies have been the most common dissection approaches for SHLNs. No study exists which compares the outcomes of these techniques in Indian population. This study is aimed to analyse the operative outcomes of ex situ in vivo technique of spleen-preserving splenic hilar lymph node dissection in patients who underwent D2 total gastrectomy for operable proximal gastric cancer in comparison with in situ in vivo technique of splenic hilar lymph node dissection. We reviewed prospectively collected data from 17 patients with operable proximal gastric cancer between September 2016 and April 2019 who underwent D2 total gastrectomy with splenic hilar lymph node dissection and studied the preoperative demographic factors, operative and postoperative outcomes comparing the different operative techniques. Patients with oesophago-gastric junction involvement, direct splenic or other adjacent organ invasion requiring multivisceral resection and gastric stump carcinoma were excluded. Overall, 17 patients underwent D2 total gastrectomy for operable gastric cancer. Mean age of presentation was 54.7 years including 13 males and 4 females. Five patients had middle third and 12 patients had upper third cancer. All patients had splenic hilar nodal clearance as follows: in situ — 14 and ex situ — 3 patients. R0 resection was achieved in all patients. Lymph node harvest tends to be higher with lower operative time and blood loss in patients with ex situ technique compared to in situ technique with similar morbidity. Ex situ in vivo technique of spleen-preserving splenic hilar lymph node dissection can be considered as both safe and feasible procedure for operable proximal gastric cancer patients in experienced centres to achieve better lymph node yield with no significant increase in morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most common cancer and third most common cause of cancer death in the world. India has low incidence of gastric cancer but mortality rates are high due to advanced nature of the disease at presentation [1]. Surgery offers the chance of cure which yields 5-year survival of 93.2 to 5.2% according to the stage (stage I to stage IV) of tumour at presentation [2]. Radical gastrectomy with D2 lymphadenectomy is the universally accepted standard of care in these patients. Choice between subtotal, total gastrectomy and extended total gastrectomy is guided by various factors including location of the tumour, differentiation and ability to achieve adequate margin of clearance in the proximal side [3]. Splenic hilar lymph node (SHLN) carries metastatic deposits in 15 to 20% cases of proximal gastric cancers and hence mandates clearance in patients undergoing total gastrectomy as a part of D2 lymphadenectomy [4,5,6]. Of the various methods described to clear these nodes, spleen-preserving approaches, i.e. leaving spleen in vivo by either of the in situ or ex situ technique are in vogue in dedicated high-volume centres and recent reports of enhanced survival benefits with the latter method of SHLN dissection (SHLND) need to be taken note of. This study is aimed to analyse the operative outcomes of ex situ in vivo technique of spleen-preserving splenic hilar lymph node dissection in Indian patients who underwent D2 radical total gastrectomy for operable proximal gastric cancer in comparison with in situ in vivo technique of splenic hilar lymph node dissection.
Methods
Patients
We reviewed prospectively collected data from 17 patients with operable proximal gastric cancer (histologically proven adenocarcinoma) between September 2016 and April 2019 who underwent D2 total gastrectomy with splenic hilar lymph node dissection and studied the preoperative demographic factors, operative and postoperative outcomes comparing the different operative techniques. Patients with oesophago-gastric junction involvement, gastric stump carcinoma and direct splenic or other adjacent organ invasion requiring multivisceral resection were excluded.
Staging
Esophagogastroduodenoscopy and biopsy, CT scan of the abdomen and pelvis were done in all patients to diagnose and stage the disease. Preincisional staging laparoscopy was performed in all patients. Cancer staging was based on the eighth edition of the Union for International Cancer Control (UICC) TNM classification system. None of the patients in this study received neoadjuvant therapy.
Surgical Procedure
By an upper midline incision abdominal exploration was done to detect metastatic disease. Intraoperatively, the location of the tumour and the presence of serosal disease and adjacent organ involvement were assessed. Omentectomy and bursectomy were routinely done. The division of right gastroepiploic, right gastric, duodenal transection, division of left gastric vessels and oesophageal transection were done sequentially. D2 lymphadenectomy included removal of stations 1 to 12a group of lymph nodes [3].
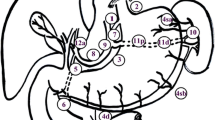
The approach of spleen-preserving SHLN dissection was at the discretion of the surgeon during the operation and splenectomy was performed for direct tumour infiltration and adherent metastatic splenic hilar lymph nodes. Ex situ splenic hilar lymph node dissection was preferred for bulkier tumours due to the perceived technical convenience in clearing the level 10 lymph nodes. In the in situ spleen-preserved group, the spleen and the pancreas were not mobilised from the retroperitoneum. Lymph nodes along the splenic artery were dissected. All the soft tissues at the splenic hilum were removed as cautiously as possible (Fig. 1). In the ex situ spleen-preserved group, splenic hilar lymphadenectomy was performed after full mobilisation of the distal pancreas and spleen. The spleen was mobilised from retroperitoneal attachments and brought forward in to the midline wound. Lymph nodes along the splenic artery and at the splenic hilum were completely dissected, with the pancreas and spleen preserved with its vascularity, and then replaced into the peritoneal cavity (Fig. 2). Reconstruction following total gastrectomy was carried out by Roux-en Y esophagojejunostomy.
Operative and Postoperative Outcomes
Postoperative surgical complications were graded according to the Clavien-Dindo classification [7] (Table 3). The operative time was measured from the time of trocar insertion to the time of abdominal closure. The amount of intraoperative blood loss was determined according to the volumes and weights of suction pumps and surgical gauze during gastrectomy. Patients who were at a disease stage >T2, N0 were administered adjuvant therapy. The adjuvant therapy included 5-fluorouracil and cisplatin.
Statistical Analysis
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented as mean and results on categorical measurements are presented in number (%). Significance is assessed at 5% level of significance. Student’s t test (two-tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (intergroup analysis) on metric parameters. Chi-square/Fisher exact test has been used to find the significance of study parameters on categorical scale between two groups. Data analysis was done by SPSS22.0 and R environment ver.3.2.2 statistical software.
Results
Demographics
Overall, 17 patients underwent D2 total gastrectomy for operable gastric cancer. Mean age of presentation was 54.7 years including 13 males and 4 females. All of the patients were of good performance status (ECOG 1 or 2). Pulmonary comorbidity, associated with smoking, was found to be the most common comorbidity (Table 1).
Pathologic Outcomes
Twelve patients had upper third and 5 patients had middle third tumours. All patients had splenic hilar nodal clearance as follows: in situ technique in 14 patients and ex situ technique in 3 patients. R0 resection was achieved in all patients. Lymph node harvest was higher in ex situ group (mean 22 nodes) when compared to in situ group (mean 17.7 nodes) but did not reach statistical significance (P = 0.122). Mean metastatic lymph node ratio (MLNR) is 0.23 in ex situ group and 0.21 in in situ group. Mean tumour size was significantly higher in ex situ group (mean 10.5 cm) than the in situ group (mean 7.6 cm) (P = 0.019) (Table 2).
Surgical Outcomes
Lower operative time (ex situ 287 min vs in situ 295 min, P = 0.612) and less blood loss (ex situ 217 ml vs in situ 286 ml, P = 0.563) was observed in ex situ group but was not statistically significant. Overall complication rate was 35.3% (6/17 patients) which included 17.7% (3/17 patients) major complications (Clavien-Dindo grade ≥3) and 5.9% (1/17 patients) in hospital mortality with no difference amongst the techniques (Table 3). Incidence of any morbidity was less in ex situ group (33.3% compared to 35.7% in situ group) though incidence of major morbidity was less in in situ group (14.3% compared to 33.3% in the ex situ group). One patient in the ex situ group had pulmonary complication requiring mechanical ventilator support for 48 h and recovered. One mortality occurred in in situ group (7.1%) due to anastomotic dehiscence of esophagojejunostomy for which emergency relaparotomy and tube oesophagostomy was done but patient succumbed to sepsis on POD 15. No difference was observed in length of ICU stay or hospital stay between two groups (Table 4).
Follow-up
All the patients are followed up with frequent outpatient visits for history and physical examination every 3–6 months, CECT chest and abdomen every 6 months for first 2 years and annually thereafter. As of December 2019, one patient in ex situ group and 9 patients in in situ group expired and one lost to follow-up and the rest of the 6 patients are alive without recurrence. Two patients in ex situ group (66.7%) and 10 patients in in situ group (71.4%) received 6 cycles of adjuvant chemotherapy of 5-fluorouracil and cisplatin.
Discussion
India falls under the low-incidence region on global gastric cancer distribution, though it stands second most common cause of cancer-related death in Indian men and women of age between 15 and 44. Distal gastric cancer is the most common in India but proximal gastric cancer contributes not less than 30%. Most of these patients present in advanced stage and surgery remains the mainstay of treatment [1].
Standard gastrectomy is the principal surgical procedure performed with curative intent which includes removal at least two thirds of the stomach with a D2 lymph node dissection. Proximal margin of 5 cm is recommended in tumours with infiltrative growth pattern. For a total gastrectomy, D0 lymphadenectomy includes anything less than D1; D1 includes dissection of level 1 to 7; D1+ includes D1 lymph nodal dissection and stations 8a, 9 and 11p; and D2 incorporates D1 lymph nodal dissection and stations 8a, 9, 10, 11p, 11d and 12a [3].
Splenic hilar lymph nodes (level 10) carry metastatic deposits in proximal gastric cancer in 15 to 20% cases and clearance of these lymph nodes offers survival benefit [4,5,6]. Japanese experience with total gastrectomy and D2 lymphadenectomy with splenectomy was with acceptable morbidity compared to Western experience and splenectomy was considered an essential part of D2 total gastrectomy. Large randomised control trial from Japan (JCOG 0110) observed that addition of splenectomy does not offer survival advantage as compared to in situ spleen-preserving SHLND in proximal gastric cancers not invading greater curvature, though total number of lymph nodes as well as splenic hilar lymph nodes were significantly higher in splenectomy group [8]. Two retrospective studies also suggest that splenectomy can be safely avoided in proximal gastric cancer invading greater curvature [9, 10]. Splenectomy is indicated when the tumour is invading the spleen directly. Hence spleen-preserving splenic hilar lymph node dissection is recommended in most of the patients requiring total gastrectomy. Dissection of No. 10 (splenic hilar lymph nodes) with or without splenectomy for cancer of the upper stomach invading the greater curvature (D2 + No. 10) is classified as a non-standard gastrectomy in latest version of Japanese gastric cancer treatment guidelines, and could be considered on the condition that it can be conducted safely. This procedure had been defined as D2 lymphadenectomy in the previous editions of the Japanese Gastric Cancer Treatment Guidelines [3, 11]. Two large retrospective studies from China that compared various methods of splenic node clearance were published recently [12, 13].
Ji et al. [12] analysed 217 patients with upper and/or middle third AGC who underwent R0 total or proximal gastrectomy with splenic hilar lymphadenectomy of whom 150 patients underwent total gastrectomy. Forty patients underwent in situ method of SHLND, 84 underwent ex situ method of SHLND and 26 underwent splenectomy as a part of D2 total gastrectomy. Number of harvested SHLNs per patient was significantly higher in the ex situ group than in the in situ group (P = 0.015) in their study but did not significantly differ in total harvested lymph nodes per patient. Postoperative hospital stay, blood loss volume and postoperative complication rate did not differ between ex situ and in situ methods. Kaplan-Meier survival analysis showed better survival outcomes with ex situ method though multivariate analysis did not show significance in terms of method of splenic hilar lymph node dissection. Authors concluded that ex situ method was more effective in dissecting SHLN compared to in situ method.
Yang et al. [13] analysed 178 patients who underwent total gastrectomy with splenic hilar lymph node clearance. In situ technique was applied in 148 patients and ex situ method applied in 30 patients. The number of total harvested lymph nodes and No. 10 lymph nodes increased significantly in ex situ group at the cost of prolonged operation time, compared to the in situ group (P < 0.05). However, there were no significant differences in terms of intraoperative estimated blood loss (P = 0.886), postoperative hospital stays (P = 0.696) and reoperation rates (P = 0.309) between the 2 groups. Although the 3-year overall survival rate for patients in the ex situ group was slightly better than that of in situ group (61.8% vs 52.0%), the estimated 5-year survival rates of in situ group and ex situ group were 45.3% and 49.5%, respectively, without statistical significance (P = 0.302). This study observed higher effectiveness of dissection of level 10 lymph nodes in ex situ group.
We found that ex situ procedure was more effective for SHLN dissection than in situ splenic-preserving procedure and did not jeopardise surgical safety. Ex situ spleen-preserving procedure allows safer and more thorough dissection under direct vision, and allowed easier identification and preservation of splenic blood vessels and leads to decreased injury to spleen and pancreas than in the in situ group, where removal of SHLNs was very difficult due to dissection in narrow and deep space near splenic hilum. Therefore, although more time was required to mobilise the spleen and pancreas tail, the time needed to dissect SHLNs was reduced as observed by the total operative time to be less in the ex situ group compared to the in situ group (P = 0.612). Similar advantages with ex situ method have been observed by Ji et al. and Chen et al. [12, 14]. Operative blood loss was also less in the ex situ group than in situ group (P = 0.563). In our study total number of lymph nodes harvested was higher in the ex situ group than in situ group (P = 0.122). Two groups showed similar morbidity rates. No complication associated with splenic hilar clearance such as splenic capsular tear, conversion to splenectomy, pancreatic fistula or splenic torsion was encountered. Single mortality in our study was due to esophagojejunostomy anastomotic leak and is not related to the choice of dissection of splenic hilar lymph nodes.
Our study also has few limitations. First, it is a retrospective study, and selection bias was difficult to avoid. The choice of lymphadenectomy procedure was decided by surgeons, who usually chose patients with larger tumours for ex situ procedure, as this method seems to be more effective means to dissect the SHLNs. The two groups did not significantly differ with regard to other clinicopathologic parameters. Second, short-term outcomes are analysed and hence survival estimates are not analysed. Third, low number of patients included in each group reduces the statistical significance in any differences observed between them.
Conclusion
D2 gastrectomy is the standard of surgical care in gastric cancer. Splenic hilar lymph nodes are dissected as an extended part of standard D2 total gastrectomy for tumours invading greater curvature of stomach. Of the various methods of achieving this goal, ex situ technique offers advantages in terms of operative and pathological outcomes though statistically not proven due to limitations of small sample size and retrospective nature of the study. Ex situ in vivo technique of spleen-preserving splenic hilar lymph node dissection can be considered as both safe and feasible procedure for operable proximal gastric cancer patients in experienced centres to achieve better lymph node yield with no significant increase in morbidity. Further large prospective randomised controlled study in multicentre setup is necessary to clarify whether ex situ technique should be considered the ideal technique for level 10 nodal clearance in proximal gastric cancer.
References
Servarayan Murugesan C et al (2018) Gastric cancer in India: epidemiology and standard of treatment. Updat Surg 70:233–239. https://doi.org/10.1007/s13304-018-0527-3
Wang W et al (2010) Prognosis of 980 patients with gastric cancer after surgical resection. Chin J Cancer 29(11):923–930
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19
Okajima K, Isozaki H (1995) Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg 19(4):537–540
Ikeguchi M, Kaibara N (2004) Lymph node metastasis at the splenic hilum in proximal gastric cancer. Am Surg 70(7):645–648
Sasada S, Ninomiya M, Nishizaki M, Harano M, Ojima Y, Matsukawa H et al (2009) Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res 29(8):3347–3351
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T et al (2016) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. https://doi.org/10.1097/SLA.0000000000001814
Nishida T, Nanashima A (2017) Significance of splenectomy for upper gastric carcinoma with invasion to the greater curvature. Int Surg 2017 102(5–6):284–292
Ohkura Y et al (2017) Efficacy of prophylactic splenectomy for proximal advanced gastric cancer invading greater curvature. World J Surg Oncol 15(1):106. https://doi.org/10.1186/s12957-017-1173-9
Japanese Gastric Cancer Association (2021) Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 24:1–21. https://doi.org/10.1007/s10120-020-01042-y
Ji X, Fu T, Bu Z et al (2016) Comparison of different methods of splenic hilar lymph node dissection for advanced upper- and/or middle-third gastric cancer. BMC Cancer 16:765. https://doi.org/10.1186/s12885-016-2814-z
Yang K, Zheng L (2015) W Zhang et al. Comparisons between different procedures of No. 10 lymphadenectomy for gastric cancer patients with total gastrectomy. Medicine 94(33):e1305. https://doi.org/10.1097/MD.0000000000001305
Chen Z, Hao H, Zhou Y, Xiang J, Cai Y (2013) Radical surgery for cardia carcinoma: total gastrectomy, D2 + No. 10 dissection, esophagojejunal Roux-en-Y anastomosis. Trans Gastrointest Cancer 2(S1):50–56. https://doi.org/10.3978/j.issn.2224-4778.2013.05.18
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalyanasundarabharathi, V.C., Kolandasamy, C., Prabhakaran, R. et al. Ex Situ In Vivo Technique of Spleen-Preserving Splenic Hilar Lymph Node Dissection in Operable Proximal Gastric Adenocarcinoma. Indian J Surg Oncol 13, 481–487 (2022). https://doi.org/10.1007/s13193-021-01487-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-021-01487-2