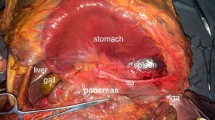
Abstract
Objective
To investigate the feasibility and safety of laparoscopic spleen-preserving splenic hilum lymph nodes (LNs) dissection for advanced proximal gastric cancer using an omnibearing method.
Methods
Between August 2013 and December 2014, 16 patients with advanced proximal gastric cancer treated in Guangdong Province Hospital of Chinese Medicine, were enrolled and subsequently underwent laparoscopic radical total gastrectomy (TG) with spleen-preserving splenic hilum LNs dissection. During dissecting Nos. 10 and 11 LNs, we divided them into two parts, namely LNs anterosuperior and posterior to the splenic vessel. The clinicopathological characteristics, intraoperative outcomes and postoperative courses were retrospectively collected and analyzed in the study.
Results
Laparoscopic surgery was successfully completed in all 16 patients without conversion to open surgery, and no perioperative death occurred. The mean operating time was 328.75 ± 46.96 min, and the mean estimated blood loss was 135.63 ± 62.07 ml. One patient experienced intraoperative bleeding due to the splenic vein injury which was successfully handled with laparoscopic vessel suturing, and one postoperative pulmonary infection was recorded. The mean time to first flatus was 3.56 ± 1.03 days with a mean 9.63 ± 1.50 days of postoperative hospital stay. The mean number of retrieved LNs was 28.31 ± 5.99, in which LNs anterosuperior to splenic artery was 2.88 ± 2.66 and LNs posterior was 1.38 ± 1.75.
Conclusion
Laparoscopic TG with spleen-preserving splenic hilum LNs dissection using an omnibearing method for advanced proximal gastric cancer was safe and technically feasible in experienced hands. Further studies in terms of its clinical significance are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is one of the most prevalent digestive tract malignancies. For advanced proximal gastric cancer, total gastrectomy (TG) with D2 lymphadenectomy is the standard surgical therapy. Apparently, lymph nodes (LNs) dissection along the splenic artery (No. 11) and the splenic hilum (No. 10) is recommended by the Japanese Gastric Cancer Treatment Guidelines [1]. Nevertheless, complete removal of the No. 10 and No. 11d LNs is technically challenging due to the tortuous splenic vessels and the high possibility of injury to the parenchyma of the spleen and pancreas. In the past decade, splenectomy with or without distal pancreatectomy was usually performed to dissect these special LNs, but high morbidity, terrible mortality and no long-term benefits prevented its clinical application.
With the development of surgical concept and the improvement of anatomical techniques, spleen-preserving No. 10 LN dissection has been gradually accepted as an alternative approach. Recently, the application of minimally invasive surgery for advanced gastric cancer is gaining popularity [2, 3]. However, laparoscopic TG with standard D2 lymphadenectomy was still not widely performed, because intracorporeal Roux-en-Y esophagojejunostomy and pancreas- and spleen-preserving splenic hilum LN dissection were mainly challenging manipulations for laparoscopic surgeons. There were a few experienced surgeons adopted the suprapancreatic approach to perform spleen-preserving splenic hilum LN dissection, while this method might not facilitate the dissection of LNs posterior to the splenic hilum.
In our study, we describe our experience of laparoscopic spleen-preserving splenic hilum LNs dissection for advanced proximal gastric cancer with an omnibearing method. Herein, the detail procedure and early results were presented.
Materials and methods
Between August 2013 and December 2014, 16 patients with proximal gastric cancer underwent laparoscopic TG with D2 lymphadenectomy at the Department of Gastrointestinal Surgery, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. The inclusion criteria were as follows: Tumor was located in the proximal of the stomach and was T2 to T4a in terms of depth of tumor invasion based on preoperative examination. Patients with a distant metastases or obvious involvement of the greater curvature and apparent nodal metastasis in the splenic hilum or along the splenic artery upon preoperative examination or/and intraoperation were excluded from this study. All patients were given details about the operative procedure and potential risks before surgery and provided written informed consent. This study protocol was approved by the ethics committee of Guangdong Province Hospital of Chinese Medicine.
Surgical procedures
The regional LNs were numbered according to the Japanese Classification of Gastric Carcinoma (JCGC) guidelines [4].
The procedure is carried out under general anesthesia with endotracheal intubation. The patient lay on the table in a supine position, with legs apart and 20° head-up tilt. The surgeon stood on the patient’s left side, the assistant stood on the patient’s right side, and the camera operator stood between the patient’s legs. CO2 pneumoperitoneum is induced after insertion of the first 10-mm trocar at the level of the umbilicus. Exploration of the abdominopelvic cavity was conducted to exclude distant metastasis. Four other working ports are inserted through the abdominal wall.
The gastrocolic ligament is divided along the border of the transverse colon using ultrasonic shears toward the pylorus. The right gastroepiploic vein and the right gastroepiploic artery were identified and ligated at its roots. Soft tissues (Nos. 4d and 6) attached to the duodenum and head of pancreas were dissected.
After overturning the gastric antrum cranially, the gastropancreatic fold was exposed. After identified the gastroduodenal artery, which was usually located in the groove between the duodenum and pancreatic head. The common hepatic artery, the proper hepatic artery, celiac trunk, splenic artery and the left gastric vessel were traced. The right gastric artery was a small branch running from the proper hepatic artery to the suprapylorus. By dissecting the tissues around the celiac trunk and its branches, the station numbers 12a, 8a, 9 and 7 were completely dissected from right to left.
LNs Anterosuperior to splenic vessel dissection
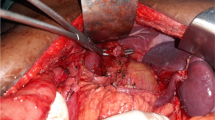
The surgeon stands between the patient’s legs. The dissection field was turned to the left toward the inferior pole of the spleen. The assistant gently overturned the stomach cephalad and kept the splenocolic ligaments, gastrosplenic ligaments under tension. The left gastroepiploic vessels are skeletonized and clamped at their roots. And then, the splenic artery and vein at the superior border of the pancreas would be exposed. The surgeon’s ultrasonic scalpel’s non-functional face closes the surface of the splenic hilum area. Starting closed to the inferior splenic pole, and continued in cephalad direction, the surgeon carefully preserved the terminal branches of the splenic vessels during dissected the perivascular tissue. And then, the splenic artery sheath was opened close to the pancreatic tail and dissected from distal portion to proximal. The anterosuperior lymphatic fatty tissue of No. 11d and No. 10 were dissected thoroughly (Fig. 1).
The duodenum was transected 2 cm distal to the pylorus using an endoscopic linear stapler. The esophagus is transected using an endoscopic linear stapler. Then, the specimen was extracted through the midline minilaparotomy incision in the epigastrium.
LNs posterior to splenic vessel dissection
The surgeon turned to the patient’s right. By dissecting the soft tissue between the retroperitoneum and the posterior aspect of the pancreatic tail, the distal pancreas can be mobilized (Fig. 2). After entering the retropancreatic space, the splenic vein was identified in the confluence of the veins splenic and inferior mesenteric. By opening the artery sheath and skeletonizing the splenic artery and vein from the proximal portion toward the distal portion, the LNs posterior to splenic vessel could be removed. In this procedure, the great pancreatic artery and the artery of pancreatic tail should be saved. Finally, the LN No. 11 and No. 10 were completely removed and all vessels in the splenic hilum area were saved (Fig. 3).
Then, the LNs located in lesser gastric curvature were dissection. A Roux-en-Y esophagojejunostomy were carried out intracorporeally using a circular stapler. An end-to-side jejunojejunostomy was performed.
Results
The clinicopathological characteristics, surgical outcomes and postoperative courses of the patients are shown in Table 1. There are 10 male and 6 female patients, with a mean age of 61.25 ± 8.21 years. The mean body mass index was 21.94 ± 2.66 kg/m2.
Laparoscopic TG with spleen-preserving splenic hilum dissection was successfully performed in all 16 patients without conversion to open procedure, and no perioperative death was recorded. One patient experienced intraoperative bleeding due to the splenic vein injury which was successfully handled with vessel saturation, and one case of postoperative pulmonary infection was cured by antibiotics. The mean operation time was 328.75 ± 46.96 min, and the mean estimated blood loss was 135.63 ± 62.07 ml. The mean time to first flatus was 3.56 ± 1.03 days with a mean 9.63 ± 1.50 days of postoperative hospital stay. The mean tumor size was 3.74 ± 1.33 cm. The mean number of retrieved LNs was 28.31 ± 5.99, in which LNs anterosuperior to splenic artery was 2.88 ± 2.66 and LNs posterior to splenic artery was 1.38 ± 1.75. Metastasis in LNs anterosuperior to splenic vessel was recorded in two patients (12.5 %), and one patient (6.25 %) occurred metastasis in LNs posterior.
Discussion
With the development of minimally invasive technique and instruments, laparoscopic distal gastrectomy for the distal gastric cancer has become more popular. However, only a few surgeons, who have acquired much experience in laparoscopic gastrectomy, perform laparoscopic total gastrectomy with D2 lymphadenectomy, because of the associated technical difficulties and concerns about splenic hilum LNs dissection. Herein, we introduced an omnibearing method to perform laparoscopic radical TG with spleen-preserving splenic hilum LNs dissection and our initial results were accepted.
In our study, the operation time was longer than other similar reporters [5–8]. The reason may be that we did not reach the platform of the learning curve. However, the estimated blood loss, the number of dissected LNs and the morbidity were satisfactory. Therefore, laparoscopic spleen-preserving splenic hilum LNs dissection with omnibearing method is technically feasible and safe. The procedure should be performed by surgeons who are experienced in open surgery and skilled in laparoscopic surgical techniques. During dissecting Nos. 10 and 11 LNs, we divided them into two parts, namely LNs anterosuperior and posterior to the splenic vessel, and they were pathologically tested, respectively. Moreover, there was no report about the clinical significance of dissection of LNs posterior to splenic vessel.
Lymph node metastasis is an important factor in the prognosis of gastric cancer. Sasada et al. [9] have reported that station No. 10 LN metastasis was a significant factor affecting the prognosis of gastric cancer. The dissection of splenic hilum LNs in gastric cancer surgery is indispensable for treating advanced proximal gastric cancers. Traditionally, TG and pancreas-preserving splenectomy has been recommended as a curative procedure for standard D2 dissection by some investigators [10–12]. However, many studies [13–17] have reported that TG with splenectomy has a high morbidity and mortality compared with TG alone, and has no benefit on patient survival. As an alternative, spleen-preserving splenic hilum LN dissection might decrease postoperative morbidity without compromising oncological principles.
Theoretically, the best option for a patient with advanced gastric cancer requiring TG is to achieve D2 LN dissection without splenectomy. However, spleen-preserving D2 LN dissection is not a simple technique, even under open condition, because of the tortuous splenic vessels and the high possibility of injury to the parenchyma of the spleen and pancreas.
Laparoscopic techniques offers important advantages when compared with open surgery for early gastric cancer: reduced intraoperative blood loss, accelerated recovery, earlier resumption of oral intake, earlier return to normal bowel function, early discharge from hospital and lower financial costs [2, 18, 19]. The same advantages have been reported after laparoscopic subtotal gastrectomy for advanced gastric cancers [20–22]. Hyung et al. [8] firstly reported the application of laparoscopic spleen-preserving No. 10 LN dissection during radical TG of proximal gastric cancer. In recent years, some investigators [23–25] have performed a D2 extended lymphadenectomy with pancreas and spleen preservation during laparoscopic total gastrectomy and showed some potential benefits.
The major difficulties of this laparoscopic procedure lie in the wide variations in the distribution of the splenic vessels and the shape of the pancreatic parenchyma among patients. This variation may increase the possibility of bleeding from branches of the splenic vessels and the postoperative pancreatic fistula. The number of splenic lobar vessels and the distance between pancreatic tail and splenic hilum should be watched when dissecting the LNs anterosuperior to splenic vessel. In general case, splenic artery divided into terminal branches in the fold of lienorenal ligament before entering into the splenic hilum. Pandey et al. [26] reported that the number of terminal branches was variable with two branches observed in 202 (63.1 %) cadavers, four branches in 60 (18.8 %), six branches in 31 (9.7 %) and more than six branches in 18 (5.6 %) cadavers. The more number of branch of splenic lobar artery, the more difficult of the dissection of splenic hilum LNs would be. It is required to distinguish between fat tissue and pancreatic tissue carefully. Furthermore, in our initial experience, the traction of splenic vessels using an elastic band could make it easier and safer to dissect the LNs around the splenic vessels. Surgeons who want to try laparoscopic dissection for splenic hilum LNs should consider the various methods available, such as a left approach [5] or a medial approach [8].
The dissection of posterior LNs of No. 11 and No. 10 was more difficult than anterosuperior side. The retropancreatic space was narrow, and the pancreas cannot be grasped, making it difficult to complete the dissection thoroughly. The use of suction/irrigation tube is our strategy. When the pancreas was pulled up toward the upper left with the aspirator, the splenic vessels will be visible. Open the artery sheath and skeletonize the splenic vessel from the proximal portion toward the distal portion with the harmonic scalpel. During the procedure, keep the surgery field clear with the suction/irrigation tubes’ function of electrocoagulation and attraction. Furthermore, always keep ultrasonic scalpel’s non-functional face closes the surface of the tissue. It is an efficient way to prevent injuring the splenic vessels and parenchyma of the spleen and pancreas. Furthermore, complete removal of the splenic LNs, part of which is located behind the pancreas, is easier by our approach than by an approach from the anterior side.
Part of No. 10 and No. 11 LNs was located behind the pancreas. It is easy to completely remove these LNs with our approach. The posterior LNs of No. 10 and No. 11 were dissected and tested alone. Studies revealed that the frequency of LN metastasis to No. 10 in proximal gastric cancer was 9–20.9 % [9, 27–29]. In our study, two out of 16 patients had splenic hilum LN metastasis (12.5 %). Only one out of 16 patients had posterior LNs of No. 11 and No. 10 metastasis (6.25 %). There were no study about the significance of posterior LNs of No. 11 and No. 10 metastasis. The future study of the significance of posterior LNs of No. 11 and No. 10 metastasis is needed.
Our retrospective study had several limitations, including relatively small sample size and did not report the long-term oncology outcomes. However, surgeons who try to conduct similar laparoscopic surgery can refer to our detailed procedure.
In conclusion, our initial results suggested that, in experienced hands, laparoscopic radical TG with spleen-preserving splenic hilum LNs dissection for proximal advanced gastric cancer with an omnibearing method is safe and technically feasible.
References
Japanese Gastric Cancer A (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123
Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251(3):417–420
Son SY, Kim HH (2014) Minimally invasive surgery in gastric cancer. World J Gastroenterol 20(39):14132–14141
Japanese Gastric Cancer A (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112
Wang JB, Huang CM, Zheng CH, Li P, Xie JW, Lin JX (2012) Laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer in left approach: a new operation procedure. World J Surg Oncol 10:241
Mou TY, Hu YF, Yu J, Liu H, Wang YN, Li GX (2013) Laparoscopic splenic hilum lymph node dissection for advanced proximal gastric cancer: a modified approach for pancreas- and spleen-preserving total gastrectomy. World J Gastroenterol 19(30):4992–4999
Lee JH, Ahn SH, Park DJ, Kim HH, Lee HJ, Yang HK (2012) Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg 36(10):2394–2399
Hyung WJ, Lim JS, Song J, Choi SH, Noh SH (2008) Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 207(2):e6–e11
Sasada S, Ninomiya M, Nishizaki M, Harano M, Ojima Y, Mastukawa H, Aoki H, Shiozaki S, Ohno S, Takakura N (2009) Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res 29(8):3347–3351
Kitamura K, Nishida S, Ichikawa D, Taniquchi H, Haqiwara A, Yamaquchi T, Sawai K (1999) No survival benefit from combined pancreaticosplenectomy and total gastrectomy for gastric cancer. Br J Surg 86(1):119–122
Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, Okajima K (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 19(4):532–536
Doglietto GB, Pacelli F, Caprino P, Bossola M, Di Stasi C (2000) Pancreas-preserving total gastrectomy for gastric cancer. Arch Surg 135(1):89–94
Brady MS, Rogatko A, Dent LL, Shiu MH (1991) Effect of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg 126(3):359–364
Adachi Y, Kamakura T, Mori M, Maehara Y, Sugimachi K (1994) Role of lymph node dissection and splenectomy in node-positive gastric carcinoma. Surgery 116(5):837–841
Okajima K, Isozaki H (1995) Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg 19(4):537–540
Lee KY, Noh SH, Hyung WJ, Lee JH, Lah KH, Choi SH, Min JS (2001) Impact of splenectomy for lymph node dissection on long-term surgical outcome in gastric cancer. Ann Surgical Oncol 8(5):402–406
Weitz J, Jaques DP, Brennan M, Karpeh M (2004) Association of splenectomy with postoperative complications in patients with proximal gastric and gastroesophageal junction cancer. Ann Surg Oncol 11(7):682–689
Vinuela EF, Gonen M, Brennan MF, Coit DG, Strong VE (2012) Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 255(3):446–456
Nakamura K, Katai H, Mizusawa J, Yoshikawa T, Ando M, Terashima M, Ito S, Takaqi M, Takaqane A, Ninomiya M, Fukushima N, Sasako M (2013) A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer (JCOG0912). Jpn J Clin Oncol 43(3):324–327
Nam BH, Kim YW, Reim D, Eom BW, Yu WS, Park YK, Ryu KW, Lee YJ, Yoon HM, Lee JH, Jeong O, Jeong SH, Lee SE, Lee SH, Yoon KY, Seo KW, Chung HY, Kwon OK, Kim TB, Lee WK, Park SH, Sul JY, Yang DH, Lee JS (2013) Laparoscopy assisted versus open distal gastrectomy with D2 lymph node dissection for advanced gastric cancer: design and rationale of a phase ii randomized controlled multicenter trial (COACT 1001). J Gastric Cancer 13(3):164–171
Moisan F, Norero E, Slako M, Varas J, Palominos G, Crovari F, Ibañez L, Pérez G, Pimentel F, Guzmán S, Jarufe N, Boza C, Escalona A, Funke R (2012) Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: a matched cohort study. Surg Endosc 26(3):661–672
Chen K, Xu XW, Mou YP, Pan Y, Zhou YC, Zhang RC, Wu D (2013) Systematic review and meta-analysis of laparoscopic and open gastrectomy for advanced gastric cancer. World J Surg Oncol 11:182
Jeong O, Jung MR, Kim GY, Kim HS, Ryu SY, Park YK (2013) Comparison of short-term surgical outcomes between laparoscopic and open total gastrectomy for gastric carcinoma: case–control study using propensity score matching method. J Am Coll Surg 216(2):184–191
Guan G, Jiang W, Chen Z, Liu X, Lu H, Zhang X (2013) Early results of a modified splenic hilar lymphadenectomy in laparoscopy-assisted total gastrectomy for gastric cancer with stage cT1-2: a case–control study. Surg Endosc 27(6):1923–1931
Bo T, Peiwu Y, Feng Q, Yongliang Z, Yan S, Yingxue H, Huaxing L (2013) Laparoscopy-assisted vs. open total gastrectomy for advanced gastric cancer: long-term outcomes and technical aspects of a case–control study. J Gastrointest 17(7):1202–1208
Pandey SK, Bhattacharya S, Mishra RN, Shukla VK (2004) Anatomical variations of the splenic artery and its clinical implications. Clin Anat 17(6):497–502
Yu W, Choi GS, Chung HY (2006) Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 93(5):559–563
Monig SP, Collet PH, Baldus SE, Schmackpfeffer K, Schröder W, Thiele J, Dienes HP, Hölscher AH (2001) Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol 76(2):89–92
Maruyama K, Gunven P, Okabayashi K, Sasako M, Kinoshita T (1989) Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg 210(5):596–602
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Wei Wang, Zhiwei Liu, Wenjun Xiong, Yansheng Zheng, Lijie Luo, Dechang Diao and Jin Wan have no conflicts of interest or financial ties to disclose.
Additional information
Wei Wang and Zhiwei Liu contributed equally to this work in the design of the study and preparation of the manuscript and should be both considered as co-first authors. Wenjun Xiong and Jin Wan contributed equally to this work in the technique design, materials collection, analysis, interpretation of data and revising the article, they should be the co-correspondence authors.
Rights and permissions
About this article
Cite this article
Wang, W., Liu, Z., Xiong, W. et al. Totally laparoscopic spleen-preserving splenic hilum lymph nodes dissection in radical total gastrectomy: an omnibearing method. Surg Endosc 30, 2030–2035 (2016). https://doi.org/10.1007/s00464-015-4438-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4438-9