Abstract
Understanding how wetland plant communities are organized in different ecosystems and spatial scales is essential to support conservation. Studies that have addressed this primary need, however, are exceptionally scarce. Here, we compared the diversity (alpha, beta and gamma) and composition of herbaceous and woody communities (treated separately and jointly) in ponds, streambanks and riverbanks in the upper Uruguay River Basin, southern Brazil. Results showed that each wetland ecosystem exhibited unique community patterns, depending on the plant group, data property (e.g., presence-absence or abundance), and community parameter. All ecosystem types had exclusive species, beta diversity explained by high rates of species turnover and balanced variation in abundance components, and particular floristic composition, revealing that each wetland type and site contributes fundamentally to the overall wetland plant diversity. These findings indicate that safeguarding wetland plant diversity depends on the adoption of holistic conservation measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands are key reservoirs of plant diversity, harboring unique, rare and endangered species and singular communities (Naiman et al. 1993; Davies et al. 2008; Oliveira-Filho et al. 2021). Specific wetland types share a particular range of abiotic features (e.g., hydrological, physicochemical, geomorphological, and spatial) and thus tend to exhibit unique plant diversity and composition patterns (Williams et al. 2004; Keddy 2010; Draper et al. 2018). Understanding how wetland plant communities are organized in different ecosystems and spatial scales is therefore crucial to support conservation (Williams et al. 2004; Socolar et al. 2016; Darwall et al. 2018).
Studies that have addressed this fundamental need, however, are exceptionally scarce. Most research has traditionally been carried out either only in lotic or lentic wetlands (or subtypes of them) such as riparian areas (e.g., Nilsson et al. 1994; Rheinhardt et al. 2013; Kuglerová et al. 2016) or ponds (e.g., Brose 2001; Rolon et al. 2012; Fernández-Aláez et al. 2020). The few contributions that collectively evaluated at least most of the variety of lentic and lotic wetlands at the regional level have only limited applicability, because they either: (i) contemplated only woody species and ecosystems with atypical characteristics in many regions of the world (e.g., tree communities in large swamps and floodplains; e.g., Pitman et al. 2014; Draper et al. 2018); (ii) focused only on “aquatic habitats” (e.g., without considering riparian areas; e.g., Davies et al. 2008; Bubíková and Hrivnák 2018); or (iii) adopted a posteriori wetland classification (e.g., Flinn et al. 2008), offering little support for tools that use a priori classifications. Thus, information is still insufficient about community patterns of different wetland types in a landscape context.
Here, our main goal was to compare the diversity and composition of plant communities (herbaceous and woody; treated separately and jointly) among freshwater wetlands in the upper Uruguay River Basin, Southern Brazil, where small and/or narrow wetlands predominate. To cover diversity across scales, we assessed both alpha (local), beta (among-site) and gamma (regional) diversity. We examined the three most common natural wetland types in the region (defined a priori): ponds (small, upland-embedded wetlands), streambanks and riverbanks (riparian areas alongside streams and rivers, respectively). We hypothesized that each wetland type shows singular patterns of plant diversity and composition. To our knowledge, this is the first time plant community patterns are contrasted among the above-mentioned wetland types.
Materials and Methods
Study Areas
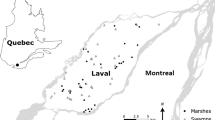
The study was conducted in a transitional region between the semi-deciduous seasonal forest and the evergreen seasonal Araucaria forest (Oliveira-Filho et al. 2015) in the upper Uruguay River Basin, Santa Catarina State, Southern Brazil (Fig. 1). The climate in the region is subtropical humid without a pronounced dry season (Alvares et al. 2014). The annual means of temperature and rainfall are 18–20 °C and 1,900–2,000 mm, respectively (Wrege et al. 2012). Soils and substrates originate from basaltic rocks and are mostly eutrophic (IBGE 1990; Cunha et al. 2006; Giehl and Jarenkow 2008), but those within and nearby ponds tend to be dystrophic (Grasel et al. 2020).
Across the landscape, we first identified three predominant natural wetland types: ponds, streambanks and riverbanks. Afterwards, we selected eight study areas per wetland type adopting the following criteria: (i) no evidence of anthropogenic habitat alteration; (ii) no sign of recent natural resource exploitation; (iii) relatively small percentage of area occupied by intensive land use activities within a 100 m radius; and (iv) minimum distance of 1,500 m between study areas (Fig. 1; Table S1). For all cases, we adopted Junk et al.’s (2014) definition and delineation proposal of Brazilian wetlands. The operational definitions and main features of the selected wetlands are presented in Table 1 and images of some are provided in Fig. S1.
Vegetation Sampling
We sampled all herbaceous species and ≥ 0.3–≤ 1 m high plants of woody species (except bryophyte, climber, and epiphyte species) – i.e., plants that roughly constitute the component commonly called “ground flora” (Goebel et al. 2012) or “herb layer” (Santos-Junior et al. 2018) – using the line intercept method (Canfield 1941; Munhoz and Araújo 2011). In each study area, we surveyed the species’ coverage (measured in cm) in 40 linear meters (i.e., 320 linear meters per wetland type) by establishing linear transects arranged parallel and equidistantly in 30 m long stretches. For each wetland type, we defined a specific sampling design based on their abiotic and biotic particularities (see above). In streambanks and riverbanks, 40 and 10 transects of 1 and 4 m were established perpendicular to the watercourses (to their left or right, starting at the stream/riverside), respectively – streams and rivers were not sampled because of the absence of plants meeting the inclusion criteria. In ponds, we applied the same transect organization adopted for riverbanks, but the sampling effort was equally divided into two areas that best represented the ecodiversity – e.g., treeless and forested patches – in order to maximize species detection (Neiff 2001; Davies et al. 2008). All surveys were conducted during the summer of 2016–2017 (December–March). Species were identified through specialized bibliography, comparisons with exsiccates of the ICN Herbarium of the Universidade Federal do Rio Grande do Sul, and expert consultations. Family names follow APG IV (2016) and PPG I (2016), while species nomenclature is based on Flora do Brasil (2020) and Flora Argentina (2021).
As we inventoried only wetlands, all sampled plants, herbaceous or woody, were considered to belong to wetland species, regardless of their abundance and frequency in wetland or terrestrial ecosystems. Our criteria are in consonance with Cronk and Fennessy’s (2001) proposal, which also considers that the term “wetland plants” is synonymous with “hydrophytes” and “macrophytes” – the authors restricted their treatment to vascular species, as is the case here. We also adopted Cronk and Fennessy’s (2001) classification of hydrophytes into life forms, i.e., emergent, submerged, floating-leaved and floating macrophytes.
Data Analysis
First, we used spline correlograms to test for spatial correlation of the study areas’ main data employed in the analyses described below – for the procedures and results, see Appendix S1. Since these tests pointed out no significant spatial correlation, all samples were taken as true replicates. We then investigated whether ponds, streambanks and riverbanks differed in their community parameters. To better comprehend the patterns of plant diversity and floristic composition, we treated herbaceous and woody species separately and together. Shrub and tree species were not analyzed separately because most ponds showed very few or no individuals of one and/or the other growth form. When applicable, species coverage, which in our case is a continuous variable, was used as a measure of abundance. Exotic species were excluded from all analyses to better describe native diversity; as these species covered only a small percentage of the transects’ total length (3.4 %), we believe that they have not decisively influenced our findings.
For descriptive purposes, we computed the absolute coverage, absolute frequency and importance value (Kent 2012) for each species in each wetland type.
Alpha diversity was assessed by four indices fitting the Hill series (Hill 1973; Morris et al. 2014) by increasing the weights given to dominant species: richness (q = 0, or zero weight to abundance), exponential of Shannon’s index (q = 1), Simpson’s inverse index (q = 2) and Berger-Parker’s inverse index (q = infinity). To compare species diversity among wetland types, we used one-way analyses of variance (ANOVAs) and pairwise comparisons. The assumptions of normality of residuals and homogeneity of variances were assessed through Shapiro-Wilk’s and Levene’s tests, respectively; some data were log-transformed to meet these premises. Data that showed normal and homoscedastic distribution were compared though parametric tests – type-II ANOVAs and Tukey’s HSD pairwise tests –, while non-parametric tests – Kruskal-Wallis ANOVAs and Dunn’s pairwise comparisons with P-values adjusted by the Holm method – were used for variables that did not meet at least one of the above-mentioned assumptions. Following the same rationale of diversity profiles, a given wetland type was considered more diverse than its counterparts regarding any species group only when showing higher values for the entire series of diversity estimates (Melo 2008; Chao and Jost 2015).
Beta diversity was compared using permutational analyses of multivariate dispersions (PERMDISPs) with post-hoc tests, where we used 9,999 permutations to assess significance and the Holm correction for multiple comparisons (Anderson 2006; Anderson et al. 2006). Such analyses were performed both for presence-absence and abundance data, for which we used Jaccard dissimilarity and Hellinger distance matrices, respectively. Additionally, to examine the subjacent processes driving species composition dissimilarity, we partitioned total beta diversity of each wetland type in two ways. First, Jaccard dissimilarities based on presence-absence data were partitioned in turnover (species replacement among sites) and nestedness (species richness differences among sites when poor sites show subsets of species from richer sites) components (Baselga 2012). Second, Ruzicka dissimilarities were partitioned in balanced variation in abundance and abundance gradients components (the abundance-based analogues to the turnover and nestedness components, respectively; Baselga 2017).
Gamma diversity was compared through diversity profiles expressed as Hill numbers (Hill 1973; q = 0–5), in line with our alpha diversity assessment strategy. These profiles were built with 95 % confidence intervals and computed according to Chao and Jost’s (2015) proposal.
Species composition was compared through permutational multivariate analyses of variance (PERMANOVAs) (Anderson, 2001) with pairwise contrasts using 9,999 permutations to assess significance and the same correspondent distance/dissimilarity matrices employed in PERMDISPs; P-values resulting from post-hoc tests were adjusted by the Holm method. Since PERMANOVAs and pairwise comparisons may be significant due to the sample groups’ location and/or dispersion, we used the results of PERMDISPs and post-hoc tests – and graphs; see below – to interpret the resulting patterns (Anderson et al. 2008). Complementarily, we examined the wetlands’ community composition uniqueness following the method of Legendre and De Cáceres (2013), which consists in partitioning total beta diversity into local contributions (LCBDs). LCBDs were computed using both species presence-absence and coverage data and the Jaccard and Hellinger dissimilarity/distance, respectively. We then investigated whether wetland types differed in their LCBDs using ANOVAs and post-hoc tests (see the analyses routine above).
Lastly, we built graphs to visualize patterns tested with ANOVAs, PERMDISPs, PERMANOVAs and pairwise tests: boxplots were constructed for alpha diversity parameters, distances to spatial median resulted from PERMDISPs and LCBDs; and non-metric multidimensional scaling (NMDS; McCune and Grace, 2002) ordinations (optimized for two dimensions) were computed based on matrices with species presence-absence and coverage data and the Jaccard and Hellinger dissimilarity/distance, respectively. To better visualize the patterns of beta diversity (multivariate spread), spider diagrams connecting sites to their sample groups’ spatial median were plotted into the NMDSs. Also, to better show the wetlands’ contribution to the investigated patterns of species composition uniqueness, LCBDs were fitted and plotted as smooth surfaces and vectors on the NMDSs.
All analyses were performed in R (R Core Team 2020) using packages ‘SpadeR’ (Chao et al. 2016), ‘lawstat’ (Gastwirth et al. 2020), ‘betapart’ (Baselga et al. 2020), ‘FSA’ (Ogle et al. 2020), ‘vegan’ (Oksanen et al. 2020) and ‘adespatial’ (Dray et al. 2020).
Results
We sampled a total of 177 species pertaining to 128 genera and 59 families (Table S2). The number of exclusive and shared species per plant group for the wetland types is shown in Fig. S2. The species with the greater importance values were: Luziola peruviana, Trichanthecium schwackeanum, Rugoloa polygonata, Commelina obliqua and Hygrophila costata in ponds; Didymochlaena truncatula, Lastreopsis effusa, Dennstaedtia globulifera, Ctenanthe muelleri and Ctenitis submarginalis in streambanks; and Goniopteris riograndensis, Ocellochloa stolonifera, Ctenitis submarginalis, Ruellia angustiflora and Ctenanthe muelleri in riverbanks (Table S2). All species are emergent, except Lemna valdiviana (sampled in ponds), which is floating.
Alpha Diversity
Streambanks and riverbanks were alike in all alpha diversity metrics for all plant groups, while ponds showed the lowest indices in general (Fig. 2a–c; Table S3). However, as higher weights were given to more abundant species, ponds remained less diverse than lotic environments in relation to woody species only (Fig. 2a–c; Table S3; see, e.g., q = infinity).
Boxplots for alpha diversity parameters in ponds (Ps), streambanks (Ss) and riverbanks (Rs) in the upper Uruguay River Basin, Southern Brazil. Boxes show the 25th and 75th percentiles, medians (thick lines) and means (black dots), while staples indicate the smallest and highest values (excluding outliers). Outliers are shown as hollow circles. Different letters above the top staples within each species group and diversity parameter indicate significant differences (P ≤ 0.05) according to Tukey’s HSD or Dunn’s pairwise comparisons (see Table S3). q = 0, 1, 2 and inf. (infinity) correspond to richness, the exponential of Shannon’s index, the Simpson’s inverse index and the Berger-Parker’s inverse index, respectively
Beta Diversity
Beta diversity of herbaceous species differed among all wetland types and showed the following pattern, both for presence-absence and coverage data: ponds > riverbanks > streambanks (Fig. 3a,d,g; Table S4). For woody species, no difference was observed (Fig. 3b,e,h; Table S4). When the global set of species was analyzed, beta diversity for presence-absence data was higher in ponds than in the two riparian ecosystems only, while beta diversity for coverage data was similar between ponds and riverbanks and higher in both when compared to streambanks (Fig. 3c,f,i; Table S4). Total beta diversity in all cases was almost entirely explained by species turnover and balanced variation in abundance components instead of nestedness and abundance gradients components (Fig. S3).
Beta diversity patterns in ponds (Ps), streambanks (Ss) and riverbanks (Rs) in the upper Uruguay River Basin, Southern Brazil. a–c) Boxplots for distances to spatial median resulted from permutational analyses of multivariate dispersions. Boxes show the 25th and 75th percentiles, medians (thick lines) and means (black dots), while staples indicate the smallest and highest values (excluding an outlier, shown as a hollow circle). Different letters above the top staples indicate significant differences (P ≤ 0.05) according to PERMDISP post-hoc tests (see Table S4). d–i) Non-metric multidimensional scaling (NMDS) ordinations based on species presence-absence (Jaccard dissimilarity; d–f) and coverage data (Hellinger distance; g–i). Spider diagrams connect sites to their sample groups’ spatial median
Gamma Diversity
Gamma diversity of herbaceous species was higher in ponds and riverbanks than in streambanks, whereas the first two showed similar diversity (Fig. 4a). Woody species were less diverse in ponds than in streambanks and riverbanks, which in turn showed overlapping diversity profiles (Fig. 4b). When all species were considered, gamma diversity was greater in riverbanks than in ponds and streambanks, while the last two had similar diversity (Fig. 4c).
Gamma diversity patterns in ponds (Ps), streambanks (Ss) and riverbanks (Rs) in the upper Uruguay River Basin, Southern Brazil, showed through diversity profiles. Shaded areas indicate the 95 % confidence intervals. q = 0, 1 and 2 correspond to richness, the exponential of Shannon’s index and the Simpson’s inverse index, respectively
Ponds exhibited most exclusive herbaceous species and also the largest number of unique species of the global set of species, while riverbanks showed the highest number of exclusive woody species (see more details in Fig. S2).
Species Composition
The composition of herbaceous and woody species considered separately and together differed among all wetland types, regardless of the data type (presence-absence or abundance) and the sample groups’ multivariate dispersions (Fig. 5a–f; Table S4). Ponds showed the highest floristic uniqueness regarding all plant groups and data types, while the two riparian ecosystems showed similar LCBDs, except for herbaceous species, whose composition was more singular in riverbanks than in streambanks (Fig. 5; Table S5).
Floristic composition patterns in ponds (Ps), streambanks (Ss) and riverbanks (Rs) in the upper Uruguay River Basin, Southern Brazil. a–f) Non-metric multidimensional scaling (NMDS) ordinations based on species presence-absence (Jaccard dissimilarity; d–f) and coverage data (Hellinger distance; g–i). Smooth surfaces and vectors are based on samples local contributions to beta diversity (LCBDs). g–i) Boxplots for LCBDs. Boxes show the 25th and 75th percentiles, medians (thick lines) and means (black dots), while staples indicate the smallest and highest values (excluding outliers). Outliers are shown as hollow circles. Different letters above the top staples within each species group and diversity parameter indicate significant differences (P ≤ 0.05) according to Tukey’s HSD pairwise comparisons (see Table S5)
Discussion
Results showed that, in general, each wetland type presented singular diversity and composition patterns, corroborating our expectation. However, when the wetland types’ features are assessed in detail, their uniqueness depends on the plant group, data property (e.g., presence-absence or abundance), and community parameter. Bellow, we discuss all these particularities.
Overall, our spatial correlation analyses suggest that niche processes, rather than dispersal limitation, essentially determined the revealed community patterns, probably due to both the relatively small spatial scale of our study and particularly to the great dispersion capacity of hydrophyte species (Santamaría 2002; Soons 2006). In fact, many studies have shown that, on spatial scales similar or even larger compared to that of our study region, dispersal limitation had little or no effects on macrophyte communities’ patterns in both lentic and lotic systems, unlike, e.g., environmental variables (e.g., Brose 2001; Alahuhta et al. 2013, 2015; Fernández-Aláez et al. 2020; Lansac-Tôha et al. 2020; but see, e.g., Rolon et al. 2012; Padial et al. 2014).
Alpha Diversity
Among the three wetland types, the two riparian ecosystems tended to show the highest values of all alpha diversity indices, which is most likely linked to their habitat complexity. For instance, streambanks and riverbanks had the highest elevation ranges, implying in spatially variable soil waterlogging or inundation regimes that, in turn, have direct and uneven impacts on physico-chemical properties – e.g., through irregular water supply and sediment deposition (Wittmann et al. 2004, 2017; Budke et al. 2007). These ecosystems thus show well-known characteristics for boosting alpha diversity, as environmental heterogeneity and gradients of disturbance and resource availability (Pollock et al. 1998; Lite et al. 2005; Budke et al. 2010). The two riparian wetland types, however, did not show statistical differences, although riverbanks tended slightly to present the highest diversity in general. The local diversity of vascular plant species in riparian areas has previously been shown to gradually increase from headwaters to downstream on relatively small spatial scales (Gould and Walker 1997; Kuglerová et al. 2015, 2016) or to show a unimodal pattern on relatively large spatial scales (Nilsson et al. 1989; Renöfält et al. 2005) – albeit severe floods may temporarily change this trend (see Renöfält et al. 2005). This continuous increase in diversity, at least until riparian areas alongside watercourses at intermediate distances from headwaters in relatively large lotic systems, was explained, e.g., by the associated increase in environmental heterogeneity, flood disturbance, nutrient availability, and sedimentation (Nilsson and Jansson 1995; Gould and Walker 1997; Kuglerová et al. 2015). As the riparian areas studied by us are predominantly narrow and adjacent to small watercourses, we believe that the changes in environmental conditions from streambanks to riverbanks may have been too small to generate a significant increase in local diversity.
Ponds, in turn, had overall the lowest alpha diversity indices, although their diversity patterns of herbaceous and woody species differed sharply from those of the other wetland types. The herbaceous component in ponds was similarly diverse to that of streambanks and riverbanks, which might be primarily attributed to the large mean canopy openness observed in most sites and the permanently wet conditions (Hassall et al. 2011; Bando et al. 2015). In addition, ponds showed relatively high within-site heterogeneity related to light incidence and, to a lesser extent, to hydrological conditions, which may have favored niche partitioning (Brose 2001; Rolon et al. 2012; Holtmann et al. 2019). The diversity of woody species in ponds, however, was much lower than that in riparian areas. This finding was not surprising, given that it is well known that soils or substrates subjected to prolonged or permanent soil waterlogging or flooding exert strong selective pressure on most woody species of the regional pool, e.g., by developing anaerobic and toxic conditions (Crawford 1992; Pezeshki and DeLaune 2012; Pitman et al. 2014). In addition, the ponds in the upper Uruguay River Basin generally have substrates with very low base saturation (i.e., poor in nutrients) and pH and high aluminum saturation, which can represent additional stressors (Delhaize and Ryan 1995; Budke et al. 2007; Grasel et al. 2020). However, the fact that a much smaller amount of herbaceous species is limited by such filters (at least, e.g., in predominantly forested regions) has been little discussed in literature. As all wetland species are derived from terrestrial ancestors (Sculthorpe 1985; Cronk and Fennessy 2001), we suspect that part of the explanation for the contrasting alpha diversity patterns of herbaceous and woody species that we find in ponds involves differences in the speed with which these plant groups evolve. According to the generation time and mitosis rate hypotheses, herbaceous and shorter species have higher rates of molecular evolution than woody and taller ones, respectively (see Smith and Donoghue 2008; Lanfear et al. 2013). Therefore, we speculate that herbaceous species, which also tend to be shorter than woody species, may have adapted faster to colonize environments with harsh hydrological conditions, which may help clarify why they outnumber woody species in the ponds we sampled.
Concerning the global set of species, ponds were again as diverse as riparian areas, mainly because of their high diversity in herbaceous species.
Beta Diversity
Beta diversity of herbaceous species was higher in ponds than in the two riparian ecosystems, which may be essentially the result of their tendency to have greater among-site environmental heterogeneity than lotic systems reflecting specific conditions of their micro-catchments (Williams et al. 2004). In lotic systems, in turn, floods reduce among-site environmental heterogeneity and hence beta diversity (Thomaz et al. 2007). Further, riverbanks had greater beta diversity than streambanks. A potential explanation is that the latter exhibited a greater number of microhabitats (e.g., hydro-geomorphological niches; Batzer et al. 2018), which may have increased the chance of recruiting rarer species of the regional pool (e.g., those associated to larger riparian areas; Kuglerová et al. 2015) and, consequently, promoted a higher dissimilarity in community composition at the landscape scale.
Beta diversity of woody communities, in contrast, was similar among all wetland types. The most likely reason why ponds did not show the greatest beta diversity again was their previously mentioned environmental harshness, which probably counterbalanced their tendency to present high beta diversity – e.g., due to their great inter-site environmental heterogeneity – by decisively limiting the establishment of woody species (Williams et al. 2004; Davies et al. 2008; Keddy 2010). Likewise, riverbanks did not repeat a greater beta diversity compared to streambanks. As suggested above, a greater local environmental heterogeneity in riverbanks than in streambanks may have favored a higher beta diversity of herbaceous species in the former. However, woody species are less sensitive to fine-scale environmental changes (Lite et al. 2005; Hagan et al. 2006), indicating that riverbanks’ intra-site environmental diversity may have been too modest for similar effects to be observed for this plant group. Indeed, Goebel et al. (2012) demonstrated that the overstory composition practically did not vary from low to high portions of riparian areas in poorly to deeply entrenched valleys, while the ground-flora exhibited high replacement rates along the same gradient, although the latter component was formed by both herbaceous and woody species.
Regarding the global set of species, beta diversity patterns largely reflected those found for herbaceous communities, since woody communities were alike among wetland types. However, the diversity of riverbanks became similar to that of streambanks and ponds in relation to the binary and coverage data, respectively, which is likely because riverbanks showed a tendency to exhibit the lowest and highest beta diversity of woody species in relation to the same data types, respectively.
Gamma Diversity
The highest gamma diversity of herbaceous species was found in ponds and riverbanks, which is mainly related to their higher beta diversity in relation to streambanks, since alpha diversity was similar among all wetland types. Despite having displayed the highest beta diversity, ponds did not exhibit greater gamma diversity compared to riverbanks chiefly due to the tendency of the former to present the lowest alpha diversity.
In terms of woody species, streambanks and riverbanks showed equivalent gamma diversity, primarily due to their similarity regarding both alpha and beta diversity. Ponds, in turn, were the poorest ecosystems, clearly mirroring the great challenges that swampy conditions impose to the establishment and development of woody species (Pezeshki and DeLaune 2012; Pitman et al. 2014).
Regarding the global set of species, riverbanks showed the highest gamma diversity, which is explained by their high diversity for all plant groups. Streambanks and ponds, on the other hand, were between the richest and poorest ecosystems, depending on the species group (herbaceous or woody), which clarifies why they exhibited similar diversity between themselves and lower than that of riverbanks when all species were considered.
Species Composition
The revealed floristic differentiation patterns probably reflect largely the magnitude of the environmental differences between the investigated ecosystem types. Hydrological characteristics are likely especially relevant, given that hydrology plays a major role in the assembly of hydrophyte communities, although other factors, including substrate or soil properties, are also important (Cronk and Fennessy 2001; Budke et al. 2007; Keddy 2010).
Among the studied wetlands, ponds and riparian areas presented the most floristically dissimilar communities, which is paralleled in the knowledge about their environmental properties. For example, while the ponds in the upper Uruguay River Basin tend to exhibit dystrophic substrates subjected to prolonged or permanent waterlogging or flooding, riparian areas in the region generally present well-drained eutrophic soils (Giehl and Jarenkow 2008; Grasel et al. 2020). Such environments, therefore, contrast in relation to a series of well-known variables to catalyze floristic differences, such as water availability, pH, base saturation, and potential acidity (Budke et al. 2007; Giehl and Jarenkow 2008). As showed here, lentic and lotic wetlands have also been shown previously to present markedly distinct community composition, as is the case of the woody component of some swamps (always waterlogged and temporarily or permanently flooded) and floodplains (temporarily flooded and predominantly well-drained) in the Amazon (Pitman et al. 2014; Draper et al. 2018).
Community composition of streambanks and riverbanks, in turn, was the least dissimilar, varying to a similar degree as found elsewhere for riparian areas adjacent to 1st−5th order watercourses (e.g., Rheinhardt et al. 2013; Kuglerová et al. 2016). The more modest floristic differences between streambanks and riverbanks than those observed between these and ponds are probably due in large part to the greater environmental similarity between streambanks and riverbanks. Although riparian areas’ habitat features tend to vary gradually from headwaters to mouth (Nilsson et al. 1994; Kuglerová et al. 2015; Batzer et al. 2018), streambanks and riverbanks probably shared several similar environmental features, including well-drained soils during most of the year and edaphic properties that predominate in the region, e.g., elevated nutritional status and very low aluminum toxicity (SBCS/CQFS 2004; Cunha et al. 2006; Giehl and Jarenkow 2008; Grasel et al. 2020). In addition, flooding in riparian areas tends to reduce inter-site environmental heterogeneity due to its homogenizing action, thereby reducing turnover in species composition (Thomaz et al. 2007).
In general, ponds showed the greatest floristic singularity, which seems to be closely related to its high environmental uniqueness at the regional level (see above). Also, the singularity of herbaceous communities was greater in riverbanks than in streambanks, probably due to the increase in the occurrence of species more associated with specific sites (e.g., those subjected to longer flooding) of larger riparian areas (Kuglerová et al. 2015; 2016; Batzer et al. 2018). The floristic uniqueness of the two riparian ecosystems’ woody communities, however, did not differ, which also determined their similarity in relation to the global set of species. These findings suggest that the environmental novelty that riverbanks add to the other riparian areas was too modest for the woody component to show a pattern of floristic uniqueness similar to that presented by the herbaceous component, since woody species are less sensitive to small-scale habitat variations (Lite et al. 2005; Hagan et al. 2006).
Finally, it is noteworthy that each studied ecosystem makes essential or disproportionately greater contributions to the conservation of some plant species at the landscape scale. This is especially true for ponds, as riparian areas, in general, tend to have a floristic composition more similar to that of uplands (see Table S2; Ruschel et al. 2005; Giehl and Jarenkow 2008; Inácio and Jarenkow 2008; Grasel et al. 2017, 2020). Some examples of species that in the study region are unique to, or much more abundant in, particular ecosystems studied, at least in terms of wetlands, include: Eleocharis contracta, Hibiscus striatus, Hydrolea spinosa, Hygrophila costata, Lemna valdiviana, Ludwigia peruviana, Luziola peruviana and Osmunda spectabilis (and many others) in ponds; Asplenium claussenii, Dennstaedtia dissecta, Dennstaedtia globulifera, Didymochlaena truncatula and Parapolystichum effusum in streambanks; and Carex sellowiana, Exostigma rivulare, Galianthe brasiliensis, Goniopteris riograndensis, Selaginella muscosa, Selaginella sulcata and Stenandrium mandioccanum in riverbanks (see Table S2).
Implications for Conservation
Our findings have important implications for wetland plant conservation. For example, ponds, streambanks, and riverbanks all showed exclusive species, beta diversity explained by high rates of turnover and balanced variation in abundance components and distinct floristic composition. This suggests that not only each wetland type, but also each singular wetland makes unique contributions to regional hydrophyte diversity (Williams et al. 2004; Socolar et al. 2016). Furthermore, community patterns of herbaceous and woody species differed in relation to several community parameters, suggesting that conservation strategies focusing on a single plant group tend to be ineffective. For instance, considering only the woody component, one could argue that ponds have the lowest conservation priority among the evaluated ecosystems because they exhibited the lowest gamma diversity. This interpretation, however, would inevitably be different after analyzing the regional diversity patterns of the herbaceous component.
Our results also point to the need to reassess instruments related to wetland management. For instance, although we have shown that ponds make unique contributions to wetland plant diversity, such ecosystems are neglected in various regions of the globe (e.g., see Williams et al. 2004; Calhoun et al. 2017; Grasel et al. 2018; Hill et al. 2018). A critical example comes from Brazil, where ponds have practically lost their legal protection on private properties after the revision of the “Forest Code” (Federal Law nº 4,771/1965; renamed as the Native Vegetation Protection Law: Federal Law nº 12,651/2012), which also reduced or removed the protection of several other wetland habitats (Brancalion et al. 2016; Grasel et al. 2018, 2019a, b) – ponds and other wetlands are also mostly neglected in protected areas of the country (see Azevedo-Santos et al. 2019).
We advocate that safeguarding wetland plant diversity necessarily involves adopting integrative conservation strategies.
Data Availability
All data can be shared upon reasonable request by contacting the corresponding author.
Code Availability
All codes can be shared by contacting the corresponding author.
References
Alahuhta J, Kanninen A, Hellsten S, Vuori K-M, Kuoppala M, Hämäläinen H (2013) Environmental and spatial correlates of community composition, richness and status of boreal lake macrophytes. Ecological Indicators 32:172–181. https://doi.org/10.1016/j.ecolind.2013.03.031
Alahuhta J, Rääpysjärvi J, Hellsten S, Kuoppala M, Aroviita J (2015) Species sorting drives variation of boreal lake and river macrophyte communities. Community Ecology 16:76:85. https://doi.org/10.1556/168.2015.16.1.9
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2014) Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ (2006)Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecology Letters 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
APG (Angiosperm Phylogeny Group) IV (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181:1–20. https://doi.org/10.1111/boj.12385
Azevedo-Santos VM, Frederico RG, Fagundes CK et al (2019) Protected areas: a focus on Brazilian freshwater biodiversity. Diversity and Distributions 25:442–448. https://doi.org/10.1111/ddi.12871
Bando FM, Michelan TS, Cunha ER, Figueiredo BRS, Thomaz SM (2015) Macrophyte species richness and composition are correlated with canopy openness and water depth in tropical floodplain lakes. Brazilian Journal of Botany 38:289–294. https://doi.org/10.1007/s40415-015-0137-y
Baselga A (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21:1223–1232. https://doi.org/10.1111/j.1466-8238.2011.00756.x
Baselga A (2017) Partitioning abundance-based multiple-site dissimilarity into components: balanced variation in abundance and abundance gradients. Methods in Ecology and Evolution 8:799–808. https://doi.org/10.1111/2041-210X.12693
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F, Logez M, Henriques-Silva R (2020) Package ‘betapart’. R package version 1.5.2. https://cran.r-project.org/web/packages/betapart/index.html. Accessed 13 Jan 2021
Batzer DP, Noe GB, Lee L, Galatowitsch M (2018) A floodplain continuum for Atlantic Coast rivers of the southeastern US: predictable changes in floodplain biota along a River’s Length. Wetlands 38:1–13. https://doi.org/10.1007/s13157-017-0983-4
Biggs J, Williams P, Whitfield M, Nicolet P, Weatherby A (2005) 15 years of pond assessment in Britain: results and lessons learned from the work of pond conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 15:693–714. https://doi.org/10.1002/aqc.745
Brancalion PHS, Garcia LC, Loyola R, Rodrigues RR, Pillar VD, Lewinsohn TM (2016) A critical analysis of the Native Vegetation Protection Law of Brazil (2012): updates and ongoing initiatives. Nature Conservation 14:1–15. https://doi.org/10.1016/j.ncon.2016.03.003
Brose U (2001) Relative importance of isolation, area and habitat heterogeneity for vascular plant species richness of temporary wetlands in east-German farmland. Ecography 24:722–730. https://doi.org/10.1111/j.1600-0587.2001.tb00533.x
Bubíková K, Hrivnák R (2018) Comparative macrophyte diversity of waterbodies in the Central European landscape. Wetlands 38:451–459. https://doi.org/10.1007/s13157-017-0987-0
Budke JC, Jarenkow JA, Oliveira-Filho AT (2007) Relationships between tree component structure, topography and soils of a riverside forest, Rio Botucaraí, Southern Brazil. Plant Ecology 189:187–200. https://doi.org/10.1007/s11258-006-9174-8
Budke JC, Jarenkow JA, Oliveira-Filho AT (2010) Intermediary disturbance increases tree diversity in riverine forest of southern Brazil. Biodiversity and Conservation 19:2371–2387. https://doi.org/10.1007/s10531-010-9845-6
Calhoun AJK, Mushet DM, Bell KP, Boix D, Fitzsimons JA, Isselin-Nondedeu F (2017) Temporary wetlands: challenges and solutions to conserving a ‘disappearing’ ecosystem. Biological Conservation 211:3–11. https://doi.org/10.1016/j.biocon.2016.11.024
Canfield RH (1941) Application of the line interception method in sampling range vegetation. Journal of Forestry 39:388–394. https://doi.org/10.1093/jof/39.4.388
Chao A, Jost L (2015) Estimating diversity and entropy profiles via discovery rates of new species. Methods in Ecology and Evolution 6:873–882. https://doi.org/10.1111/2041-210X.12349
Chao A, Ma KH, Hsieh TC, Chiu C-H(2016) Package ‘SpadeR’. R package version 0.1.1. https://cran.r-project.org/web/packages/SpadeR/index.html. Accessed 13 Jan 2021
Crawford RMM (1992) Oxygen availability as an ecological limit to plant distribution. Advances in Ecological Research 23:93–185. https://doi.org/10.1016/S0065-2504(08)60147-6
Cronk JK, Fennessy MS (2001) Wetland plants: biology and ecology. CRC Press, Boca Raton
Cunha NG, Silveira RJC, Severo CRS (2006) Estudo de solos do município de Derrubadas – RS. EMBRAPA, Pelotas
Darwall W, Bremerich V, De Wever A et al (2018) The Alliance for Freshwater Life: a global call to unite efforts for freshwater biodiversity science and conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 28:1015–1022. https://doi.org/10.1002/aqc.2958
Davies B, Biggs J, Williams P, Whitfield M, Nicolet P, Sear D, Bray S, Maund S (2008) Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agriculture, Ecosystems & Environment 125:1–8. https://doi.org/10.1016/j.agee.2007.10.006
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiology 107:315–321. https://doi.org/10.1104/pp.107.2.315
Draper FC, Coronado ENH, Roucoux KH et al (2018) Peatland forests are the least diverse tree communities documented in Amazonia, but contribute to high regional beta-diversity. Ecography 41:1256–1269. https://doi.org/10.1111/ecog.03126
Dray S, Bauman D, Blanchet G et al (2020) Package ‘adespatial’. R package version 0.3–8. https://cran.r-project.org/web/packages/FSA/index.html. Accessed 13 Jan 2021
Fernández-Aláez M, García-Criado F, García-Girón J, Santiago F, Fernández-Aláez C (2020) Environmental heterogeneity drives macrophyte beta diversity patterns in permanent and temporary ponds in an agricultural landscape. Aquatic Sciences 82:20. https://doi.org/10.1007/s00027-020-0694-4
Flinn KM, Lechowicz MJ, Waterway MJ (2008) Plant species diversity and composition of wetlands within an upland forest. American Journal of Botany 95:1216–1224. https://doi.org/10.3732/ajb.0800098
Flora Argentina (2021) Flora Argentina. Instituto de Botánica Darwinion. http://www.floraargentina.edu.ar/. Accessed 08 May 2021
Flora do Brasil (2020) Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/. Accessed 24 Mar 2021
Gastwirth JL, Gel YR, Hui WLW, Lyubchich V, Miao W, Noguchi K (2020) Package ‘lawstat’. R package version 3.4. https://cran.r-project.org/web/packages/lawstat/index.html. Accessed 13 Jan 2021
Giehl ELH, Jarenkow JA (2008) Gradiente estrutural no componente arbóreo e relação com inundações em uma floresta ribeirinha, rio Uruguai, sul do Brasil. Acta Botanica Brasilica 22:741–753. https://doi.org/10.1590/S0102-33062008000300012
Goebel PC, Pregitzer KS, Palik BJ (2012) Influence of flooding and landform properties on riparian plant communities in an old-growth northern hardwood watershed. Wetlands 32:679–691. https://doi.org/10.1007/s13157-012-0300-1
Gould WA, Walker MD (1997) Landscape-scale patterns in plant species richness along an arctic river. Canadian Journal of Botany 75:1748–1765. https://doi.org/10.1139/b97-889
Grasel D, Spezia MB, Oliveira AD (2017) Fitossociologia do componente arborescente-arbóreo de uma floresta estacional no vale do rio Uruguai, sul do Brasil. Ciência Florestal 27:153–167. https://doi.org/10.5902/1980509826455
Grasel D, Mormul RP, Bozelli RL, Thomaz SM, Jarenkow JA (2018) Brazil’s Native Vegetation Protection Law threatens to collapse pond functions. Perspectives in Ecology and Conservation 16:234–237. https://doi.org/10.1016/j.pecon.2018.08.003
Grasel D, Fearnside PM, Rovai AS, Vitule JRS, Rodrigues RR, Mormul RP, Sampaio FDF, Jarenkow JA (2019a) Brazil’s Native Vegetation Protection Law jeopardizes wetland conservation: a comment on Maltchik et al. Environmental Conservation 46:121–123. https://doi.org/10.1017/S0376892918000474
Grasel D, Fearnside PM, Vitule JRS, Bozelli RL, Mormul RP, Rodrigues RR, Wittmann F, Agostinho AA, Jarenkow JA (2019b) Brazilian wetlands on the brink. Biodiversity and Conservation 28:255–257. https://doi.org/10.1007/s10531-018-1666-z
Grasel D, Giehl ELH, Wittmann F, Jarenkow JA (2020) Tree community patterns along pond-upland topographic gradients, upper Uruguay River basin, southern Brazil. Folia Geobotanica 55:109–126. https://doi.org/10.1007/s12224-020-09368-2
Hagan JM, Pealer S, Whitman AA (2006) Do small headwater streams have a riparian zone defined by plant communities? Canadian Journal of Forest Research 36:2131–2140. https://doi.org/10.1139/X06-114
Hassall C, Hollinshead J, Hull A (2011) Environmental correlates of plant and invertebrate species richness in ponds. Biodiversity and Conservation 20:3189–3222. https://doi.org/10.1007/s10531-011-0142-9
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Hill M, Hassall C, Oertli B et al (2018) New policy directions for global pond conservation. Conservation Letters 11:e12447. https://doi.org/10.1111/conl.12447
Holtmann L, Kerler K, Wolfgart L, Schmidt C, Fartmann T (2019) Habitat heterogeneity determines plant species richness in urban stormwater ponds. Ecological Engineering 138:434–443. https://doi.org/10.1016/j.ecoleng.2019.07.035
IBGE (Instituto Brasileiro de Geografia e Estatística) (1990) Geografia do Brasil: região Sul. IBGE, Rio de Janeiro
Inácio CD, Jarenkow JA (2008) Relações entre a estrutura da sinúsia herbácea terrícola e a cobertura do dossel em Floresta estacional no sul do Brasil. Revista Brasileira de Botânica 31:41–51. https://doi.org/10.1590/S0100-84042008000100005
Junk WJ, Piedade MTF, Lourival R et al (2014) Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conservation: Marine and Freshwater Ecosystems 24:5–22. https://doi.org/10.1002/aqc.2386
Keddy PA (2010) Wetland ecology: principles and conservation. Cambridge University Press, Cambridge
Kent M (2012) Vegetation description and analysis: a practical approach. Wiley
Kuglerová L, Jansson R, Sponseller RA, Laudon H, Malm-Renöfält B (2015) Local and regional processes determine plant species richness in a river-network metacommunity. Ecology 96:381–391. https://doi.org/10.1890/14-0552.1
Kuglerová L, Dynesius M, Laudon H, Jansson R (2016) Relationships between plant assemblages and water flow across a boreal forest landscape: a comparison of liverworts, mosses, and vascular plants. Ecosystems 19:170–184. https://doi.org/10.1007/s10021-015-9927-0
Lanfear R, Ho SYW, Jonathan Davies T, Moles AT, Aarssen L, Swenson NG, Warman L, Zanne AE, Allen AP (2013) Taller plants have lower rates of molecular evolution. Nature Communications 4:1879. https://doi.org/10.1038/ncomms2836
Lansac-Tôha FM, Bini LM, Heino J et al (2020)Scale-dependent patterns of metacommunity structuring in aquatic organisms across floodplain systems. Journal of Biogeography. https://doi.org/10.1111/jbi.14044
Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16:951–963. https://doi.org/10.1111/ele.12141
Lite SJ, Bagstad KJ, Stromberg JC (2005) Riparian plant species richness along lateral and longitudinal gradients of water stress and flood disturbance, San Pedro River, Arizona, USA. Journal of Arid Environments 63:785–813. https://doi.org/10.1016/j.jaridenv.2005.03.026
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Melo AS (2008) O que ganhamos ‘confundindo’ riqueza de espécies e equabilidade em um índice de diversidade? Biota Neotropica 8:21–27. https://doi.org/10.1590/S1676-06032008000300001
Morris EK, Caruso T, Buscot F et al (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecology and Evolution 4:3514–3524. https://doi.org/10.1002/ece3.1155
Munhoz CBR, Araújo GM (2011) Métodos de amostragem do estrato herbáceo-subarbustivo. In: Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira Neto JAA (eds) Fitossociologia no Brasil: métodos e estudos de casos, 1st edn. Editora UFV, Viçosa, pp 213–230
Naiman RJ, Décamps H, Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecological Applications 3:209–212. https://doi.org/10.2307/1941822
Neiff JJ (2001) Diversity in some tropical wetland systems of South America. In: Gopal W, Junk WJ, Davis JA (eds) Biodiversity in wetlands: assessment, function and conservation, 1st edn. Backhuys Publishers, Leiden, pp 157–186
Nilsson C, Jansson R (1995) Floristic differences between riparian corridors of regulated and free-flowing boreal rivers. Regulated Rivers: Research & Management 11:55–66. https://doi.org/10.1002/rrr.3450110106
Nilsson C, Grelsson G, Johansson M, Sperens U (1989) Patterns of plant species richness along riverbanks. Ecology 70:77–84. https://doi.org/10.2307/1938414
Nilsson C, Ekblad A, Dynesius M, Backe S, Gardfjell M, Carlberg B, Hellqvist S, Jansson R (1994) A comparison of species richness and traits of riparian plants between a main river channel and its tributaries. Journal of Ecology 82:281–295. https://doi.org/10.2307/2261296
Ogle D, Wheeler P, Dinno A (2020) Package ‘FSA’. R package version 0.8.31. https://cran.r-project.org/web/packages/FSA/index.html. Accessed 13 Jan 2021
Oksanen J, Blanchet FG, Friendly M et al (2020) Package ‘vegan’. R package version 2.5-7. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 13 Jan 2021
Oliveira-Filho AT, Budke JC, Jarenkow JA, Eisenlohr PV, Neves DRM (2015) Delving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forests. Journal of Plant Ecology 8:242–260. https://doi.org/10.1093/jpe/rtt058
Oliveira-Filho AT, Dexter KG, Pennington RT, Simon MF, Bueno ML, Neves DM (2021) On the floristic identity of Amazonian vegetation types. Biotropica. https://doi.org/10.1111/btp.12932
Padial AA, Ceschin F, Declerck SAJ et al (2014) Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS One1 9:e111227. https://doi.org/10.1371/journal.pone.0111227
Pezeshki SR, DeLaune RD (2012) Soil oxidation-reduction in wetlands and its impact on plant functioning. Biology 1:196–221. https://doi.org/10.3390/biology1020196
Pitman NCA, Andino JEG, Aulestia M, Cerón CE, Neill DA, Palacios W, Rivas-Torres G, Silman MR, Terborgh JW (2014) Distribution and abundance of tree species in swamp forests of Amazonian Ecuador. Ecography 37:902–915. https://doi.org/10.1111/ecog.00774
Pollock MM, Naiman RJ, Hanley TA (1998) Plant species richness in riparian wetlands – a test of biodiversity theory. Ecology 79:94–105. https://doi.org/10.1890/0012-9658(1998)079[0094:PSRIRW]2.0.CO;2
PPG (Pteridophyte Phylogeny Group) I (2016) A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution 54:563–603. https://doi.org/10.1111/jse.12229
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/. Accessed 13 Jan 2021
Renöfält BM, Nilsson C, Jansson R (2005) Spatial and temporal patterns of species richness in a riparian landscape. Journal of Biogeography 32:2025–2037. https://doi.org/10.1111/j.1365-2699.2005.01328.x
Rheinhardt R, Wilder T, Williams H, Klimas C, Noble C (2013) Variation in forest canopy composition of riparian networks from headwaters to large river floodplains in the southeast coastal plain. USA Wetlands 33:1117–1126. https://doi.org/10.1007/s13157-013-0467-0
Rolon AS, Rocha O, Maltchik L (2012) Do effects of landscape factors on coastal pond macrophyte communities depend on species traits? Aquatic Botany 103:115–121. https://doi.org/10.1016/j.aquabot.2012.07.004
Ruschel AR, Guerra MP, Moerschbacher BM, Nodari RO (2005) Valuation and characterization of the timber species in remnants of the Alto Uruguay River ecosystem, southern Brazil. Forest Ecology and Management 217:103–116. https://doi.org/10.1016/j.foreco.2005.05.054
Santamaría L (2002) Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica 23:137–154. https://doi.org/10.1016/S1146-609X(02)01146-3
Santos-Junior R, Müller SC, Waechter JL (2018) Diversity and floristic differentiation of South Brazilian coastal plain Atlantic forests based on herb layer life-forms. Flora 249:164–171. https://doi.org/10.1016/j.flora.2018.11.007
SBCS/CQFS (Sociedade Brasileira de Ciência do Solo/Comissão de Química e Fertilidade do Solo) (2004) Manual de adubação e de calagem para os estados do Rio Grande do Sul e de Santa Catarina. SBCS/CQFS, Porto Alegre
Sculthorpe CD (1985) The biology of aquatic vascular plants. Edward Arnold (Publishers) Ltd., London
Smith SA, Donoghue MJ (2008) Rates of molecular evolution are linked to life history in flowering plants. Science 322:86–89. https://doi.org/10.1126/science.1163197
Socolar JB, Gilroy JJ, Kunin WE, Edwards DP (2016) How should beta-diversity inform biodiversity conservation? Trends in Ecology & Evolution 31:67–80. https://doi.org/10.1016/j.tree.2015.11.005
Soons MB (2006) Wind dispersal in freshwater wetlands: knowledge for conservation and restoration. Journal of Vegetation Science 9:271–278. https://doi.org/10.1111/j.1654-109X.2006.tb00676.x
Thomaz SM, Bini LM, Bozelli RL (2007) Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia (incorporating JAQU) 579:1–13. https://doi.org/10.1007/s10750-006-0285-y
Williams P, Whitfield M, Biggs J, Bray S, Fox G, Nicolet P, Sear D (2004) Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biological Conservation 115:329–341. https://doi.org/10.1016/S0006-3207(03)00153-8
Wittmann F, Junk WJ, Piedade MTF (2004) The várzea forests in Amazonia: flooding and the highly dynamic geomorphology interact with natural forest succession. Forest Ecology and Management 196:199–212. https://doi.org/10.1016/j.foreco.2004.02.060
Wittmann F, Marques MCM, Damasceno Júnior G et al (2017) The Brazilian freshwater wetscape: changes in tree community diversity and composition on climatic and geographic gradients. PLoS One 12:e0175003. https://doi.org/10.1371/journal.pone.0175003
Wrege MS, Steinmetz S, Reisser Júnior C, Almeida IR (2012) Atlas climático da Região Sul do Brasil: estados do Paraná, Santa Catarina e Rio Grande do Sul. EMBRAPA, Brasília
Acknowledgements
We thank Manueli Blatt Spezia for fieldwork assistance, Luíz Fernando Esser for producing Fig. 1, Vander Kaufmann for providing watercourses shapefiles from the Santa Catarina State used in Fig. 1, and the following researchers for help in identifying species and/or resolving nomenclatural issues: André Luís de Gasper, Ilsi Iob Boldrini, Marco Octávio de Oliveira Pellegrini, Maria Salete Marchioretto, Sérgio Augusto de Loreto Bordignon, Monica Gomes Buchoski, Silviane Cocco Pesamosca, Danilo Soares Gissi, João Marcelo Alvarenga Braga, Martin Molz, Carlos Alberto Ferreira Júnior, Emerson Ricardo Pansarin, Isabelle Gomes Cardoso Machado da Costa, Jefferson Prado, Luis Adriano Funez, Maria de Fátima Freitas, Regina Celis Lopes Affonso and Marília Cristina Duarte.
Funding
DG received a PhD fellowship (grant number 1601741) from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
DG, ELHG, FW and JAJ planned the article; DG collected and analyzed the data; DG drafted the paper with help of all authors; all authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Wetland Vegetation.
Supplementary Information
ESM 1
(PDF 1.73 MB)
Rights and permissions
About this article
Cite this article
Grasel, D., Giehl, E.L.H., Wittmann, F. et al. Patterns of Plant Diversity and Composition in Wetlands Across a Subtropical Landscape: Comparisons Among Ponds, Streambanks and Riverbanks. Wetlands 41, 90 (2021). https://doi.org/10.1007/s13157-021-01487-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-021-01487-6