Abstract
Understanding the link between ecosystem respiration (Reco) and its influential factors is necessary to evaluate the sources of gaseous carbon loss in coastal wetlands. Seablite (Suaeda salsa Pall.) is the main vegetation type pioneering temperate coastal wetlands in northeast China, and is generally an understudied wetland type. To evaluate the influence of environmental factors on Reco, a multi-year in-situ experiment was carried out during the growing seasons of 2012 to 2014. Total CO2 efflux was measured and separated further into soil microbial and belowground root respiration (Rs + r) and plant respiration (Rplant). Reco displayed strong seasonal variation, with effluxes as high as 845 to 1150 mg CO2 m−2 h−1 during summer months and as low as 32 to 111 mg CO2 m−2 h−1 during spring (when new shoots are sprouting) and fall (when plants are senescing) months. Aboveground plant structures contributed on average 79% to total plant biomass, and accounted for most of the Reco measured; i.e., 62–96% was associated as Rplant. Plant activity was strongly seasonal, accordingly driving Reco, with 1 g of soil-emergent S. salsa biomass (dry weight) producing approximately 1.58 mg CO2 per hour toward Reco during mid-summer. When water level was below the soil surface, Rs + r was exponentially correlated to air temperature. Because Reco for S. salsa marsh in the Liaohe Delta is controlled by plant growth cycles, inundation regime, and air temperature, this finding may be applied for national carbon budget estimation purposes from S. salsa wetlands throughout Northeast China and potentially close a key gap in understanding the role of this large wetland area in contributing to respiratory CO2 emissions globally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands are known to sequester carbon at high rates, and many are regulated by salinity to emit less methane than inland wetlands due to the greater availability of sulfate (Chmura et al. 2003; Holm et al. 2016; Lu et al. 2016). Ecosystem respiration (Reco) is believed to be the dominant gaseous carbon emissions process from coastal wetlands, weakening the carbon sink function of coastal wetlands that have the highest uptake of CO2 (Nicholls 2004; Smith et al. 1983). Reco includes sources of CO2 routed through or originating from emergent plant structures (Rplant), and those sources of CO2 associated with soil microbial and belowground root respiration (Rs + r). CO2 emissions through Reco would be countered by plant (incl. algal) uptake of CO2 through seasonally driven rates of photosynthesis. Thus, when disentanglement of seasonal patterns of respiratory CO2 losses is a study goal, Rplant and Rs + r should be quantified separately because each process can have its own unique seasonal pattern and interactive response linked to plant metabolic activity (Li et al. 2010).
Considering that CO2 generated by plant and microbial respiration is much greater globally than CO2 generated from anthropogenic activities (Raich et al. 2010), these fluxes from natural and managed wetlands are inherently important to understand their role in regulating the climate cycle through positive feedbacks or negative feedbacks (Cox et al. 2000; Davidson and Janssens 2006; Melillo et al. 2002; Mitsch et al. 2008). Coastal wetlands have been the focus of much attention since large amounts of carbon can be stored in tidal wetlands (known as “blue carbon”) and in inland non-tidal coastal wetlands, but with a notable reduction in net gaseous CO2 (and CH4) emissions under some active management regimes (Chen et al. 2016; Jankowski et al. 2017; Rodríguez et al. 2017; Wang et al. 2016).
Reco in coastal wetlands is influenced by many environmental factors including soil and air temperature (Arora et al. 2016; Juszczak et al. 2013), soil properties (Hassink 1992), salinity (Neubauer et al. 2013), plant type (Xu et al. 2014), root biomass (Krauss et al. 2012), and hydrologic conditions (Guan et al. 2011). Environmental factors change greatly with time, which creates bias on evaluating Reco if the full range of changing environmental conditions is not included in determination (Marínmuñiz et al. 2015). In addition, Rplant and soil microbial respiration have different responses to temperature and water level change (Hall and Hopkins 2015; Wu et al. 2017).
Modeling is an effective way to understand and evaluate CO2 exchange between ecosystems and the atmosphere (Giltrap et al. 2010; Kandel et al. 2013), given that empirical assessment often misses extreme environmental conditions. By simulating biogeochemical activities, process-based models are capable of interpreting material and energy flow from one pool to another (Giltrap et al. 2010; Metzger et al. 2015; St-Hilaire et al. 2010). However, as more processes are considered through iterative research, the number of parameters of relevance to modeling can increase, which makes models more complicated and more difficult to apply across scales (Wang and Chen 2012). Moreover, biological processes are not typically fully integrated within models, rather statistical relationships are used to imply cause and effect, leading to imperfect model structure and larger uncertainty in model projections (Larocque et al. 2008). In contrast, empirical models are easier to deploy for evaluating respiration because driving variables are connected to observations via mathematical formulas (Yuste et al. 2005).
Partitioning in-situ Reco into different components and determining the variables controlling each component is challenging but potentially important (Li et al. 2010). For this approach, traditional chamber methods have advantages as flux measurements are direct and linked over small spatial scales to environmental measurements (Dyukarev 2017; Pumpanen et al. 2004). This approach does abandon a degree of reality accomplished through eddy covariance methods (Aubinet et al. 2012; Nicolini et al. 2018). However, models can be applied effectively to scale chamber-based assessments to larger scales.
Suaeda salsa Pall. is a pioneer herbaceous species of tidal marshes and is very tolerant to salinity (Cui et al. 2008; Guan et al. 2011). It naturally grows in highly saline soils including intertidal zones of Europe and East Asia as well as saline and alkaline soils of beaches and lakeshores in northern China. The growing season Reco rate of S. salsa wetlands in the Liaohe Delta and the Yellow River Delta averaged 335 to 402 mg CO2 m−2 h−1 (Ye et al. 2016) and approximately 193 mg CO2 m−2 h−1 (Chen et al. 2016), respectively, attesting to the variability in Reco encountered over spatial scales. Temperature is believed to be the dominant controlling factor of Reco at the local scales from which those studies were conducted, and several exponential correlations between temperature and Reco have been developed for this community type (Xie et al. 2014). However, water level also determines soil aerobic versus anaerobic condition by enhancing or restricting oxygen availability, respectively, and plant biomass also contributes to Reco through emergent plant structures and roots embedded below the soil surface (Olsson et al. 2015). Due to a mix of temporal and spatial characteristics of plant distribution and environmental factors in S. salsa wetlands across their geographic range, observing and measuring Reco of S. salsa marshes across this range would be cost-prohibitive (Sánchez-Cañete et al. 2017).
To gain a better understanding of Reco from a coastal wetland covered by S. salsa and to evaluate the contributions of plant and soil fluxes to Reco, a multi-year in-situ experiment was carried out during the growing seasons of 2012, 2013, and 2014. First, we quantified the influence of temperature, biomass, and water table on Reco and component respiratory fluxes (Rplant and Rs + r). Second, we developed a rapid assessment method (ecosystem model) to estimate system-scale Reco in S. salsa marshes of the Liaohe Delta to aid future efforts in scaling beyond experimental sites, and over potentially different environmental conditions projected for this wetland type into the future. This rapid evaluation model will have potential application in regional and national carbon budget estimation for S. salsa wetlands with lower costs than direct empirical assessment.
Materials and Methods
Study Area
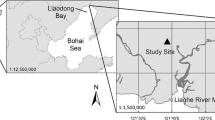
This study was conducted in the Liaohe Delta (121°25′–123°31′ E, 40°79′–41°27′ N) of Northeast China (Fig. 1). Natural wetlands in the Liaohe Delta cover about 2610 km2, which account for about 69% of the delta area (Ji et al. 2009). In addition, rice agriculture (non-natural wetlands) comprises approximately 3287 km2 in the Liaohe Delta. The Liaohe Delta is located in a temperate continental monsoon zone with mean air temperature of 8.3 °C, and mean annual precipitation of 612 mm with most rainfall occurring in summer. The mean annual evaporation rate is 1705 mm, and the mean annual sunshine duration is approximately 2769 h (Luo et al. 2003). Remarkably, the year 2013 tied with 2007 as the sixth warmest since global records began in 1850. The global average surface temperature was 0.50 °C ± 0.10 °C above the 1961–1990 average and 0.03 °C (0.05 °F) above the 2001–2010 average. The year of 2013 was also warmer than both 2011 and 2012, which, though marked by cooling La Niña conditions, were 0.43 °C and 0.46 °C above average, respectively (World Meteorological Organization 2013). In addition to the highest temperature in 2013, very wet soil conditions resulting from hydrometeorological events (HDE) were also noticeable and resulted in the worst flooding in 50 years for this region (World Meteorological Organization 2013, 2014). In contrast to the previous two years, the Liaohe Delta region experienced notably low precipitation and drought in 2014.
The Liaohe Delta comprises what is believed to be the largest reed (Phragmites australis (Cav.) Trin. ex Steud.) wetland in the world with a total area of approximately 800 km2 (Brix et al. 2014). Located at 16 km west of the Liaohe River mouth, our study site (121°36′42.66″E, 40°52′16.95”N) was a newly restored wetland on a former fallow tidal flat, which was recently colonized by S. salsa. Saueda salsa wetlands comprise only 32 km2 in the Liaohe Delta but provide seasonal color to the region during flowering that draws tourists from all over China.
The average semi-diurnal tidal range in the area is 2.7 m. The soil on the study sites is a silty clay loam with a sand, silt and clay content of 20%, 65% and 15%, respectively, and a soil bulk density of approximately 1.3 g cm−3. Soil total and organic carbon content is low, averaging 9.5 g kg−1 and 6.4 g kg−1, respectively, and total nitrogen content is 1.1 g kg−1. Soil pH is 7.3 ± 0.4 (std. err.) and soil pore water salinity is 17 ± 2 psu (Ding et al. 2012).
In 2012, surface soil samples on our specific study sites were taken using a ring collar of 100 cm3 volume over a 2 cm depth. The soil texture was measured by a Malvern laser particle size analyzer. Soil bulk density, carbon concentration, and nitrogen concentration was measured using the methods of Ye et al. (2016). The soil pH was measured by a conventional pH electrode after extraction in deionized water, and the soil pore water salinity was measured by a portable salinometer in a monitoring well once per month. Soil pore water \( {HCO}_3^{-} \)/\( {CO}_3^{2-} \) were tested using a standard acid solution titration method. The average value was 323 mg \( {HCO}_3^{-} \)/L with no \( {CO}_3^{2-} \) in the soil pore water (range, 177–502 mg \( {HCO}_3^{-} \)/L).
CO2 Flux Measurements
CO2 fluxes were measured using a field-portable infrared gas analyzer (Li-8100A, LI-COR Biosciences, Inc., Lincoln, NE, U.S.A.) with a commercial survey dark chamber (8100–103). CO2 measuring range was 0 to 3000 ppm with errors less than 1.5%. Circular survey collars (10 cm tall by 20 cm diameter) were inserted 3 to 5 cm into the soil 2 h before measurement began to limit the influences of recent disturbance. The survey collar measured an area of 318 cm2. The total volume of the flux chamber was calculated as the sum of the volume of the commercial survey chamber system (~4843 cm3) plus the volume inside the collar factoring insertion depth of each collar individually. CO2 concentrations were recorded at 1 Hz during 90 s measurement periods, and individual flux rates were the average of two measurements per chamber conducted in immediate series (10 s apart; 90 s each) to ensure similarity over at least two cycles (Mukhopadhyay and Maiti 2014). Prior to each field trip, the infrared gas analyzer was calibrated and checked for zero drift using CO2-free nitrogen gas (Dyukarev 2017).
CO2 fluxes (F, mg CO2 m−2 h−1) were calculated according to the following equation:
Where dc/dt (mol h−1) is the slope of the linear regression line for CO2 concentration over time before chamber saturation; M (mg mol−1) is the molecular mass of CO2; P (in kPa) is the barometric pressure; T (in Kelvin) is the absolute temperature during sampling; V (in Liters) is the total volume of the enclosure measuring space; S (in m2) is the ground area of the dark chamber. Finally, V0 (22.4 L/mol), T0 (273.15 K) and P0 (101.3 kPa) are the gas mole volume, absolute air temperature, and atmospheric pressure under standard conditions for gas, respectively (Song et al. 2009).
Experimental Design
Fluxes of CO2 were measured approximately monthly during the growing seasons of 2012, 2013, and 2014 (Fig. 2), for a total of 16 months of measurements over the three years. The soils of the Liaohe Delta wetlands are frozen to depths of 15 cm during the months of December to March (Ye et al. 2016). During each observation period, six plots were established along a visual gradient of aboveground biomass. The measurements were performed during 10:00 to 14:00 under full sunlight (Table S1). For each of the six plots every month, the Reco, Rs + r and Rplant were measured twice, then averaged. The measuring procedures were included as follows:
-
(1)
Measurement of ecosystem respiratory CO2 flux (Reco): Including all vegetation and soil area by a dark chamber that prevents photosynthesis;
-
(2)
Measurement of cut plant material (Rplant): The respiration of cut plant material. For this component, all S. salsa vegetation was cut at 1 to 2 cm above the soil surface within the collar, and immediately (within 2 min) placed into a sealed dark survey collar for respiratory CO2 flux measurement;
-
(3)
Measurement of soil CO2 flux within the survey chamber but without standing plants (Rs + r): after removing living plants, CO2 flux was measured again by using a dark chamber that measures only soil microbial respiration plus respiration of roots in the soils. If the soil was inundated, this test measured the CO2 exchange between the surface water and the atmosphere within the survey collar;
-
(4)
Additional measurements: An extra procedure was carried out during August and September of 2012 when the soil was inundated. Plants were removed 1–2 cm above the surface water, and the respiratory CO2 flux was measured by the dark chamber technique (Fig. 2). After this extra measurement, the regular measurement of Rs + r was conducted. Results and additional descriptions of the additional measurements are provided in the supplementary materials (Fig. S1) accompanying this manuscript.
Observation period and procedures from 2012 to 2014. Observation periods are marked as filled grey patches, and vertical blue patches indicate the relative water level of a corresponding observation period. Months with continuous blue rectangles refer to inundation of all six plots; months half covered in blue rectangles refer to inundation of only some of the plots. Lack of blue rectangles equates to no inundation. Experimental procedures (Reco, Rs + r, Rplant, and additional fluxes) are re-iterated visually on the bottom panel. The Reco measures the ecosystem respiration, which contains the plant respiration and soil respiration; The Rplant is the measured respiration on the plant material after cutting within 2 min; The Rs + r measures the soil respiration and the root respiration; The additional measurement is designed to quantify CO2 emitted from the plant stems
During all measurements, the air temperature was recorded by a temperature sensor which was integrated in the survey chamber, and the water inundation regime (flooded or not) was recorded. In April of 2014, no living plants were grown in the study site, therefore, only soil CO2 effluxes (Reco) were measured.
Biomass Measurements
All harvested S. salsa plant material was dried to a constant mass at 65 °C in a convection oven for estimation of aboveground biomass (AGB). A 15 cm deep surface soil sample was taken within each survey collar after CO2 flux measurements were completed during each sampling period. Living roots of S. salsa were collected in the core, separated from the soil column and dried at 65 °C to constant mass to measure belowground biomass (BGB).
Statistical Analysis and Modeling
All monthly data are presented as arithmetic means among plots with corresponding standard errors among plots. Correlation analyses were conducted using SPSS v20.0 to examine the relationships between the fluxes and the measured environmental variables. In all tests, the differences were considered significant at p < 0.05. Least square curve fitting was applied using Grapher v10.0 (Golden Software, Golden, CO, U.S.A.) to quantify the influence of environmental factors. An empirical model, which is capable of assessing ecosystem respiration of S.salsa wetlands with simple environmental variables, was developed using Spyder 3.2.4 on a Python 3.6 platform. The model was based on the field observations of respiration in various treatments and their corresponding air temperature, plant biomass, and inundation regime.
Results and Discussion
Ecosystem Respiration
The average contribution of soil and root respiration (Rs + r) and plant respiration (Rplant) to Reco was 16% and 84%, respectively, based on our observed data. However, the relative contributions of Rs + r and Rplant varied both within and between seasons, with Rs + r and Rplant often, but not always, corresponding to the pattern for Reco (Fig. 3).
Seasonal variation in ecosystem respiration (Reco), soil and live root respiration (Rs + r), and plant respiration (Rplant) during the growing seasons of 2012–2014. The tag “F” indicates the site is fully inundated and the “P” tag means the site is partly inundated, and no tag means the site is not flooded
Reco varied significantly over the growing season with peak values in August when the weather was hot (Fig. 3); this was also generally the case for Rs + r and Rplant except for Rs + r at the end of 2012 and Rplant at the end of 2014. Interestingly, despite the slight seasonal pattern deviations for Rs + r and Rplant, seasonal patterns of Reco were nearly identical between years, although peak Reco varied between 845 mg CO2 m−2 h−1 in 2014 and 1150 mg CO2 m−2 h−1 in 2013. During mid-summer (July and August) there was high spatial variation in Reco (as indicated by the relatively large variation among measurements) due to the variations in plant biomass within the collars and also differences in water table depth at the time of the specific measurements. To some degree, this reflects the natural spatial patterns of Suaeda salsa development within its range. It is worth mentioning that the Reco in 2013 was more variable than the other two years, which was apparently caused by larger variations in the aboveground biomass (Fig. S7).
Rs + r generally varied in concert with Reco with highest rates in July–August, except in 2012 when Rs + r was low (< 100 mg CO2 m−2 h−1) in July and August. This corresponds to a period where the soil surface in all six measuring plots was inundated, i.e. had standing water on the soil surface. This was also the case in June–July in 2013, which also had very low Rs + r rates. The inundation probably reduces Rs + r somewhat because of the prevailing anoxic conditions in the soil during inundation. However, emission of CO2 to the atmosphere through the water surface might also be reduced because CO2 is highly soluble in water and enters into an equilibrium with the bicarbonate buffer system (Berglund and Berglund 2011), which is especially prominent in saline water (i.e., the porewater of our sites was ~50% of full-strength seawater). Based on the porewater values of \( {HCO}_3^{-} \)/L previously measured (323 mg \( {HCO}_3^{-} \)/L with no detectable \( {CO}_3^{2-} \)), the concentration gradient for dissolving CO2 into porewater would be high in these S. salsa wetlands. Compared to the rates of Rs + r and Rplant, the rate of gas exchange between the surface water and the atmosphere was relatively low in our study.
Our observations also suggest that a major effect of inundation to the S. salsa marsh respiration is the physical blocking of the gas transport from the soil to the atmosphere. Hence, the measured CO2 flux from Rs + r to the atmosphere is very sensitive to water level variations just around the soil surface. This phenomenon was also reported in a cool temperate bog located in Mer Bleue, Canada (Lafleur et al. 2005; Pugh et al. 2018). Thus, surface water provides a physical barrier to CO2 efflux, serving to trap CO2 until water drawdown occurs (Krauss et al. 2012) or ebullition events occur, as has been demonstrated previously for CH4 (Devol et al. 1990). Nevertheless, the highest Rs + r rates (>400 mg CO2 m−2) as measured by our dark chamber techniques were recorded in August of 2013 and 2014 when the water table was below the soil surface and temperatures were high.
Rplant generally peaked in August when temperatures were high and when the plant biomass was maximal, except in 2014 where Rplant was highest in September. Rplant generally varied in concert with Reco and often, particularly during inundated periods, contributed to most of the ecosystem respiration (approximately 93%), while both Rs + r and Rplant contributed to Reco when the water table was below the soil surface (approximately 38% Rs + r and 62% Rplant). Reco from additional S. salsa wetlands in the Liaohe Delta was in the range of what we found, and also peaked in June to August, depending on the year (Ye et al. 2016), corresponding strongly to peak seasonal aboveground biomass.
Plant Biomass
Suaeda salsa is an annual herb that germinates and starts to grow in late April. The plants then follow the normal seasonal vegetation growth cycle for cold temperate regions, with flowering beginning in July and maturation of seeds occurring around late September (Mori et al. 2010). In the current study, the biomass production of S. salsa largely followed this pattern, reaching total biomass of 530–930 g dry mass m−2 among years (Fig. 4). Overall, the aboveground biomass comprised about 79% of the total biomass (i.e., aboveground plus belowground to a depth of 15 cm), but the proportion varied during the growing season. In the spring and early summer, roots contributed a larger proportion (25% to 35%) of the total biomass whereas in the late summer and autumn, roots only contributed to 15–20% of the total biomass. This indicates that the roots of S. salsa develop prior to peak aboveground biomass, and are thus slightly out of phase, suggesting an important role for early growing season root growth initiation which also influences Reco in many coastal wetland types (Krauss et al. 2012). After mid-September, the aboveground biomass remains stable probably because roots at this stage are now able to support the biomass of the entire plant. Mao et al. (2011) reported that roots contributed 8% to ~13% of total Suaeda salsa biomass in the Yellow River Delta, China, which is similar to our findings.
Seasonal variation of S. salsa biomass during three growing seasons. Error bars indicate the standard error of the mean at each sampling period (n = 6). The inset graph presents the proportion of aboveground biomass (AGB) relative to total biomass. The dashed line indicates the overall average AGB proportion
Influencing Environmental Factors on Rs + r and Rplant
Air temperature varied between 3 °C and 33 °C during the measurement period. Rs + r was lower than 200 mg CO2 m−2 h−1 when the air temperature was below 14 °C (Fig. 5), which is consistent with the findings of Ye et al. (2016). When the low fluxes measured during inundated periods were excluded, we found that Rs + r was exponentially correlated with air temperature on a seasonal scale, which has also been reported in several other studies (Bäckstrand et al. 2010; Xie et al. 2014). If we did not remove fluxes of Rs + r that were measured during inundated periods, the correlation would be significantly weakened (Fig. 5), suggesting a strong statistical interaction between air temperature and inundation that needs to be considered in the modeling (Krauss et al. 2012). Rs + r also correlated weakly with air temperature probably because the combined effects of soil temperature, water table, and plant biomass on Rs + r were not considered in this correlation (Flanagan et al. 2002; Reth et al. 2005; Zhang et al. 2016). Rs + r measured from plots with large plant biomass was significantly higher than the exponentially predicted values, indicating that roots in the soils contributed significantly to Rs + r, and when root biomass was large, influenced Rs + r (and Reco) independently of air temperature (Fig. 5), and potentially independently of other environmental variables.
During inundated periods, Rs + r rates were low because water blocked both oxygen and CO2 transport (Yang et al. 2014). Water can both absorb and emit CO2 depending on the \( {\mathrm{HCO}}_3^{-} \)/\( {\mathrm{CO}}_3^{2-} \) balance in the surface water and the dissolved balance of CO2 between the surface water and the atmosphere (Wanninkhof and Knox 2003). Suaeda salsa has very limited aerenchyma in its tissue, and no plant-mediated gas transport has been detected in this species (Brix et al. 1996). Besides, Rs + r under inundated regimes were much lower than Rs + r during unflooded periods, and was also much lower than the Rplant quantitatively.
Plants are reported to account for 35% to 90% of the total ecosystem respiration in wetlands (Johnson et al. 2000). However, regardless of this ratio, plant respiration is regarded as the main source of spatial Reco variation as soil respiration is often more homogeneous (Han et al. 2007). In the Yellow River Delta, Han et al. (2014) did not find a significant relationship between Reco and biomass during the growing season in a S. salsa wetland. This may be because they did not partition Reco as we did here, although the difference between Rplant and Reco was less than 10% during fully inundated periods except for a difference of 112 mg CO2 m−2 h−1 in July 2013 (Fig. 3). Rs + r was lower than 100 mg CO2 m−2 h−1 during all fully inundated periods, which suggests that the S. salsa plants contributed the most to the Reco.
We observed a significant linear correlation between AGB and Rplant for all measured months (p < 0.01) (Fig. 6). The slopes of the regression lines vary over a growing season. The ratios of Rplant/AGB were generally low (< 0.3 mg CO2 per g dry mass per hour) after October, probably because of plant senescence in the fall. However, measurements were not carried out on the same day of each month every year, and plant phenology might vary within a month. Therefore, we separated each observation by Julian day to explore seasonal variation, and the slopes of the linear curves for each measurement are shown in Fig. 7. Indeed, the most consistent cause of inter-measurement differences in the ratio of Rplant/AGB was Julian day. Some studies prefer to use the air or soil temperature as the proxy to evaluate seasonal parameters, while the accumulated temperature has been shown to be better for evaluations of plant phenology (Cannell and Smith 1983). Since we did not measure meteorological variables on site continuously over annual cycles, we are still able to predict respiration parameters versus S. salsa plant biomass because of the significance of seasonal time represented by Julian day as a proxy.
The relationship between plant respiration rate (Rplant) and aboveground biomass (AGB) in each month during the three growing seasons. The dash line indicates the upper limit and the lower limit of 99.99% confidence. All points without 99.99% confidence were moved as outliers (marked as “x”). Linear correlations were significant at <0.01 level. The slope of each month was extracted to represent plant activity
Modeling of Components of Ecosystem CO2 Fluxes
As a mathematical summary to Fig. 2, Reco is calculated as the sum of Rplant and Rs + r as follows:
Please note also that this study is not about net radiative balance, in which case, Reco would have to be balanced with net uptake of CO2 from photosynthesis for that analysis to work.
Soil and Root Respiration
Rs + r is determined by water level and air temperature, which is
Where kWT represents the influence of water table on Rs + r (in mg CO2 m−2 h−1), and f(T) indicates the influence of air temperature (T in °C) on Rs + r. Equation 3 displays the influence of water level (h in cm) on kWT, with the parameter a = 4.6, which was calculated by assuming h = 1 cm and kWT being set up with a small number (0.01), indicating that water level of 1 cm could completely block the flux of CO2 as soil respiration because of the barrier provided by the water, while −1 cm water level could enable full gaseous exchange between soil and atmosphere (Fig. 8a). Equation 4 indicates the relationship between air temperature and Rs + r during non-inundated periods, which is represented by an exponential curve, where Rs + r is in mg CO2 m−2 h−1, F0 = 16.49, and b = 0.113 (Fig. 5). Parameter b describes the temperature sensitivity of Rs + r and F0 (in mg CO2 m−2 h−1) represents what Rs + r would be at 0 °C. Equation 2 to 4 include the influence of temperature and water table on Rs + r. An exponential temperature response has been commonly reported by many papers such as Bäckstrand et al. (2010) and Xie et al. (2014). The influence of water table is based on a logistic equation which was originally described by Verhulst and Pierre-François (1838).
Key parameters and driving variables. a kWT in relation to water level change near the soil surface; b kact (same as Rplant/AGB, in mg CO2 h−1 g dry weight−1) in relation to Julian day, the points were monthly individual linear fitting slopes of Rplant versus AGB using the same method as in fig. 6. The fitting equation is shown via Eq. 6, where Dm = 214.83 (Julian day) and Ds = 76.63(days); and c soil and live root respiration (Rs + r) in relation to air temperature when the soil surface was not covered by water
Plant Respiration
Rplant in S. salsa can be determined by
where mp (g dry mass m−2) is the biomass of S. salsa and kact (mg CO2 h−1 g dry mass−1) is the amount of CO2 that 1 g of plant material can produce in 1 h based on our study, and is used here to indicate the influence that seasonal plant activity has on Rplant.
kact (referring to the dry mass specific plant respiration rate in Fig. 7) has a seasonal signature as well, related to environmental variables such as air temperature; however, air temperatures alone were not as useful in predicting Rplant as kact. A Gaussian equation between Julian day (D in eq. 6) and kact was used to evaluate the seasonal and annual variation in kact. According to our observation and analysis (Fig. 8b), the best fit parameters of kamax (in mg CO2 h−1 g dry mass−1), Dm (as a specific day), and Ds (in days) were 1.49 mg CO2 h−1 g dry mass−1, Day 214.83, and 76.63 days, respectively. The points in Fig. 8b are the slopes of linear fitting (Rplant versus AGB) during each month of separate years.
Reco in 2012 was significantly underestimated because plant activity in 2012 was higher than the other two years (Fig. 9). The estimated Rs + r rate includes living root respiration as well as soil organic matter respiration (microbial) according to our model, if we assume that the living roots respire as fast as the aboveground parts. With the record of belowground biomass and the parameter kact, the contribution of each component can be estimated. At our study sites, Rs + r had an average contribution of 23.9% to Reco according to the model calculation. Similarly, the modeled aboveground Rplant constitutes an average of 55.2% of Reco. The plant biomass determines the spatial variation in Reco, and is therefore suggesting that our model is widely applicable to other temperate S. salsa wetlands through plant biomass, water table depth, and air temperature modelling alone. As Moore and Dalva (1993) reported, the effects of climatic change on gas flux from peatlands are more likely to be associated with changes in the water table than with changes in thermal regime. It is important to note that ecosystem respiration consists of autotrophic respiration and heterotrophic respiration. The former comes from plant and the later comes from soil microbial respiration. The S. salsa was usually the only vegetation in S. salsa wetlands as few plant species survives the high salinity condition. The structural characteristics of S. salsa can be slightly variable as patches develop seasonally to eventually form continuous coverage (as we show) but its rhizosphere microbiome is likely to be relatively similar across sites. Therefore, we suggest that the model presented here can be widely used in other temperate S. salsa wetlands.
Observed respiration versus modeled respiration. The triangle marks represent observed plant respiration (Rplant), while the square marks represent observed soil and root respiration (Rs + r). The green and tawny bars indicate the modelled Rplant and Rs + r, respectively. The modelled and observed ecosystem respiration (Reco) of 2012, 2013 and 2014 are colored red, yellow and blue, respectively
To demonstrate the idea of our modelling approach, Fig. 10 shows the seasonal trend of temperature and living biomass. As a check, an inundated regime was projected onto this relationship in July. This figure shows that the seasonal variation of Reco is influenced by temperature, biomass and water inundation regime. When the soil is inundated, the Rs + r is low. The temperature is controlling the Rs + r and the growth of the plants, thus changing the plant biomass and hence Rplant.
More complicated environmental variables on plant activity, such as water table and temperature, processes of gas diffusion, and water \( {HCO}_3^{-} \)/\( {CO}_3^{2-} \) balance are not included in this model due to the limitation of our field observation. More observations or data from other S. salsa wetlands are needed to test this model on a larger scale. However, with easily obtained environmental variables (AGB, air temperature, and water regime), Reco (and by extension Rplant and Rs + r) can be estimated at a large spatial scale, making assessments of area-scaled CO2 emissions from this wetland type, such as conducted by Ye et al. (2016), more cost-effective in the future. This model provides a regional rapid assessment protocol for Reco within S. salsa marshes. The necessary environmental variables can even be obtained through remote sensing.
Conclusions
Ecosystem respiration (Reco) of a S. salsa wetland in the Liaohe Delta, China, was observed to range from 23 to 2995 mg CO2 m−2 h−1, with significant seasonal variation. Flux partitioning confirmed that Reco was correlated with plant biomass, water regime, and air temperature. Plant biomass and plant activity controlled the plant respiration, and further dominated the Reco during inundated period. Both soil and plant contributed to Reco when the water level was below the soil surface. Soil and root respiration was exponentially correlated with air temperature with a sensitivity of 0.113 °C−1. In addition, S. salsa produces as much as 1.58 mg CO2 g−1 dry mass h−1 during mid-summer. Air temperature, plant biomass, and hydrological regime are essential to estimate Reco using our proposed rapid assessment method. With regional data calculated from remote sensing, the method may be used to evaluate Reco of S. salsa marshes on a large scale in the Liaohe Delta, and potentially in other similar cold temperate wetland types.
References
Arora B, Spycher NF, Steefel CI, Molins S, Bill M, Conrad ME, Dong W, Faybishenko B, Tokunaga TK, Wan J (2016) Influence of hydrological, biogeochemical and temperature transients on subsurface carbon fluxes in a flood plain environment. Biogeochemistry 127(2):1–30
Aubinet M, Vesala T, Papale D (2012) Eddy covariance: a practical guide to measurement and data analysis. Springer:365–376
Bäckstrand K, Crill PM, Jackowicz-Korczyñski M, Mastepanov M, Christensen TR, Bastviken D (2010) Annual carbon gas budget for a subarctic peatland, northern Sweden. Biogeosciences 7(1):95–108
Berglund Ö, Berglund K (2011) Influence of water table level and soil properties on emissions of greenhouse gases from cultivated peat soil. Soil Biology and Biochemistry 43(5):923–931
Brix H, Sorrell BK, Schierup H-H (1996) Gas fluxes achieved by in situ convective flow in Phragmites australis. Aquatic Botany 54(2):151–163
Brix H, Ye S, Laws EA, Sun D, Li G, Ding X, Yuan H, Zhao G, Wang J, Pei S (2014) Large-scale management of common reed, Phragmites australis , for paper production: A case study from the Liaohe Delta, China. Ecological Engineering 73:760–769
Cannell M, Smith R (1983) Thermal time, chill days and prediction of budburst in Picea sitchensis. Journal of Applied Ecology 20(3):951–963
Chen L, Liu ZT, Han GX, Chu XJ, Sun BY, Liu HF, Li JW (2016) Effects of environmental and biotic factors on soil respiration in a coastal wetland in the Yellow River Delta, China. Chinese Journal of Applied Ecology 27(6):1795–1803
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Global Biogeochemical Cycles 17(4):1111–1132
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408(6809):184–187
Cui B, He Q, Zhao X (2008) Ecological thresholds of Suaeda salsa to the environmental gradients of water table depth and soil salinity. Acta Ecologica Sinica 28(4):1408–1418
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440(7081):165–173
Devol AH, Richey JE, Forsberg BR, Martinelli LA (1990) Seasonal dynamics in methane emissions from the Amazon River floodplain to the troposphere. Journal of Geophysical Research Atmospheres 95(D10):16417–16426
Ding Y, Ye S, Zhao Q (2012) Nutrients and carbon sequestration in the newly created wetlands of the Yellow River Delta. Geological review 58(1):183–189
Dyukarev EA (2017) Partitioning of net ecosystem exchange using chamber measurements data from bare soil and vegetated sites. Agricultural and Forest Meteorology 239:236–248
Flanagan LB, Wever LA, Carlson PJ (2002) Seasonal and interannual variation in carbon dioxide exchange and carbon balance in a northern temperate grassland. Global Change Biology 8(7):599–615
Giltrap DL, Li C, Saggar S (2010) DNDC: a process-based model of greenhouse gas fluxes from agricultural soils. Agriculture, Ecosystems & Environment 136(3–4):292–300
Guan B, Yu J, Wang X, Fu Y, Kan X, Lin Q, Han G, † ZL (2011) Physiological responses of halophyte Suaeda salsa to water table and salt stresses in coastal wetland of Yellow River Delta. CLEAN – Soil Air Water 39(12):1029–1035
Hall S, Hopkins DW (2015) A microbial biomass and respiration of soil, peat and decomposing plant litter in a raised mire. Plant Soil and Environment 61(9):405–409
Han G, Zhou G, Xu Z, Yang Y, Liu J, Shi K (2007) Soil temperature and biotic factors drive the seasonal variation of soil respiration in a maize ( Zea mays L.) agricultural ecosystem. Plant and Soil 291(1–2):15–26
Han G, Luo Y, Li D, Xia J, Xing Q, Yu J (2014) Ecosystem photosynthesis regulates soil respiration on a diurnal scale with a short-term time lag in a coastal wetland. Soil Biology and Biochemistry 68(1):85–94
Hassink J (1992) Effects of soil texture and structure on carbon and nitrogen mineralization in grassland soils. Biology and Fertility of Soils 14(2):126–134
Holm GO, Perez BC, Mcwhorter DE, Krauss KW, Johnson DJ, Raynie RC, Killebrew CJ (2016) Ecosystem level methane fluxes from tidal freshwater and brackish marshes of the Mississippi River Delta: implications for coastal wetland carbon projects. Wetlands 36(3):401–413
Jankowski KL, Törnqvist TE, Fernandes AM (2017) Vulnerability of Louisiana's coastal wetlands to present-day rates of relative sea-level rise. Nature Communications 8:14792
Ji Y, Zhou G, New T (2009) Abiotic factors influencing the distribution of vegetation in coastal estuary of the Liaohe Delta, Northeast China. Estuaries and Coasts 32(5):937–942
Johnson LC, Shaver GR, Cades DH, Rastetter E, Nadelhoffer K, Giblin A, Laundre J, Stanley A (2000) Plant carbon-nutrient interactions control CO2 exchange in Alaskan wet sedge tundra ecosystems. Ecology 81(2):453–469
Juszczak, Radoslaw, Michalak-Galczewska, Maria, Humphreys, Elyn, Olejnik, Janusz, Acosta (2013) Ecosystem respiration in a heterogeneous temperate peatland and its;sensitivity to peat temperature and water table depth. Plant and Soil 366(1–2):505–520
Kandel TP, Elsgaard L, Lærke PE (2013) Measurement and modelling of CO2 flux from a drained fen peatland cultivated with reed canary grass and spring barley. Global Change Biology Bioenergy 5(5):548–561
Krauss KW, Whitbeck JL, Howard RJ (2012) On the relative roles of hydrology, salinity, temperature, and root productivity in controlling soil respiration from coastal swamps (freshwater). Plant and Soil 358(1–2):265–274
Lafleur PM, Moore TR, Roulet NT, Frolking S (2005) Ecosystem respiration in a cool temperate bog depends on peat temperature but not water table. Ecosystems 8(6):619–629
Larocque GR, Bhatti JS, Gordon AM, Luckai N, Wattenbach M, Liu J, Peng C, Arp PA, Liu S, Zhang CF (2008) Uncertainty and Sensitivity Issues in Process-based Models of Carbon and Nitrogen Cycles in Terrestrial Ecosystems. Developments in Integrated Environmental Assessment 3
Li X, Fu H, Guo D, Li X, Wan C (2010) Partitioning soil respiration and assessing the carbon balance in a Setaria italica (L.) Beauv. Cropland on the loess plateau, northern China. Soil Biology and Biochemistry 42(2):337–346
Lu W, Xiao J, Liu F, Zhang Y, Liu CA, Lin G (2016) Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: a meta-analysis of eddy covariance data. Global Change Biology 23(3):1180–1198
Luo H, Huang F, Zhang Y (2003) Space-time change of marsh wetland in Liaohe delta area and its ecological effect. Journal of Northeast Normal University 35(2):100–105
Mao P, Chen W, Liu Y, Wang G, Chen J, Han G, Zhang Z, Yu J (2011) Biomass allocation in Suaeda salsa population in different habitats of coastal zone. Ecology and Environmental Sciences 20:1214–1220
Marínmuñiz JL, Hernández ME, Morenocasasola P (2015) Greenhouse gas emissions from coastal freshwater wetlands in Veracruz Mexico: effect of plant community and seasonal dynamics. Atmospheric Environment 107(3):107–117
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Morrisseau S (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298(5601):2173–2176
Metzger C, Jansson P-E, Lohila A, Aurela M, Eickenscheidt T, Belelli-Marchesini L, Dinsmore K, Drewer J, van Huissteden J, Drösler M (2015) CO2 fluxes and ecosystem dynamics at five European treeless peatlands–merging data and process oriented modeling. Biogeosciences 12(1):125–146
Mitsch WJ, Tejada J, Nahlik A, Kohlmann B, Bernal B, Hernández CE (2008) Tropical wetlands for climate change research, water quality management and conservation education on a university campus in Costa Rica. Ecological Engineering 34(4):276–288
Moore T, Dalva M (1993) The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. Journal of Soil Science 44(4):651–664
Mori S, Akiya M, Yamamura K, Murano H, Arao T, Kawasaki A, Higuchi K, Maeda Y, Yoshiba M, Tadano T (2010) Physiological role of sodium in the growth of the halophyte Suaeda salsa (L.) pall. Under high-sodium conditions. Crop Science 50(6):2492
Mukhopadhyay S, Maiti SK (2014) Soil CO2 flux in grassland, afforested land and reclamine overburden dumps: a case study. Land Degradation and Development 25(3):216–227
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10(12):8171–8183
Nicholls RJ (2004) Coastal flooding and wetland loss in the 21st century: changes under the SRES climate and socio-economic scenarios. Global Environmental Change 14(1):69–86
Nicolini G, Aubinet M, Feigenwinter C, Heinesch B, Lindroth A, Mamadou O, Moderow U, Mölder M, Montagnani L, Rebmann C, Papale D (2018) Impact of CO2 storage flux sampling uncertainty on net ecosystem exchange measured by eddy covariance. Agricultural and Forest Meteorology 248:228–239
Olsson L, Ye S, Yu X, Wei M, Krauss KW, Brix H (2015) Factors influencing CO2 and CH4 emissions from coastal wetlands in the Liaohe Delta, Northeast China. Biogeosciences 12(16):4965–4977
Pugh CA, Reed DE, Desai AR, Sulman BN (2018) Wetland flux controls: how does interacting water table levels and temperature influence carbon dioxide and methane fluxes in northern Wisconsin? Biogeochemistry 137(1–2):15–25
Pumpanen J, Kolari P, Ilvesniemi H, Minkkinen K, Vesala T, Niinistö S, Lohila A, Larmola T, Morero M, Pihlatie M (2004) Comparison of different chamber techniques for measuring soil CO2 efflux. Agricultural and Forest Meteorology 123(3):159–176
Raich JW, Potter CS, Bhagawati D (2010) Interannual variability in global soil respiration, 1980-94. Global Change Biology 8(8):800–812
Reth S, Reichstein M, Falge E (2005) The effect of soil water content, soil temperature, soil pH-value and the root mass on soil CO2 efflux – a modified model. Plant and Soil 268(1):21–33
Rodríguez JF, Saco PM, Sandi S, Saintilan N, Riccardi G (2017) Potential increase in coastal wetland vulnerability to sea-level rise suggested by considering hydrodynamic attenuation effects. Nature Communications 8:16094
Sánchez-Cañete EP, Scott RL, van Haren J, Barron-Gafford GA (2017) Improving the accuracy of the gradient method for determining soil carbon dioxide efflux. Journal of Geophysical Research: Biogeosciences 122(1):50–64
Smith C, DeLaune R, Patrick W Jr (1983) Carbon dioxide emission and carbon accumulation in coastal wetlands. Estuarine, Coastal and Shelf Science 17(1):21–29
Song C, Xu X, Tian H, Wang Y (2009) Ecosystem–atmosphere exchange of CH4 and N2O and ecosystem respiration in wetlands in the Sanjiang plain, northeastern China. Global Change Biology 59(3):692–705
St-Hilaire F, Wu J, Roulet NT, Frolking S, Lafleur PM, Humphreys ER, Arora V (2010) McGill wetland model: evaluation of a peatland carbon simulator developed for global assessments. Biogeosciences 7(11):3517–3530
Verhulst, Pierre-François (1838) Notice sur la loi que la population poursuit dans son accroissement. Correspondance Mathématique et Physique (10):113–121
Wang G, Chen S (2012) A review on parameterization and uncertainty in modeling greenhouse gas emissions from soil. Geoderma 170:206–216
Wang GD, Wang M, Lu XG, Jiang M (2016) Surface elevation change and susceptibility of coastal wetlands to sea level rise in Liaohe Delta, China. Estuarine, Coastal and Shelf Science 180:204–211
Wanninkhof R, Knox M (2003) Chemical enhancement of CO2 exchange in natural waters. Limnology and Oceanography 41(4):689–697
World Meteorological Organization (2013) WMO Statement on the status of the global climate in 2013
World Meteorological Organization (2014) WMO Statement on the status of the global climate in 2014
Wu J, Guan K, Hayek M, Restrepo-Coupe N, Wiedemann KT, Xu X, Wehr R, Christoffersen BO, Miao G, Silva RD (2017) Partitioning controls on Amazon forest photosynthesis between environmental and biotic factors at hourly to interannual timescales. Global Change Biology 23(3):1240–1257
Xie X, Zhang MQ, Zhao B, Guo HQ (2014) Dependence of coastal wetland ecosystem respiration on temperature and tides: a temporal perspective. Biogeosciences 11(3):539–545
Xu X, Zou X, Cao L, Zhamangulova N, Zhao Y, Tang D, Liu D (2014) Seasonal and spatial dynamics of greenhouse gas emissions under various vegetation covers in a coastal saline wetland in Southeast China. Ecological Engineering 73:469–477
Yang G, Chen H, Wu N, Tian J, Peng C, Zhu Q, Zhu D, He Y, Zheng Q, Zhang C (2014) Effects of soil warming, rainfall reduction and water table level on CH4 emissions from the Zoige peatland in China. Soil Biology and Biochemistry 78(0):83–89
Ye S, Krauss KW, Brix H, Wei M, Olsson L, Yu X, Ma X, Wang J, Yuan H, Zhao G (2016) Inter-annual variability of area-scaled gaseous carbon emissions from wetland soils in the Liaohe Delta, China. PLoS One 11(8):e0160612
Yuste JC, Janssens IA, Ceulemans R (2005) Calibration and validation of an empirical approach to model soil CO2 efflux in a deciduous forest. Biogeochemistry 73(1):209–230
Zhang Q, Sun R, Jiang G, Xu Z, Liu S (2016) Carbon and energy flux from a Phragmites australis wetland in Zhangye oasis-desert area, China. Agricultural and Forest Meteorology 230–231:45–57
Acknowledgements
This study was jointly funded by the National Key R&D Program of China (2016YFE0109600), Ministry of Land and Resources program: “Special foundation for scientific research on public causes” (Grant No. 201111023), National Natural Science Foundation of China (Grant Nos. 41240022 & 40872167), China Geological Survey (Grant Nos. DD20189503, GZH201200503 and DD20160144), and in-kind contributions from the U.S. Geological Survey (through Project Annex No. 6, CH-02.0600) Land Carbon Program and Environments Program. Funding for L. Olsson was provided by Sino-Danish Center for Education and Research and the Danish Council for Independent Research – Natural Sciences (Project 4002-00333B) via a grant to HB. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement of the U.S. Government.
We thank Guangming Zhao, Hongming Yuan, Jin Wang, Xigui Ding, Xiongyi Miao, Jin Liu and other staff of our working group for field and laboratory assistance. We also thank the staff of Reed Institute of Panjin City for the help and convenience they offered. We thank Edward A. Laws from Louisiana State University for assistance with data analysis. Finally, we thank Olivier Pringault, Scott F. Jones, and an anonymous reviewer for the suggestions to improve our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 802 kb)
Rights and permissions
About this article
Cite this article
Yu, X., Ye, S., Olsson, L. et al. A 3-Year In-Situ Measurement of CO2 Efflux in Coastal Wetlands: Understanding Carbon Loss through Ecosystem Respiration and its Partitioning. Wetlands 40, 551–562 (2020). https://doi.org/10.1007/s13157-019-01197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01197-0