Abstract
Foundation plant species play an important functional role in modifying microenvironment through vegetation structure and by influencing soil properties. Mangroves and salt marsh differ in vegetation structure, yet offer similar ecosystem services. This work aimed to understand how different vegetation classes and abiotic factors along a latitudinal gradient of spatial and temporal complexity affect soil CO2 efflux. Soil CO2 efflux, biomass, soil properties, and soil and air temperature were measured in July 2015 and January 2016 in situ at 10 sites along a 342-km latitudinal gradient on the Atlantic coast of Florida. Mean CO2 flux values ranged from 0.41 ± 0.19 to 2.03 ± 0.19 μmol m−2 s−1 across vegetation classes, and efflux values in mangrove plots were not significantly different between sampling dates, whereas ecotone and salt marsh efflux values were. On average, CO2 flux was 1.11 ± 0.18 μmol m−2 s−1 greater in July than in January across plots. There was no significant trend between efflux and aboveground biomass and a positive trend between belowground biomass and CO2 efflux. Edaphic parameters (organic matter content (%), soil N, organic C, and pH) were comparable across all vegetation class soils and there was a positive trend between soil temperature and flux. Soil efflux had an inverse relationship with latitude; in January, there is an increase in efflux with a decrease in latitude, while in July, efflux increases with an increase in latitude. Consequently, we assert that differences in CO2 efflux were due to soil temperature and species productivity along the latitudinal gradient studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon (C) cycling and storage are important ecosystem services provided by mangrove forests (Alongi 2014; Kristensen et al. 2008; Twilley et al. 1992), and carbon dioxide (CO2) efflux from mangrove soils is a significant component of the global C budget (e.g., Alongi 2014; Donato et al. 2011). Net soil C sequestration is a key source of uncertainty in coastal wetland C budgets (Bauer et al. 2013) due to variability associated with both physical and biological factors (e.g., Reddy and DeLaune 2008) that vary along spatial and temporal gradients. The Atlantic coast of Florida moves from a sub-tropical habitat dominated by mangroves in the south to a temperate habitat dominated by salt marsh in the north, with a gradient of change evident in both habitat structure (e.g., Simpson et al. 2017) and Köppen climatic classification (Climate-Data.org 2017). Mild winter temperatures are currently facilitating the expansion of woody mangroves into herbaceous and gramonoid salt marshes along the Atlantic coast of Florida (Cavanaugh et al. 2014), thus providing an excellent opportunity to quantify variability in soil CO2 efflux along a latitudinal gradient of change.
Vegetation type plays an important role in regulating soil efflux through controlling a variety of environmental factors. Communities may modify microenvironment and resource availability by altering the dynamics of biomass, organic matter, and nutrient cycling (Dias et al. 2010; Han et al. 2014; Howes et al. 1986; Maestre and Cortina 2003; Raich et al. 1997; Wang et al. 2009), which are intricately tied with CO2 efflux. Mangrove and salt marsh belowground biomass pools and soil biogeochemistries differ suggesting that replacement of salt marsh with mangroves might alter ecosystem C dynamics (Comeaux et al. 2012; Doughty et al. 2016; Leopold et al. 2013; Lewis et al. 2014; Osland et al. 2013; Patterson and Mendelssohn 1991). Temperature is one of the key regulators influencing biogeochemical processes in wetlands and air temperature varies spatially and temporally along the latitudinal gradient. Air temperature drives soil temperature (Zheng et al. 1993), and there have been many studies showing a positive correlation between temperature and soil efflux (Raich and Schlesinger 1992). Sediment temperatures have been shown to influence CO2 efflux in mangrove ecosystems (Chen et al. 2012; Leopold et al. 2015; Liang et al. 2013) and when coupled with vegetation type will likely alter soil CO2 efflux across latitudes (Inglett et al. 2011).
Understanding the factors that influence soil CO2 efflux is of critical importance to global C cycles. Soil CO2 flux represents the second largest source of terrestrial C release to the atmosphere on a global scale (Harden et al. 2017; Jobbagy and Jackson 2000; Raich and Schlesinger 1992; Schlesinger and Andrews 2000) and mangroves are among the world’s most productive ecosystems, with their net primary production (NPP) estimated at 218 ± 72 Tg C year−1 (Bouillon et al. 2008). Soil efflux is directly correlated with NPP, which is also intricately tied with C storage, suggesting that understanding the spatial and temporal drivers of soil CO2 efflux will be highly valuable for predicting future C storage potential. While soil CO2 efflux is included in budgets of total C storage (e.g., Alongi 2014), the rate of CO2 loss through efflux and the high degree of variability across latitudes is often overlooked. Quantifying CO2 flux in wetland systems and understanding the factors influencing C storage and exchange has become increasingly important because there is still a limited understanding of the factors controlling the temporal and across-ecosystem variability of soil efflux (Reichstein et al. 2003). However, this knowledge is necessary to develop process-based models and to produce reliable regional estimates of soil CO2 efflux.
In this study, we investigated soil CO2 efflux over a latitudinal gradient of spatial and temporal complexity. We hypothesized that alterations in biotic (community structure) and abiotic (temperature) drivers, which are expected to change in response to global climate change (IPCC 2014), will likely modify soil CO2 efflux rates and C dynamics of coastal ecosystems. We sought to answer the following questions: (1) do differences in mangrove and salt marsh productivity lead to differences in soil CO2 efflux and, (2) how does soil temperature affect soil CO2 efflux along a gradient of spatial and temporal complexity? Quantifying soil efflux along a latitudinal gradient enables inferences on how changes in habitat structure will alter C dynamics of these important coastal ecosystems.
Methods
Study Sites
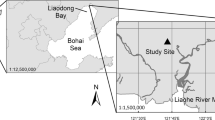
Ten sites, spanning 342 km (26°–29° N) along the east coast of Florida, were sampled to observe soil CO2 efflux rates in structurally different habitats along a latitudinal gradient (Fig. 1). The spatial expanse of sampling sites allowed for quantification and comparison of ecosystem processes in four types of contrasting vegetation structures (class): (a) ecotonal mangroves, (b) pure salt marsh, (c) pure fringing mangroves, and (d) pure interior mangroves. In total, 54 plots were sampled across the ten sites. At each plot, soil CO2 efflux, above- and belowground biomass, and environmental variables were measured, and soil cores were collected.
Within each of the ten sites, 10 × 10-m permanent plots were established in the fringe and interior. Pure salt marsh and ecotonal (salt marsh and mangrove) plots were sampled in the north (28°–29° N), while pure mangrove stands were sampled in the south (26°–27° N) (Fig. 1). The five northern sites had six plots per site, three plots in the fringe (ecotone), and three in the interior (salt marsh). Pure salt marsh stands contained monocultures or polycultures of herbaceous or graminoid salt marsh species and ecotonal stands were composed of a mixture of short-statured (dwarf) Avicennia germinans, Laguncularia racemosa, and Rhizophora mangle mangroves and salt marsh species. Salt marsh species included Batis maritima, Distichlis spicata, Salicornia bigelovii, Salicornia virginica, Spartina alterniflora, and Sueda linearis. Northern sites had an average winter temperature of 21.91 ± 0.20 °C (ranging from − 0.77 to 28.63 °C) and average summer temperature of 30.04 ± 0.14 °C (ranging from 12.02 to 38.34 °C). The sites also had an average precipitation of 3.73 ± 1.27 mm day−1 in January 2016 and 5.00 ± 1.27 mm day−1 in July 2015.
In the five southern sites, there were three sites with six plots (three plots in the fringe (mangrove) and three plots in the interior (mangrove)) (see Fig. 2 in Simpson et al. 2017), while two of the sites only had three plots in the fringe. Southern sites were dominated by all three species of mangrove and were devoid of salt marsh species. Trees found fringing the seaward edge were typically tall (~ 5 m), while those in the landward interior were shorter (~ 3 m) and denser in stature. Southern sites had an average winter temperature of 21.97 ± 0.21 °C (ranging from 5.04 to 36.96 °C) and an average summer temperature of 27.73 ± 1.06 °C (ranging from 17.68 to 42.25 °C). Southern sites had an average precipitation of 6.60 ± 1.02 mm day−1 in January 2016 and 5.08 ± 1.27 mm day−1 in July 2015.
Soil CO2 efflux (μmol m−2 s−1) and soil temperature (°C) across vegetation classes in January 2016 and July 2015. A two-way ANOVA revealed an interaction between vegetation class and time on CO2 efflux (F7,103 = 4.37, p = 0.0049). Soil temperature was significantly different across vegetation class and sampling date (January and July) (F7,95 = 5.78, p = 0.0011). Values ± SE are presented. SE was left off of soil temperatures for visual clarity. Different letters denote significantly different groups
Field Sampling
Soil CO2 Efflux
Soil CO2 efflux was measured using a LI-COR 6400 portable photosynthesis system configured with the LI-COR soil CO2 flux chamber (LI-COR Corp, Lincoln, NE, USA). The chamber was set to penetrate only 0.5 mm into the soil to avoid damaging surface roots. Settings for soil flux measurements were determined at each site following standard procedure (LI-COR Inc. 2003). At each plot, three CO2 efflux measurements were taken and averaged together. Conspicuous microbial mats or biofilms were removed before taking measurements as suggested by Lovelock (2008). Crab burrows and pneumatophores were excluded from the chamber, which have been shown to alter biogeochemical processes and facilitate flux from soils (Bouillon et al. 2008; Kristensen et al. 2008; Pülmanns et al. 2014; Troxler et al. 2015). Sampling was done over 10 days (one site per day) and all measurements were taken at low tide. Interior plots were measured first, then fringe plots as a means to keep tidal range and exposure duration constant between plots and across sites. Sampling was conducted once in July 2015 and once in January 2016 to capture any temporal variability across the seasonal endmembers.
Biomass Measurements
To measure the aboveground biomass pool in mangroves, diameter at breast height (DBH) measurements was taken on all trees to calculate biomass through species-specific allometric equations (Supplementary Table 1). Mangrove root biomass was calculated using the general formula by Komiyama et al. (2008). Aboveground biomass estimates for salt marsh plant species were determined via destructive harvesting of one 1-m2 subplot in each plot. Soil cores (20 cm in length and 5 cm in diameter) were taken to estimate salt marsh belowground biomass. Biomass measurements were then scaled up to Mg ha−1.
Soil and Environmental Characteristics
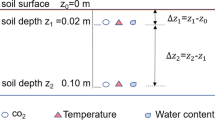
At each plot, 50-cm soil cores were collected using a 10-cm inner diameter stainless steel corer. Soil cores were taken from each plot in April 2015 and subsamples of a known volume were oven-dried at 70 °C to a constant mass to determine bulk density (BD). Bulk density (g cm−3) of each sample was calculated by dividing the oven-dried mass by the volume of the sample. Samples were ball milled with a Mixer/Mill 8000D (SPEX, Metuchen, New Jersey, USA) to ensure homogeneity prior to analysis for total organic C (TC), total N (TN), and loss on ignition (LOI). Subsamples of the homogenized soils were combusted using a CE–440 elemental analyzer (Exeter Analytical, Inc. Chelmsford, Massachusetts, USA) for TC and TN. Remaining subsamples were combusted at 500 °C for 4 h in a Lindberg/Blue MTM Moldatherm box furnace (Thermo Fischer Scientific, Waltham, Massachusetts, USA) for LOI measurements. OC (%) pools were obtained as the product of total organic soil C (TC) and bulk density to 50 cm. Total organic soil C per sampled depth interval was calculated for each core then summed and scaled up to determine total soil C (Mg C ha−1) at each sampling location.
Porewater salinity, pH, and soil temperature were measured during each sampling trip. Porewater was extracted from the ground at 15 cm using a sipper (McKee et al. 1988) and measured with a refractometer for salinity. pH and soil temperature were measured in situ at 5 cm depth in each sampling location with an IQ 150 (Spectrum Technologies, Inc., Aurora, IL, USA). Air temperature was recorded from January 2015 to January 2016 in every plot, with HOBO pendant temperature loggers (Onset Computer Corporation, Bourne, Massachusetts, USA). Additionally, regional air temperatures for January 2015–2016 and regional daily precipitation for July 2015 and January 2016 were obtained online from Weather Underground.
Soil strength, a proxy of belowground decomposition, was measured in each plot, in July 2015, at a depth of 15 cm with a shear vane tester (Geotech Society, Inc. N.Z.). Soil shear stress (hereafter referred to as soil strength) measurements give a relative value of plant decomposition based on the presumption that the integrity of root matrix is directly related to shear vane values (Turner et al. 2009). The handheld instrument measures maximum resistance (torque) when a rod fitted with vanes is rotated at a standard soil depth. Soil strength was calculated as: τ (kPa) = M/K, where M is the torque to shear the soil and K is a constant, calculated by the manufacturer, depending on dimensions and shape of the vane.
Statistical Analysis
A two-way ANOVA was used to test for interactions between sampling time (January or July) and vegetation class (fringe, interior, ecotonal, salt marsh) on soil CO2 efflux. In addition, a two-way ANOVA was used to test for interactions between sampling time (January or July) and latitude (26°–29° N) on soil CO2 efflux, soil temperature, and monthly precipitation. Soil variables (LOI, BD, OC, soil N, soil C, C/N, soil strength) were analyzed across latitude using one-way ANOVAs. Normality was assessed using the Shapiro–Wilks test and homogeneity of samples was assessed using Levene’s test. When required, variables were log or square root transformed to comply with normality and homogeneity of variances when testing linear models. When significant differences were found, pair-wise comparisons were explored with Tukey’s honestly significant differences test. Alpha (α) was set at ≤ 0.05.
Linear regression correlations were used to explore interactions between soil efflux and environmental characteristics (soil temperature, pH, salinity, soil C, soil N, C/N) across all sites. Interactions between biomass components and soil efflux were also explored with simple linear regressions. Stepwise regression modeling was used to test the effects of multiple environmental parameters (soil temperature, pH, salinity, soil C, soil N, soil strength, biomass) on soil efflux. Analyses were performed using JMP 5.0. (S.A.S Inc., Cary, North Carolina, USA). Throughout the manuscript, data are reported as mean ± standard error.
Results
Vegetation Class
CO2 efflux values in mangrove plots (fringe and interior) were not significantly different between sampling dates, whereas northern plot (ecotone and salt marsh) efflux values were F7, 103 = 4.57, p = 0.0049 (Fig. 2). On average, CO2 efflux was 1.11 ± 0.18 μmol m−2 s−1 greater in July than in January across plots. Additionally, mangrove efflux was significantly different from salt marsh efflux in January (p = 0.0226). There was a significant difference in aboveground biomass (AGB) across vegetation classes (p = < 0.0001) (Table 1); however, there was no significant trend between efflux and AGB. Efflux values ranged from 0.41 ± 0.19 to 2.03 ± 0.19 μmol CO2 m−2 s−1, which is in line with other reported values in the literature (e.g., Chen et al. 2010, 2014; Lovelock 2008; Poungparn et al. 2009; Liang et al. 2013; Troxler et al. 2015). Belowground biomass (BGB) was significantly different across vegetation classes (Table 1), and as BGB increases, there was an increase in soil CO2 efflux (R2 = 0.22, p = < 0.001).
LOI, salinity, pH, soil N, and OC (%) were not significantly different across vegetation classes, whereas soil strength, BD, and C/N were (Table 1). Salinity and pH were not significant across vegetation classes and explained minimal variability within CO2 efflux (R2 = 0.18, p = < 0.0001; R2 = 0.11, p = 0.002, respectively). Soil strength values were highest in interior plots and lowest in salt marsh plots (F3,103 = 26.6, p = < 0.0001), and BD was significantly different between fringe and ecotonal plots (F3,103 = 3.69, p = 0.0143). Soil C/N values were low (< 15) in ecotonal and salt marsh and greater in mangrove plots (F3,103 = 24.1, p = < 0.0001). Soil C was significantly different across vegetation classes, ecotonal and salt marsh sites having significantly less soil C than mangrove sites (fringe and interior) (F3,103 = 4.086, p = 0.0087). All plots averaged 27.3 ± 0.36 °C in July. In January, fringe and interior plot soil temperatures averaged 17.8 ± 0.97 °C, whereas ecotone and salt marsh averaged 4.5 ± 0.87 °C (F7,95 = 5.78, p = 0.0011) (Fig. 2).
Latitude
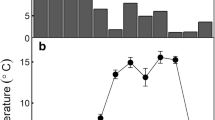
Differences in efflux across the latitudinal gradient were investigated because vegetation class translates to vegetation variation across latitude. Fringe and interior mangrove plots equate to southern plot locations (26–27° N) and ecotone and salt marsh plots were those found in 28–29° N (Fig. 1). Soil efflux has an inverse relationship with latitude; in January, there is an increase in efflux with a decrease in latitude, while in July, efflux increases with an increase in latitude (F3,98 = 11.03, p = < 0.0001) (Fig. 3a). Soil CO2 efflux does not vary between sampling dates at 26° N, whereas efflux was greater in July than in January across the latitudes of 29°–27° N (Fig. 3a). The plots at 29° N had the greatest difference in soil efflux between January and July (0.35 ± 0.14 to 1.84 ± 0.15 μmol CO2 m−2 s−1).
Soil temperatures were significantly different between January and July sampling dates; however, within the sampling dates, soil temperature was variable across latitude (F7,96 = 51.58, p = < 0.0001) (Fig. 3b). Soil temperature was greater in July than in January across all latitudes (F1,99 = 463.81, p = < 0.0001) and accounts for 46% of the variability seen in soil CO2 efflux. As soil temperature increases, soil efflux and variability within increase (R2 = 0.46, p = < 0.0001; y = 0.08x–0.62) (Fig. 4). Air temperatures were cooler in January than in July and decreased with increase in latitude (F7,12,331 = 526, p = < 0.0001). Additionally, soil strength is significantly different across latitude, with higher stress measurements in southern latitudes (F3,102 = 11.8, p = < 0.0001) (Table 2). Soil C (Mg C ha−1) also decreases with an increase in latitude (F3,102 = 4.74, p = 0.004), which may be foreshadowed by the similar trend seen in soil C/N (F3,102 = 19.3, p = < 0.0001) (Table 2). Precipitation was not significantly different across latitude (p = 0.1610) or time (p = 0.9105).
Discussion
Woody encroachment in coastal ecosystems is likely to alter the soil microenvironment through dynamic interactions of biomass structure and resource availability (Dias et al. 2010; Han et al. 2014; Howes et al. 1985; Maestre and Cortina 2003; Raich et al. 1997; Wang et al. 2009). Biomass pools were variable across vegetation classes, and correlation with soil efflux was highly variable as seen in other studies (e.g., Comas and Eissenstat 2004; Jackson et al. 2009; Lovelock et al. 2006; Lovelock 2008; Shi et al. 2006). Both AGB and BGB were significantly greater in mangrove plots than in ecotonal and salt marsh (Table 1) and there was a strong positive relationship between BGB and efflux. Experiments in a range of ecosystems have echoed this finding; increases in BGB equate to increases in soil efflux (e.g., Geng et al. 2012; Hanson et al. 2000; Jackson et al. 2009). Differences in root biomass may lead to a higher rate of efflux by living fine roots due to high metabolic activity and high rates of nutrient uptake (Pregitzer et al. 2002). Additionally, the soil microenvironment which regulates soil CO2 efflux was similar across all vegetation class soils (Table 1). Edaphic variables, which are linked to productivity and organic matter inputs of an ecosystem (Reddy and DeLaune 2008), are expected to change with encroachment of woody species into herbaceous and succulent dominated systems. While vegetation class structure and biomass ratios differ, organic matter content (%), soil N, organic C, and pH were comparable across all vegetation class soils (Table 1). Lack of differences suggests that the soil microenvironment is slow to change with mangrove encroachment (e.g., Henry and Twilley 2013; Perry and Mendelssohn 2009), thus highlighting the importance of other spatial and temporal drivers on soil efflux along the Atlantic coast of Florida.
Soil temperature has a well-documented influence on CO2 efflux in mangrove soils (Chen et al. 2012; Leopold et al. 2015; Liang et al. 2013) and fluxes exhibit a striking seasonal pattern with higher rates during summer months (Alongi et al. 2005; Chen et al. 2016; Howarth and Teal 1979; Kirwan and Blum 2011). Soil efflux trends with soil temperature across vegetation zones (Fig. 2) and vegetation zone reflects differences in latitude along the gradient studied. Many global- and regional-scale analyses on the influence of air temperature on mangrove productivity have used latitude as a surrogate variable (Alongi 2009). Soil temperature is relatively constant along the latitudinal gradient during prospective sampling dates; yet, soil efflux has an inverse relationship with latitude. In January, there is an increase in efflux with a decrease in latitude, while in July, efflux increases with an increase in latitude. This inverse relationship may be driven in part by biomass productivity of the system.
Mangrove and salt marsh species habitat varies spatially and productivity differs temporally. Herbaceous and succulent salt marsh make up 50–60% of northern plots (28–29° N), whereas mangroves make up 100% of the southern plots (26–27° N). Salt marsh species have seasonal pulsed turnover rates (Hopkinson et al. 1978), which contrast to mangroves long lived aboveground biomass pools (Tomlinson 1986). Seasonal differences in productivity will lead to differences in the amount of organic matter available for mineralization. High turnover rates in northern latitudes may contribute more organic matter available for microbial breakdown and mineralization, leading to higher rates of CO2 efflux. Peak soil efflux is expected in the summer months, due to high soil temperatures and the availability of labile substrates (Howes et al. 1985; Teal and Howes 1996). Additionally, litter quality significantly affects organic matter decomposition (Couteaux et al. 1995; Mooshammer et al. 2012; Strickland et al. 2009) and the dominant species in northern plots were S. alterniflora, B. martima, S. virginica, and A. germinans, all with low litter C/N (35.4 ± 1.74, 18.37 ± 0.20, 27.05 ± 0.40, and 26.4 ± 0.86, respectively) as compared to R. mangle (42.5 ± 0.52), the dominant species in southern plots. Low litter C/N supports increases in decomposition, and increased CO2 efflux rates and subsequent decomposition in northern latitudes were supported by low soil strength values and soil C/N (Table 1). Northern plots have the greatest occurrence of salt marsh species and A. germinans pneumatophores. Pneumatophores oxidate the rhizosphere through oxygen leakage from roots (McKee et al. 1988), thus allowing for greater exchange of oxygen in surface and anoxic sediments. Large inputs of labile organic matter and a greater exchange of oxygen suggest that surface soils of northern plots have greater C turnover than southern plots which are composed mainly of R. mangle. Physical and chemical changes in these surface soils may be contributing to the loss of C via leaching/remineralization. High organic matter turnover and CO2 flux in July is met by a large decrease in plant productivity (Hopkinson et al. 1978) and efflux in January, as soil temperatures decrease significantly. Soil efflux involves fluctuating populations of numerous organisms, each undergoing a complex series of reactions, some of which may have different temperature sensitives (Rees et al. 1988). Decreasing soil temperatures can halt microbial breakdown and decrease microbial species richness (Lynch et al. 2004) and when accompanied by decreases in plant productivity, can lead to decreases in soil efflux.
Reductions in mineralization rates may lead to decreases in soil efflux and may increase C accumulation in the system. Hence, the dynamic nature of soil temperature and efflux in northern latitudes has likely diminished C accumulation potential. Increases in latitude are echoed with decreases in average soil C (Mg C ha−1) pools (Table 2). Mangrove plots in the south, which are not subject to such large temporal changes in soil temperature as seen in the northern plots, and the resulting flux of CO2 to the atmosphere, have 23.7% higher soil C pools (Mg C ha−1) (Fig. 3; Table 2). This may be influenced in part by plant productivity, extended growing season, and highly recalcitrant litter. Higher soil strength in southern mangrove plots suggests increased live rooting volume, which helps bind sediments and promotes C accumulation (Baustian et al. 2012). The high rates of mangrove productivity seen in southern plots, coupled with anoxic soils, have contributed to peat development and the high soil C observed. However, increased CO2 efflux in northern latitudes due to increases in soil temperature and species turnover would likely lead to a slower C accumulation in these systems.
Understanding the impacts of spatial and temporal variables on soil efflux, and ultimately soil respiration, is important for an accurate prediction of the future terrestrial C balance in the context of changing climate. Soil CO2 respiration is the sum of heterotrophic and autotrophic respiration in the soil (e.g., of roots, microbes, and soil fauna). In the long term, all CO2 produced in the soil must be emitted by the surface and soil CO2 efflux must correspond to soil respiration. However, in the short term, soil CO2 efflux deviates from total soil respiration due to many spatial and temporal reasons (Maier et al. 2011; Roland et al. 2015). Our study provides values of soil CO2 flux over a range of environmental conditions and illustrates the importance of both spatial and temporal drivers in mangrove ecosystem C balance by identifying important sources of variability in C flux. Fifty-six percent of the variability in soil efflux could be explained by a combination of soil temperature, pH, soil C, and belowground biomass, with soil temperature explaining 46% of the model. Sources of variability may be regulated by the microenvironment and are hard to elucidate over such a large spatial expanse, and the differences across the latitudinal gradient are highly variable and location-dependent. The factors that drive variation in CO2 efflux, and ultimately soil respiration, will influence sources and sinks of C along the Atlantic coast of Florida. Our results provide a foundation for better understanding of the effects of vegetation class and soil temperature on soil efflux and C cycling in a changing landscape. Within the context of climate change, studies on the spatial and temporal drivers of soil efflux are especially important because they can help scientists and environmental managers better anticipate and prepare for the ecological consequences of changing climatic conditions (Glick et al. 2011; Stein et al. 2014).
References
Alongi, D.M., J. Pfitzner, L.A. Trott, F. Tirendi, P. Dixon, and D.W. Klumpp. 2005. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang estuary, China. Estuarine, Coastal and Shelf Science 63 (4): 605–618.
Alongi, D. 2009. The energetics of mangrove forests. Springer Science & Business Media. https://books.google.com/books?hl=en&lr=&id=tHM54IKQSv4C&oi=fnd&pg=PR3&dq=Alongi+2009&ots=snscJi797g&sig=33V7SjKG9N6BZDEKfUpZOaC3OXM#v=onepage&q=Alongi%202009&f=false
Alongi, D.M. 2014. Carbon cycling and storage in mangrove forests. Annual Review of Marine Science 6 (1): 195–219.
Bauer, J.E., W.J. Cai, P.A. Raymond, T.S. Bianchi, C.S. Hopkinson, and P.A. Regnier. 2013. The changing carbon cycle of the coastal ocean. Nature 504 (7478): 61–70.
Baustian, J.J., I.A. Mendelssohn, and M.W. Hester. 2012. Vegetation's importance in regulating surface elevation in a coastal salt marsh facing elevated rates of sea level rise. Global Change Biology 18 (11): 3377–3382.
Bouillon, S., A.V. Borges, E. Castañeda-Moya, K. Diele, T. Dittmar, N.C. Duke, E. Kristensen, S.Y. Lee, C. March and, J.J. Middelburg, V.H. Rivera-Monroy, T.J. Smith III, and R.R. Twilley. 2008. Mangrove production and carbon sinks: A revision of global budget estimates. Global Biogeochemical Cycles 22(2): 1–12.
Cavanaugh, K.C., J.R. Kellner, A.J. Forde, D.S. Gruner, J.D. Parker, W. Rodriguez, and I.C. Feller. 2014. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proceedings of the National Academy of Sciences 111 (2): 723–727.
Chen, G.C., N.F.Y. Tam, and Y. Ye. 2010. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Science of the Total Environment 408 (13): 2761–2767.
Chen, G.C., N.F. Tam, and Y. Ye. 2012. Spatial and seasonal variations of atmospheric N 2 O and CO 2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biology and Biochemistry 48: 175–181.
Chen, G.C., Y.I. Ulumuddin, S. Pramudji, S.Y. Chen, B. Chen, Y. Ye, D.Y. Ou, Z.Y. Ma, H. Huang, and J.K. Wang. 2014. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Science of the Total Environment 487: 91–96.
Chen, G., B. Chen, D. Yu, N.F. Tam, Y. Ye, and S. Chen. 2016. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environmental Research Letters 11 (12): 124019.
Climate-Data.org. Köppen climatic classifications of Florida. https://en.climate-data.org/region/1000/ Accessed on August 31st, 2017.
Comas, L.H., and D.M. Eissenstat. 2004. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Functional Ecology 18 (3): 388–397.
Comeaux, R.S., M.A. Allison, T.S. Bianchi, and T.S. 2012. Mangrove expansion in the Gulf of Mexico with climate change: Implications for wetland health and resistance to rising sea levels. Estuarine, Coastal and Shelf Science 96: 81–95.
Couteaux, M.M., P. Bottner, and B. Berg. 1995. Litter decomposition, climate and liter quality. Trends in Ecology & Evolution 10 (2): 63–66.
Dias, A.T.C., J. Van Ruijven, and F. Berendse. 2010. Plant species richness regulates soil respiration through changes in productivity. Oecologia 163 (3): 805–813.
Donato, D.C., J.B. Kauffman, D. Murdiyarso, S. Kurnianto, M. Stidham, and M. Kanninen. 2011. Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 4 (5): 293–297.
Doughty, C.L., J.A. Langley, W.S. Walker, I.C. Feller, R. Schaub, and S.K. Chapman. 2016. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estuaries and Coasts 39 (2): 385–396.
Geng, Y., Y. Wang, K. Yang, S. Wang, H. Zeng, F. Baumann, P. Kuehn, T. Scholten, and J.S. He. 2012. Soil respiration in Tibetan alpine grasslands: Belowground biomass and soil moisture, but not soil temperature, best explain the large-scale patterns. PloS one 7(4), p.e34968.
Glick, P., B.A. Stein, and N.A. Edelson. 2011. Scanning the conservation horizon: A guide to climate change vulnerability assessment, 168 p. Washington, DC: National Wildlife Federation.
Han, G., Q. Xing, Y. Luo, R. Rafique, J. Yu, and N. Mikle. 2014. Vegetation types alter soil respiration and its temperature sensitivity at the field scale in an estuary wetland. PLoS One 9 (3): e91182.
Hanson, P.J., N.T. Edwards, C.T. Garten, and J.A. Andrews. 2000. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48 (1): 115–146.
Harden, J.W., J. Sanderman, and G. Hugelius. 2017. Soils and the Carbon Cycle. In Soils and the carbon cycle. The International Encyclopedia of Geography.
Henry, K.M., and R.R. Twilley. 2013. Soil development in a coastal Louisiana wetland during a climate-induced vegetation shift from salt-marsh to mangrove. Journal of Coastal Research 29 (6): 1273–1283.
Hopkinson, C.S., J.G. Gosselink, and R.T. Parrando. 1978. Aboveground production of seven marsh plant species in coastal Louisiana. Ecology 59 (4): 760–769.
Howarth, R.W., and J.M. Teal. 1979. Sulfate reduction in a New England salt marsh. Limnology 24.
Howes, B.L., J.W.H. Dacey, and J.M. Teal. 1985. Annual carbon mineralization and belowground production of Spartina alterniflora in a New England salt marsh. Ecology 66 (2): 595–605.
Howes, B.L., J.W.H. Dacey, and D.D. Goehringer. 1986. Factors controlling the growth form of Spartina alterniflora: feedbacks between above-ground production, sediment oxidation, nitrogen and salinity. The Journal of Ecology 881–898.
Inglett, K.S., P.W. Inglett, and R.K. Reddy. 2011. Soil microbial community composition in a restored calcareous subtropical wetland. Soil Science Society of America Journal 75 (5): 1731–1740.
IPCC 2014. Field, C.B., V.R. Barros, D.J. Dokken, K.J. Mach, M.D. Mastrandrea, T.E. Bilir, and B. Girma. 2014. Climate change 2014: Impacts, adaptation, and vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 1132.
Jackson, R.B., C.W. Cook, J.S. Pippen, and S.M. Palmer. 2009. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90 (12): 3352–3366.
Jobbágy, E.G., and R.B. Jackson. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10 (2): 423–436.
Kirwan, M.L., and L.K. Blum. 2011. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosciences 8 (4): 987–993.
Komiyama, A., J.E. Ong, and S. Poungparn. 2008. Allometry, biomass, and productivity of mangrove forests: A review. Aquatic Botany 89 (2): 128–137.
Kristensen, E., S. Bouillon, T. Dittmar, and C. Marchand. 2008. Organic carbon dynamics in mangrove ecosystems: A review. Aquatic Botany 89 (2): 201–209.
Leopold, A., C. Marchand, J. Deborde, C. Chaduteau, and M. Allenbach. 2013. Influence of mangrove zonation on CO 2 fluxes at the sediment–air interface (New Caledonia). Geoderma 202: 62–70.
Leopold, A., C. Marchand, J. Deborde, and M. Allenbach. 2015. Temporal variability of CO2 fluxes at the sediment-air interface in mangroves (New Caledonia). Science of the Total Environment 502: 617–626.
Lewis, D.B., J.A. Brown, and K.L. Jimenez. 2014. Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt-marshes. Estuarine, Coastal and Shelf Science 139: 11–19.
Liang, J.I.N., L.U. Chang-Yi, Y.E. Yong, and Y.E. Gong-Fu. 2013. Soil effluxin a subtropical mangrove wetland in the Jiulong River estuary, China. Pedosphere 23 (5): 678–685.
LI-COR Inc. 2003. 6400–09 Soil CO2 Flux Chamber Instruction Manual. https://www.licor.com/documents/iqpbvjolxbhrf52mlimp
Lovelock, C.E., R.W. Ruess, and I.C. Feller. 2006. Fine root respiration in the mangrove Rhizophora mangle over variation in forest stature and nutrient availability. Tree Physiology 26 (12): 1601–1606.
Lovelock, C.E. 2008. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 11 (2): 342–354.
Lynch, J.M., A. Benedetti, H. Insam, M.P. Nuti, K. Smalla, V. Torsvik, and P. Nannipieri. 2004. Microbial diversity in soil: Ecological theories, the contribution of molecular techniques and the impact of transgenic plants and transgenic microorganisms. Biology and Fertility of Soils 40 (6): 363–385.
Maestre, F.T., and J. Cortina. 2003. Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Applied Soil Ecology 23 (3): 199–209.
Maier, M., H. Schack-Kirchner, E.E. Hildebrand, and D. Schindler. 2011. Soil CO2 efflux vs. soil respiration: Implications for flux models. Agricultural and Forest Meteorology 151 (12): 1723–1730.
McKee, K.L., I.A. Mendelssohn, and M.W. Hester. 1988. Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. American Journal of Botany 75 (9): 1352–1359.
Mooshammer, M., W. Wanek, J. Schnecker, B. Wild, S. Leitner, F. Hofhansl, and K.M. Keiblinger. 2012. Stoichiometric controls of nitrogen and phosphorus cycling in decomposing beech leaf litter. Ecology 93 (4): 770–782.
Osland, M.J., N. Enwright, R.H. Day, and T.W. Doyle. 2013. Winter climate change and coastal wetland foundation species: Salt-marshes vs. mangrove forests in the southeastern United States. Global Change Biology 19 (5): 1482–1494.
Patterson, C.S., and I.A. Mendelssohn. 1991. A comparison of physicochemical variables across plant zones in a mangal/salt marsh community in Louisiana. Wetlands 11 (1): 139–161.
Perry, C.L., and I.A. Mendelssohn. 2009. Ecosystem effects of expanding populations of Avicennia germinans in a Louisiana salt-marsh. Wetlands 29 (1): 396–406.
Poungparn, S., A. Komiyama, A. Tanaka, T. Sangtiean, C. Maknual, S. Kato, and P. Patanaponpaiboon. 2009. Carbon dioxide emission through soil respiration in a secondary mangrove forest of eastern Thailand. Journal of Tropical Ecology 25 (4): 393–400.
Pregitzer, K.S., J.L. DeForest, A.J. Burton, M.F. Allen, R.W. Ruess, and R.L. Hendrick. 2002. Fine root architecture of nine north American trees. Ecological Monographs 72 (2): 293–309.
Pülmanns, N., K. Diele, U. Mehlig, and I. Nordhaus. 2014. Burrows of the semi-terrestrial crab Ucides cordatus enhance CO2 relases in a north Brazilian mangrove forest. PLoS One 9 (10): e109532. https://doi.org/10.1371/journal.pone.0109532.
Raich, J.W., and W.H. Schlesinger. 1992. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44 (2): 81–99.
Raich, J.W., A.E. Russell, and P.M. Vitousek. 1997. Primary productivity and ecosystem development along an elevational gradient on Mauna Loa, Hawai‘i. Ecology 78 (3): 707–721.
Reddy, K.R., and R.D. DeLaune. 2008. Biogeochemistry of wetlands: Science and applications. Boca Raton, Florida: CRC press.
Rees, A.T., M.M. Burrell, T.G. Entwistle, J.B. Hammond, D. Kirk, and N.J. Kruger. 1988. Effects of low temperature on the respiratory metabolism of carbohydrates by plants. In Symposia of the Society for Experimental Biology 42: 377–393.
Reichstein, M., A. Rey, A. Freibauer, J. Tenhunen, R. Valentini, J. Banza, and R. Joffre. 2003. Modeling temporal and large scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Global Biogeochemical Cycles 17 (4).
Roland, M., S. Vicca, M. Bahn, T. Ladreiter-Knauss, M. Schmitt, and I.A. Janssens. 2015. Importance of nondiffusive transport for soil CO2 efflux in a temperate mountain grassland. Journal of Geophysical Research: Biogeosciences 120 (3): 502–512.
Schlesinger, W.H., and J.A. Andrews. 2000. Soil respiration and the global carbon cycle. Biogeochemistry 48 (1): 7–20.
Shi, P.L., X.Z. Zhang, Z.M. Zhong, and H. Ouyang. 2006. Diurnal and seasonal variability of soil CO2 efflux in a cropland ecosystem on the Tibetan plateau. Agricultural and Forest Meteorology 137 (3): 220–233.
Simpson, L.T., T.Z. Osborne, L.J. Duckett, and I.C. Feller. 2017. Carbon storages along a climate induced coastal wetland gradient. Wetlands 1-13.
Stein, B.A., P. Glick, N.A. Edelson, and A. Staudt. 2014. Climate-smart conservation: Putting adaptation principles into practice. Washington, DC: National Wildlife Federation.
Strickland, M.S., C. Lauber, N. Fierer, and M.A. Bradford. 2009. Testing the functional significance of microbial community composition. Ecology 90 (2): 441–451.
Teal, J.M., and B.L. Howes. 1996. Interannual variability of a salt-marsh ecosystem. Limnology and Oceanography 41 (4): 802–809.
Tomlinson, P.B. 1986. The botany of mangroves. Cambridge: Cambridge University Press. https://books.google.com/books/about/The_Botany_of_Mangroves.html?id=uwT6SMY-oNAC, 419p.
Turner, R.E., B.L. Howes, J.M. Teal, C.S. Milan, E.M. Swenson, and D.D. Goehringer-Toner. 2009. Salt marshes and eutrophication: An unsustainable outcome. Limnology and Oceanography 54 (5): 1634–1642.
Troxler, T.G., J.G. Barr, J.D. Fuentes, V. Engel, G. Anderson, C. Sanchez, C., and S.E. Davis. 2015. Component-specific dynamics of riverine mangrove CO2 efflux in the Florida coastal Everglades. Agricultural and Forest Meteorology 213: 273–282.
Twilley, R.R., R.H. Chen, and T. Hargis. 1992. Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water, Air, and Soil Pollution 64 (1–2): 265–288.
Wang, L., P. D'Odorico, S. Manzoni, A. Porporato, and S. Macko. 2009. Carbon and nitrogen dynamics in southern African savannas: The effect of vegetation-induced patch-scale heterogeneities and large scale rainfall gradients. Climatic Change 94 (1-2): 63–76.
Zheng, D., E.R. Hunt Jr, and S.W. Running. 1993. A daily soil temperature model based on air temperature and precipitation for continental applications. Climate Research 183-191.
Acknowledgements
The authors would like to thank Florida State Parks, the Merritt Island National Wildlife Refuge, Guana–Tolmato–Matanzas National Estuarine Research Reserve, and Canaveral National Seashore for permits and unabridged access to their parks. We also thank L.J. Duckett, M.L. Lehmann, and Z.R. Foltz for field and lab assistance, and S.K. Chapman and two anonymous reviewers for their edits and suggestions, which greatly improved this manuscript. This is contribution no. 1088 of the Smithsonian Marine Station.
Funding
This research was funded by the National Aeronautics and Space Administration (NASA) Climate and Biological Response program (NNX11AO94G) and the National Science Foundation (NSF) MacroSystems Biology program (EF1065821).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dennis Swaney
Electronic supplementary material
Supplementary Table 1
(DOC 31 kb)
Rights and permissions
About this article
Cite this article
Simpson, L., Osborne, T.Z. & Feller, I.C. Wetland Soil Co2 Efflux Along a Latitudinal Gradient of Spatial and Temporal Complexity. Estuaries and Coasts 42, 45–54 (2019). https://doi.org/10.1007/s12237-018-0442-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0442-3