Abstract
Fish introduced into wetlands can impact amphibian populations through predation on eggs and larvae. While relationships among hydroperiod, habitat complexity and predation on amphibian larvae have been examined in relatively natural freshwater ecosystems, they have not been explicitly considered in urban landscapes. We examined these relationships in 64 urban wetlands in southern Australia using non-native fish and aquatic invertebrates as predators. Larvae of three out of six frog species detected during our study were captured in wetlands containing fish. With other variables held constant, the mean number of tree frog (Litoria spp.) larvae in the wetland with the highest abundance of predatory fish was predicted to be only 0.8–3.2 % of the number of larvae in a fishless wetland. We also found a negative relationship between predatory invertebrates and larval abundance. The abundance of tree frog larvae was greatest in ephemeral wetlands where predatory fish were generally absent. Our results suggest that traditional models of amphibian distribution along pond-permanence gradients may not be applicable in urban ecosystems due to modified hydrology favoring permanent wetlands. To conserve amphibians in urban areas, we recommend draining wetlands periodically to remove exotic fish, and conserving or restoring ephemeral wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation and hydroperiod influence the composition of larval amphibian communities in wetlands (Wilbur 1987; Werner et al. 2007). Predatory fish and aquatic invertebrates can eliminate larvae of some species and alter amphibian assemblage structure (Heyer et al. 1975; Hero et al. 1998). The introduction of fish into geographic areas outside their natural range is regarded as a major driver of the decline and disappearance of some amphibian species, through predation on their eggs and larvae (Collins and Storfer 2003; Kats and Ferrer 2003). For example, introduction of mosquitofish (Gambusia spp.) has been implicated in the decline of several amphibian species (Gamradt and Kats 1996; Hamer et al. 2002). There is also evidence that introduced invertebrate predators such as crayfish (Cambaridae) can reduce the distribution and abundance of some amphibian species (Gamradt and Kats 1996; Riley et al. 2005), but the geographic extent of this impact appears to be far less than that of exotic fish. Wellborn et al. (1996) suggested that fish predation on amphibian eggs and larvae will be greatest in permanent wetlands and decrease along a hydroperiod gradient towards ephemeral wetlands, which do not support permanent populations of fish because they periodically dry. In these fish-free ephemeral wetlands, aquatic invertebrates are the primary predators of amphibian larvae (Wellborn et al. 1996).
Accordingly, wetland hydroperiod can have a strong effect on amphibian assemblages, due in part to its influence on occurrence of predatory fish and aquatic invertebrates, and also due to life history requirements of amphibians, i.e., the duration of the larval phase (Pechmann et al. 1989; Wellborn et al. 1996). For example, Semlitsch et al. (1996) found that wetland hydroperiod was a significant predictor of number and diversity of metamorphosing amphibians. Elsewhere, studies have shown that while fish and invertebrate predation can affect amphibian species, the strength of the effect can be mitigated by either wetland ephemerality (Werner et al. 2007) or structural complexity of the aquatic habitat (Tarr and Babbitt 2002; Baber and Babbitt 2004; Hartel et al. 2007). Increased structural complexity in wetlands (e.g., dense aquatic vegetation) may reduce fish-larvae or invertebrate-larvae encounters by providing habitat refugia, and subsequently decrease the number of larvae killed or injured by these aquatic predators.
Humans often introduce exotic fish into waterways (Kats and Ferrer 2003; Pyke 2005). Because the occurrence of non-native fish increases with an increase in the degree of human development around a wetland (Copp et al. 2005), it is expected that exotic fish species in particular would have major impacts on amphibian occurrence and abundance in urban wetlands. By contrast, while some invertebrate predators decrease the abundance of amphibian larvae in urban waterways (Riley et al. 2005), they do not appear to negatively affect amphibian occurrence (Babbitt et al. 2003).
The degradation of aquatic habitats in urban landscapes caused by the introduction of predatory fish is recognized as a key threat to amphibians (Hamer and McDonnell 2008). Urbanization often results in the conversion of fish-free ephemeral wetlands to deeper and more permanent wetlands that contain fish (Kentula et al. 2004). For example, losses in natural ephemeral wetlands and gains in permanent wetlands in the Willamette Valley, Oregon, U.S.A., have facilitated the spread of non-native fish and reduced amphibian occurrence in the region (Pearl et al. 2005). In some regions, predatory fish are also more common in permanent wetlands in urban areas than in rural areas (Rubbo and Kiesecker 2005). These studies suggest that the distribution of amphibian species along urbanization gradients may be primarily influenced by hydroperiod because of the relationship between predatory fish and wetland permanency.
However, increased aquatic vegetation that provides refuge sites in urban wetlands may decrease the risk of predation by non-native fish and enable some species to persist in wetlands with long hydroperiods (Pearl et al. 2005). Despite the potential for aquatic vegetation and shortened hydroperiods to mitigate the impact of predatory fish on larval amphibians in urban wetlands, no studies conducted in these areas have sought to identify interactive effects between habitat attributes and fish predation. Furthermore, no previous studies have explicitly assessed Wellborn et al.’s (1996) model of mechanisms generating assemblage structure in amphibian communities in urban wetlands by investigating the relative importance of native (invertebrate) and non-native (fish) predators.
Here, we assess the relationships among larval amphibian occurrence and abundance and aquatic predators, wetland hydroperiod and aquatic vegetation in wetlands distributed along an urban-rural gradient. While previous studies have assessed the influence of these parameters on amphibian communities in relatively natural ecosystems (e.g., Werner et al. 2007; Both et al. 2011), we sought to test whether the mechanisms of Wellborn et al. (1996) are equally applicable in human-modified landscapes. Our approach provides insight into how urbanization may affect a traditional model of amphibian distribution along the pond-permanence gradient. We also provide recommendations for managing amphibian communities in urban wetlands.
Methods
Study Area
We conducted our study in the Greater Melbourne area of Victoria in south-eastern Australia. The current human population of the region is 3.9 million and is predicted to increase to 5 million by 2051 (McDonnell and Holland 2008; Australian Bureau of Statistics 2010). Melbourne has a temperate climate, with an annual mean precipitation of 639 mm; precipitation is distributed evenly throughout the year (Stern 2005). The area of remnant wetlands in the inner and outer suburbs of Melbourne totals 4546 ha (McDonnell and Holland 2008).
Amphibian Surveys
We conducted tadpole surveys at 65 wetlands in public parks and gardens. Sixty-two wetlands were originally selected by Parris (2006) using maps and aerial photographs, and stratified by wetland size, presence or absence of a vertical pond wall, and landscape context. The remaining three wetlands were selected by Hamer and Parris (2011) and were located within 100 m of the original sites. We further stratified our sample of 65 wetlands by the proportion of road cover within a 500-m radius along an urban-rural gradient (see Hamer and Parris (2011) for a map of the study area). The availability of wetlands for sampling within the larger set surveyed by Parris (2006) was reduced by drought conditions experienced during our study. The 65 wetlands we surveyed included remnant wetlands on the rural fringe, ornamental ponds in city parks, old quarries and stormwater retention ponds. Wetland sites varied considerably in size (range: 2–51104 m2).
We conducted four aquatic surveys at the 65 wetlands over two breeding seasons in spring, summer and autumn (November–December 2007, March–April 2008, August–September 2008 and November–December 2008) to include the full range of breeding periods of the 14 frog species recorded in the Greater Melbourne area (Hero et al. 1991). We randomized the sequence that wetland sites were visited.
We quantified the abundance of amphibian species using two survey techniques at each wetland: 1) bottle traps left in wetlands overnight; and 2) dip-netting following trap retrieval in the mornings. Bottle traps were plastic funnel traps made from 1.25-L soft drink bottles (Richter 1995) and dip nets were 1.4 mm mesh on a triangular frame (35 × 30 × 30 cm). The total number of bottle traps deployed was proportional to a wetland’s surface area (two traps minimum, plus one additional trap per doubling of surface area >25 m2, maximum 12 traps; modified sampling protocol from Adams et al. 1997). Dip-net surveys were time-constrained; a minimum 2 min was spent dip-netting, plus two additional minutes per doubling of surface area >25 m2 to a maximum of 22 min. We stratified traps and dip-net sweeps proportionally among microhabitat types, because larval amphibians are often microhabitat specialists (Shaffer et al. 1994). No bottle traps were deployed in wetlands if the water depth was <10 cm.
We identified larvae to species in the field following Anstis (2002) and released individuals once identified. Specimens that could not be identified in the field were anaesthetized and preserved in 70 % ethanol for later identification. The larvae of the southern brown tree frog (Litoria ewingii) and whistling tree frog (L. verreauxii) were grouped under the category “Litoria spp.”, hereafter referred to as “tree frogs”, because tadpoles of the two species are morphologically similar and cannot be readily distinguished.
Fish Sampling, Aquatic Invertebrates and Wetland Variables
We quantified predatory fish catch per unit effort (CPUE) by dividing the number of predatory fish captured in bottle traps during a survey period by the number of bottle traps deployed during that period. We defined predatory fish as those species recorded in the study area that are known, based on previous studies (e.g., Morgan and Buttemer 1996; Pyke and White 2000), to eat the eggs or tadpoles of any frog species found in south-eastern Australia. We measured the proportion of the wetland surface area covered by aquatic vegetation (i.e., % emergent + % submerged vegetation = total % aquatic vegetation) at wetlands from October–December 2007 (range: 0–192 %). We defined emergent and submerged vegetation as aquatic vegetation that extended above or below the water surface, respectively. Both emergent and submerged vegetation can exist in the same area of a wetland, so total % aquatic vegetation can exceed 100 %. We measured hydroperiod at wetlands throughout the study and scored the variable according to the proportion of surveys (n/4) in which a wetland held water. Wetlands with a hydroperiod score <1 dried on at least one occasion and were classified as ephemeral; wetlands with a score = 1 were classified as permanent.
As with predatory fish, we quantified CPUE of predatory aquatic invertebrates by dividing the number of predatory invertebrates captured during a survey period by the number of bottle traps deployed during that period. Predatory invertebrates included: giant water bugs (Belostomatidae), diving beetles (Dytiscidae), water scorpions and needle bugs (Nepidae), backswimmers (Notonectidae), dragonfly and damselfly larvae (Odonata) and freshwater crayfish (Parastacidae; Van Buskirk 2005; Wells 2007; Werner et al. 2007). Because not all aquatic invertebrates represent an equal risk of predation to anuran larvae, we calculated a score of predation risk among these taxa, similar to that used by Van Buskirk (2005). The mean CPUE of each of the invertebrate taxa were weighted by a danger rating, according to their size and ability to handle tadpoles as prey items, as follows: giant water bugs (× 0.50), diving beetles (adults: × 0.75; larvae: × 0.50), water scorpions and needle bugs (× 0.50), backswimmers (× 0.25), dragonfly larvae (× 0.50), damselfly larvae (× 0.25), and freshwater crayfish (× 1.00).
Statistical Analyses
We quantified CPUE of amphibian larvae by dividing the number of larvae captured during a survey period by the number of bottle traps deployed during that period. We did not combine CPUE from bottle trap and dip-net surveys into a single measure, because the former method was passive area-constrained sampling while the latter was active time-constrained sampling, and these different sampling efforts cannot be reliably standardized for effort (Shulse et al. 2010). Instead, we used information from our dip-net surveys to supplement bottle trap data. When a species was captured by dip-netting but not bottle-trapping, we assigned the site a standard CPUE of 0.01 so that detection of the species was acknowledged, but its abundance was lower than the minimum CPUE recorded in bottle traps (this value was one-half the minimum CPUE possible from bottle trapping; see Van Buskirk 2005; Babbitt et al. 2009). Assigning a CPUE of 0 to these sites (n = 5), which would result from using bottle trap data only, may produce biased estimates of the effects of the explanatory variables on occupancy and abundance. We then calculated a mean value from trapping over 1–3 periods. Survey data from one survey period (March–April 2008) were eliminated from the analysis due to the low number of individuals captured. Because one of the 65 wetlands held water only on this occasion, we removed it from the dataset, leaving 64 wetlands for analysis (51 permanent and 13 ephemeral wetlands).
We assessed the similarity of the larval frog assemblages according to three factors: 1) predatory fish present or absent; 2) low versus high predatory invertebrate abundance; and 3) permanent versus ephemeral wetlands. We first constructed a similarity matrix, based on the relative abundance (square-root transformed) of tadpoles in wetlands where at least one species was captured, using the Bray-Curtis coefficient (Bray and Curtis 1957). Eliminating wetlands where no species were captured resulted in a similarity matrix between pairs of 32 wetlands. Only species captured at >1 wetland were included in the matrix. We defined high invertebrate abundance as abundances that were greater than half the maximum CPUE of the 32 wetlands. We then used non-metric multi-dimensional scaling (nMDS) to represent the relative dissimilarities in species assemblages among the 32 wetlands according to the three factors (Clarke and Warwick 1994). We used the “stress” value as a measure of goodness-of-fit of the overall structure of the sample relationships in two-dimensional space, and we interpreted this value according to the rules-of-thumb given in Clarke and Warwick (1994).
We performed a one-way analysis of similarities (ANOSIM) on the data in the similarity matrix (999 permutations) to determine if there were differences in the assemblages between the three factors (Clarke and Warwick 1994). For those tests that had a high Global R value, we then assessed the contribution of each species to the average sample dissimilarity between wetlands with different levels of the factor using the SIMPER (similarity percentages) routine (Clarke and Warwick 1994). The SIMPER routine calculates the overall percentage contribution each species makes to the average dissimilarity between two groups, so that species can be listed in decreasing order of their importance in discriminating the two sample sets (Clarke and Gorley 2001). Analyses were conducted using Primer v5 (Clarke and Gorley 2001).
We used Bayesian, zero-inflated negative binomial regression (ZINB) in OpenBUGS 3.0.3 (Spiegelhalter et al. 2007) to explore relationships among predatory fish and predatory invertebrate abundance, proportional cover of aquatic vegetation and wetland hydroperiod, and the probability of occurrence and relative abundance of larval amphibians for species with a naive detection rate >0.25. Ecological data sets composed of counts often contain a large proportion of zero values so that the data do not readily fit standard distributions (Martin et al. 2005). This zero inflation can result from either ‘true zero counts’ caused by the real ecological effect of interest, and ‘false zero counts’ caused by the failure to detect a species when it is present (Martin et al. 2005); both sources of error will cause bias in parameter estimates and their confidence intervals (Tyre et al. 2003). We used a ZINB model to account for both excess zeros and overdispersion (i.e., large counts causing inflated variance) in the relative abundance data. We used a conditional (or hurdle) model in which the first model estimated the probability that the species was present at a wetland using logistic regression (1), and the second model estimated the expected relative abundance of the species, conditional on presence, using negative binomial regression (2) (Welsh et al. 1996):
where: p(x i ) is the probability that an observation i is generated by the negative binomial, expressed as a function of the explanatory variables (x) through a logit transformation; λ(z i ) is the relative abundance of individuals at wetland i expressed as a function of the explanatory variables (z) through a log transformation; and β0 and β1 are the regression coefficients for each explanatory variable (Martin et al. 2005). The negative binomial model included a parameter (ϕ) to account for overdispersion and excess zeros in the count data.
We included the four explanatory variables (abundance of predatory fish, abundance of predatory invertebrates, proportional cover of aquatic vegetation and hydroperiod) in different combinations in the ZINB models to assess competing hypotheses concerning the effects of these variables on the probability of occurrence and relative abundance of amphibian larvae at a wetland. We hypothesized that the probability of occurrence and abundance would 1) decrease with increasing predator abundance; 2) decrease with increasing wetland hydroperiod; and 3) increase with increasing cover of aquatic vegetation. We also hypothesized that there would be an interaction between predator abundance and aquatic vegetation. Each variable was included in a model that represented one of these hypotheses. We assessed two linear interactions; 1) abundance of predatory fish × aquatic vegetation cover, and 2) abundance of predatory invertebrates × aquatic vegetation cover, as aquatic vegetation may increase the survival of larval anurans in the presence of predatory fish and invertebrates (Tarr and Babbitt 2002; Baber and Babbitt 2004). We also assessed one quadratic term (hydroperiod) as there is evidence from natural systems that larval amphibian abundance peaks in wetlands of intermediate hydroperiod (Snodgrass et al. 2000a), which fits with the model of Wellborn et al. (1996). In previous work we described the response of the amphibian community in the study area to non-native fish, aquatic vegetation and hydroperiod, but our response variables were species richness and community composition, and we did not include interaction terms (Hamer and Parris 2011). We checked for intercorrelations between the variables, and did not include proportional cover of aquatic vegetation and hydroperiod in the same model as they were highly correlated (Spearman’s rho, ρ = −0.47; Online Resource 1). Data on CPUE of predatory fish were ln(x + 1)-transformed prior to analysis.
The wetlands included in this study tended to occur in spatial clusters with some wetlands <20 m apart. Thus, the presence and relative abundance of larvae of a species at a site may be influenced by their occurrence/abundance at one or more neighboring sites, resulting in spatial autocorrelation which may lead to biased estimation of model coefficients (Wintle and Bardos 2006). We therefore added a categorical ‘cluster term’ as a random effect in each model (Parris 2006). We assigned the 64 wetlands in the study area to one of 22 clusters, each containing 1–17 wetlands. Each cluster was comprised of wetlands ≤2 km apart that were not separated by busy roads (i.e., barriers to dispersal). In total, we made inferences based on 14 models, which included one model containing a constant and random effect term only (cluster model), another containing a constant only (null model), and 12 containing the explanatory variables (Table 1).
We used OpenBUGS to generate 100,000 samples from the posterior distribution of each model after discarding an initial ‘burn-in’ of 10,000 samples. We centered the explanatory variables by subtracting the mean from each variable, which improves the efficiency of the Monte Carlo Markov Chain sampling by reducing the correlation between successive samples (McCarthy 2007). We used vague priors (mean, standard deviation) for the intercept term (a ∼ dnorm(0, 0.001)), overdispersion parameter (ϕ ∼ dgamma(0.1, 0.1)) and the regression coefficients (β(x), β(z) ∼ dnorm(0, 0.1)). We ran three replicate Markov chains for each model and checked that convergence was reached for all variables on the basis of the Brooks-Gelman-Rubin statistic (i.e., R < 1.001; Gelman and Rubin 1992; Brooks and Gelman 1998), and by visual inspection of the chain histories. Bayesian credible intervals (95 % confidence intervals, BCIs) were obtained from the 2.5th and 97.5th percentiles of the posterior distribution.
We assessed the relative importance of the four explanatory variables by calculating the multiplicative effect (with BCIs) of each variable on the probability of occurrence and relative abundance across the range of the variable. Calculating multiplicative effects allowed us to determine the magnitude and precision of the relationship between occurrence/relative abundance and the variables, which is more ecologically meaningful than relying on statistical significance (McCarthy 2007; see also Cumming and Finch 2005). The multiplicative effect size of each explanatory variable in the logistic regression (occurrence) models was calculated as the change in the probability of occurrence across the range of that variable, assuming an average probability of occurrence of 0.5:
where \( \mathrm{Pmax}={1 \left/ {{\left[ { \exp \left( {-{\beta_i}\times \mathrm{ma}{{\mathrm{x}}_i}} \right)+1} \right]}} \right.} \) and \( \mathrm{Pmin}={1 \left/ {{\left[ { \exp \left( {-{\beta_i}\times \mathrm{mi}{{\mathrm{n}}_i}} \right)+1} \right]}} \right.} \), and max i and min i are the maximum and minimum values observed for that variable, respectively. A multiplicative effect size of 0 corresponds to no change in the probability of occurrence. An explanatory variable with E i substantially different from 0 (either negative or positive) is likely to have a strong effect on the probability of occurrence.
The multiplicative effect size of the negative binomial regression (abundance) was calculated as the exponent of the standardized coefficient:
where E i is the multiplicative effect of variable i, β i is the regression coefficient of variable i, and range i is the range of values for variable i. In this case, a multiplicative effect size of 1 corresponds to no change in the relative abundance, so an explanatory variable with E i substantially different from 1 is expected to have a strong effect on abundance (with E i > 1 = positive effect; E i < 1 = negative effect). Wide BCIs indicate low precision and hence greater uncertainty in the estimates of model coefficients.
We also assessed the relative fit of the models against model complexity using the Deviance Information Criterion (DIC; Spiegelhalter et al. 2002). We considered the best-supported models to be those with a ΔDIC < 2 (ΔDIC = DIC–minimum (DIC)), models with a ΔDIC of 2–10 to have less support, and those with a ΔDIC > 10 to have essentially no support (Spiegelhalter et al. 2007; Anderson 2008).
Results
Larval Frog Assemblages
The mean CPUE of the larvae of six frog species captured during our surveys ranged from 0.00 to 0.02 in wetlands where predatory fish were present, and from 0.01 to 1.59 in wetlands where fish were absent (Fig. 1). Tree frog larvae had the highest rate of occurrence and relative abundance at the 64 wetlands, whereas the larvae of the Victorian smooth froglet (Geocrinia victoriana) had the lowest (Online Resource 2). Tree frogs, southern bullfrog (Limnodynastes dumerilii) and striped marsh frog (L. peronii) were the only species captured in wetlands where predatory fish were present. The relative abundance of the larvae of all species except tree frogs was highest in ephemeral wetlands where predatory fish were absent (Online Resource 3).
Predator Assemblages
Predatory fish consisted of two non-native species, mosquitofish (Gambusia holbrooki) and redfin perch (Perca fluviatilis). No other predatory fish were encountered. Mosquitofish accounted for 99.9 % of the total number of predatory fish captured in bottle traps while three redfin perch were captured at one site. Backswimmers accounted for 53.1 % of the total number of predatory invertebrates captured while crayfish accounted for only 1.8 % of captures (Online Resource 4). Predatory fish were present in only 2 out of 13 (15.4 %) ephemeral wetlands, while they were present in 22 out of 51 (43.1 %) permanent wetlands. In contrast, predatory invertebrates were present in 10 ephemeral wetlands (76.9 %) and in 36 permanent wetlands (70.6 %). However, the CPUE of predatory fish and predatory invertebrates was not strongly correlated with hydroperiod (Spearman’s rho, ρ = 0.27 and −0.26, respectively; Online Resource 1).
Composition of Larval Frog Assemblages
Wetlands with and without predatory fish had different larval assemblages (ANOSIM: Global R = 0.28; Fig. 2). The average dissimilarity (SIMPER) between these two groups was 72.6, with 32.9 (45.3 %), 14.8 (20.4 %), 12.2 (16.8 %) and 10.2 (14.0 %) contributed by tree frogs, striped marsh frog, southern bullfrog and common eastern froglet (Crinia signifera), respectively. Tree frogs contributed most to the average similarity of larval assemblages in wetlands without predatory fish (79.9 %), followed by common eastern froglet (8.4 %) and southern bullfrog (7.1 %). Tree frogs also contributed most to the average similarity of assemblages in wetlands with predatory fish present (63.6 %), followed by striped marsh frog (36.4 %). These results support our prediction, that the presence of predatory fish in a wetland modified the composition of larval frog assemblages.
Non-metric multi-dimensional scaling (nMDS) ordination of 32 wetlands based on Bray-Curtis similarities of the relative abundance of five tadpole species (Crinia signifera, Limnodynastes dumerilii, L. peronii, Litoria spp. and Paracrinia haswelli), according to: a predatory fish present (open circles) and absent (shaded triangles); b low predatory invertebrate abundance (open circles) and high predatory invertebrate abundance (shaded triangles); and c permanent wetlands (open circles) and ephemeral wetlands (shaded triangles). Relationships are depicted in two-dimensional space; stress = 0.12
We found no evidence of a large difference in the composition of larval assemblages between wetlands with different invertebrate abundance (Global R = −0.13), or between permanent and ephemeral wetlands (Global R = 0.04).
Based on these results and the number of detections, we used CPUE of tree frogs to assess the relationships between predatory fish, predatory aquatic invertebrates, aquatic vegetation and hydroperiod, and the occurrence and relative abundance of larvae in a wetland.
Probability of Occurrence and Relative Abundance of Tree Frog Larvae
Relationships with Predators
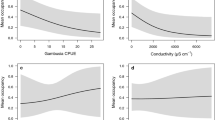
Tree frog larvae were less likely to occur at a wetland as the CPUE of predatory fish increased, with an average multiplicative effect size of −0.405 (range of 95 % CI: −0.783–0.138) across the five models that included this variable (Fig. 3a). Thus, the probability of occurrence at wetlands with the highest CPUE of fish was about 41 % lower than that at wetlands with the lowest CPUE (i.e., no fish). There was also a strong negative association between the CPUE of predatory fish and the relative abundance of tree frog larvae (conditional on presence), with predicted multiplicative effect sizes between 0.008 and 0.032 (range of 95 % CI: 0.000–1.660; Fig. 3b). Thus, the wetland with the highest CPUE of predatory fish (21.0 fish per bottle trap) was predicted to have 0.8–3.2 % of the mean number of larvae in a wetland with no fish present, holding other variables constant. The predicted relationship showed a steep decline in abundance of tree frog larvae with increasing CPUE of predatory fish at a wetland (Fig. 4a).
The multiplicative effect of four explanatory variables (mean and BCIs) on the a occurrence and b relative abundance of tree frog (Litoria spp.) larvae at 64 wetlands in the study area. See Table 1 for model numbers. Effect sizes >0 or 1 indicate a positive effect of the explanatory variable on occurrence or relative abundance, respectively; effect sizes <0 or 1 indicate negative effects
Predicted relative abundance (solid line) and BCIs (dashed lines) of tree frog (Litoria spp.) larvae as a function of mean CPUE of a predatory fish; and b predatory invertebrates (weighted by a danger rating). Mean values and BCIs were estimated from model 7 while holding the other explanatory variables at their mean value
Interestingly, we found a positive relationship between the CPUE of predatory invertebrates and the occurrence of tree frog larvae (Fig. 3a), with an average multiplicative effect size of 0.565 (range of 95 % CI: −0.153–0.893) across the five models that included this variable. However, there was a negative relationship between the CPUE of predatory invertebrates and the relative abundance of tree frog larvae. While the latter relationship was not quite as strong as that of predatory fish, it was still substantial with multiplicative effect sizes between 0.015 and 0.096 (Fig. 3b). The predicted relationship showed a gradual decline in larval abundance over the range of predatory invertebrate abundance observed at the wetlands (Fig. 4b).
Relationships with Hydroperiod
While there was some evidence that tree frog larvae were less likely to occur as wetland hydroperiod increased, the wide BCIs encompassing 1 indicated this association was uncertain (Fig. 3a). But we found a clear negative relationship between wetland permanence (hydroperiod) and relative abundance, with multiplicative effect sizes between 0.015 and 0.051 (Fig. 3b). Thus, permanent wetlands were predicted to have 2–5 % of the mean number of larvae detected at the most ephemeral wetland, when holding the other explanatory variables constant. We did not find evidence of a quadratic effect of hydroperiod in either the logistic model (mean, BCI: –1.53, –6.38–3.48) or the negative binomial model of relative abundance (0.00, –6.20–6.20).
Relationships with Aquatic Vegetation
We found evidence of a strong positive relationship between aquatic vegetation and the occurrence of tree frog larvae, with multiplicative effect sizes between 0.71 and 0.76 (Fig. 3a). Thus, the probability of tree frog larvae occurring at a wetland increased by 71–76 % between a wetland with no vegetation and the wetland with the highest proportional cover of aquatic vegetation, when holding the other variables constant. There was limited evidence for a positive association between aquatic vegetation and the relative abundance of tree frog larvae. Two out of five models predicted a small positive relationship, with the other three predicting an effect size close to 1 (no change), and all estimates had wide BCIs encompassing 1 (Fig. 3b).
Interaction Between Predator Abundance and Aquatic Vegetation
We did not find evidence of an interaction between the CPUE of predatory fish and aquatic vegetation in either the logistic model (mean, BCI: 1.92, −1.87–5.79) or the negative binomial model of relative abundance (−0.36, −5.88–5.17). Again, there was no interaction between invertebrate CPUE and aquatic vegetation in either model (mean, BCI: 0.91, −3.23–5.50 and 1.71, −2.35–5.74, respectively).
Model Support and DIC Values
Two models of the probability of occurrence and relative abundance of tree frog larvae received more support than the remaining six models based on DIC values (ΔDIC < 2.0: models 7 and 11; Table 1). These two models included the CPUE of predatory fish, and the CPUE of predatory invertebrates and wetland hydroperiod as explanatory variables. There was also some support for seven other models that included various combinations of the four explanatory variables (ΔDIC = 2.0–5.0), including the two interaction models (models 6 and 14) and the quadratic model (model 9). There was essentially no support for the null model (model 1) or for the model including only the cluster term (model 2; ΔDIC > 10.0).
Discussion
The composition of tadpole assemblages differed between wetlands with no predatory fish and wetlands with fish, and there was a strong negative relationship between CPUE of predatory fish and the relative abundance of tree frog larvae. The relationship between predatory fish and larval occurrence was also negative but less certain, and a similar result was found with species richness earlier (Hamer and Parris 2011). Although no experimental studies have been conducted, tree frog larvae may suffer high rates of fish predation because they actively swim and feed throughout the water column (Peterson et al. 1992; Anstis 2002), which would make them particularly vulnerable to attack from foraging mosquitofish (Pyke 2005). The swimming behavior of southern brown tree frog larvae also increases its risk of attack by odonate larvae (Peterson et al. 1992), and we observed a negative relationship between the CPUE of predatory invertebrates and relative abundance of tree frog larvae. In contrast, the probability of occurrence of tree frog larvae at a wetland increased with an increasing abundance of predatory invertebrates, probably because high invertebrate abundance at a wetland is correlated with increasing aquatic vegetation, which also favors occupancy by tree frogs. The relationship between predatory invertebrates and tree frog larvae may be slightly weaker because mosquitofish are fast swimmers that actively search for prey (Pyke 2005), whereas predatory invertebrates often employ a passive ambush foraging mode thereby limiting their foraging area and reducing encounter rates with prey (Wellborn et al. 1996).
Some studies suggest that habitat complexity (e.g., aquatic vegetation) may mediate the level of predation by mosquitofish on tadpoles to some extent (Morgan and Buttemer 1996), particularly for relatively inactive species (Baber and Babbitt 2004). There was a positive relationship between the extent of aquatic vegetation in a wetland and the occurrence of tree frog larvae, as there was for species richness earlier (Hamer and Parris 2011). However, the ability of vegetation to mitigate the impact of predatory fish on larvae was uncertain, as indicated by the very wide BCIs around the predicted effect sizes for the interaction model that overlapped zero. Mosquitofish forage efficiently in structurally-complex habitats (Casterlin and Reynolds 1977) and are likely to encounter tree frog larvae even in wetlands with a high cover of aquatic vegetation. Mosquitofish are therefore likely to be reducing the number of tree frog larvae surviving to metamorphosis at wetlands in our region and where they have been introduced elsewhere. Aquatic habitat complexity has been shown to reduce predation rates by invertebrates on amphibian larvae, although this will depend on the foraging mode of the predator and the use of protective microhabitats by amphibians (Babbitt and Tanner 1997; Tarr and Babbitt 2002).
Our results are largely consistent with the permanence-predation mechanism proposed by Wellborn et al. (1996); predatory fish were more prevalent in permanent wetlands, while predatory invertebrates were more common in ephemeral wetlands. We found no evidence of a peak in larval occurrence or abundance in wetlands of intermediate hydroperiod (wetlands with a hydroperiod score <1 but >0.25). However, this may be due to the low variability in the hydroperiod of wetlands we observed, which resulted in a small sample of wetlands of intermediate hydroperiod (n = 11). In many urban ecosystems, wetland hydroperiod is likely to be increased by modified hydrology caused by impervious surfaces (e.g., roads, parking lots, buildings and pavements) in the surrounding landscape (Kentula et al. 2004; Lee et al. 2006). Therefore, the permanence gradient that underpins the theoretical model of Wellborn et al. (1996) may become skewed towards a greater prevalence of permanent wetlands in urban areas.
The relative abundance of tree frog larvae was higher in ephemeral wetlands, and predatory fish were absent from a higher proportion of ephemeral wetlands. Occurrence was also higher in ephemeral wetlands but there was greater uncertainty. A similar result was found with species richness in our earlier study (Hamer and Parris 2011). There is likely to be less predation on larvae by fish predators in ephemeral wetlands because they dry out periodically, eliminating fish populations (Wellborn et al. 1996). Other studies of larval amphibian distribution have demonstrated an increased presence of predatory fish in permanent wetlands in urban areas, coinciding with a decrease in species occupancy (Pearl et al. 2005; Rubbo and Kiesecker 2005). In our study there was a negative correlation between wetland permanency and cover of aquatic vegetation (i.e., ephemeral wetlands supported greater aquatic vegetation cover). Ephemeral wetlands may also contain more food resources (Harris 1999). Nonetheless, reproduction in ephemeral wetlands is often risky as wetland drying can lead to substantial losses of amphibian larvae (Semlitsch et al. 1996; Skelly 1996). While draining urban wetlands to remove silt and clear unwanted vegetation can remove fish and invertebrate predators, it can also eliminate entire cohorts of amphibian larvae if draining occurs during the period when larvae are present in wetlands.
While we previously found that tree frogs had an intermediate response to the abundance of predatory fish when assessed within the context of community composition (Hamer and Parris 2011), the strong negative relationship between tree frogs and fish observed when modeled individually highlights the importance of using complementary measures of species response in urban landscapes, as advocated by Hamer and McDonnell (2008). Even when species are modeled individually there can be discrepancies. For example, although there was a negative relationship between CPUE of predatory invertebrates and the relative abundance of tree frog larvae, we found a positive relationship between invertebrate CPUE and the occurrence of tree frog larvae. We therefore recommend modeling multiple response variables in amphibian studies. Because our study was correlative, inferences as to the effects of predators, vegetation, and hydroperiod on response variables may only be achieved through experimental approaches that control other confounding variables. An experimental approach would also provide greater inference when recommending management actions to increase the suitability of wetlands as amphibian breeding sites.
Our results here and previous (Hamer and Parris 2011) indicate that management actions should focus on eliminating predatory fish from wetlands to increase the suitability of urban wetlands as amphibian breeding sites. Because humans frequently introduce non-native fish into urban wetlands (Brönmark and Edenhamn 1994; Copp et al. 2005; Pyke 2008), educating the public regarding the negative impacts of exotic fish on frog species may also be a useful management strategy. Our results showed that aquatic vegetation may increase the occurrence of tree frog larvae, and so planting aquatic vegetation is likely to provide breeding sites for tree frogs. Encouraging vegetation growth and providing mosquitofish-free wetlands has been shown to bolster amphibian reproductive output in wetlands designed for mitigation and restoration in non-urban landscapes (Shulse et al. 2012). We recommend adopting similar measures at wetland restoration sites in urban areas. This study also highlights the potential importance of ephemeral wetlands, which are often overlooked for conservation (Snodgrass et al. 2000b). Many ephemeral wetlands in urban areas are frequently dredged or dammed, increasing their permanency and therefore propensity to support populations of predatory fish (Adams 1999; Pearl et al. 2005). Protecting or restoring ephemeral wetlands, which are naturally fish-free, should therefore be a management priority.
References
Adams MJ (1999) Correlated factors in amphibian decline: exotic species and habitat change in western Washington. Journal of Wildlife Management 63:1162–1171
Adams MJ, Richter KO, Leonard WP (1997) Surveying and monitoring amphibians using aquatic funnel traps. In: Olson DH, Leonard WP, Bury RB (eds) Sampling Amphibians in Lentic Habitats. Northwest Fauna Number 4, Society for Northwestern Vertebrate Biology, Olympia, Washington, pp 47–54
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer Science + Business Media, New York
Anstis M (2002) Tadpoles of South-eastern Australia: a guide with keys. Reed New Holland, Sydney
Australian Bureau of Statistics (2010) Year Book Australia, 2009–10, No. 91. Australian Bureau of Statistics, Canberra, Australia
Babbitt KJ, Tanner GW (1997) Effects of cover and predator identity on predation of Hyla squirella tadpoles. Journal of Herpetology 31:128–130
Babbitt KJ, Baber MJ, Tarr TL (2003) Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Canadian Journal of Zoology 81:1539–1552
Babbitt KJ, Baber MJ, Childers DL, Hocking D (2009) Influence of agricultural upland habitat type on larval anuran assemblages in seasonally inundated wetlands. Wetlands 29:294–301
Baber MJ, Babbitt KJ (2004) Influence of habitat complexity on predator–prey interactions between the fish (Gambusia holbrooki) and tadpoles of Hyla squirella and Gastrophryne carolinensis. Copeia 2004:173–177
Both C, Cechin SZ, Melo AS, Hartz SM (2011) What controls tadpole richness and guild composition in ponds in subtropical grasslands? Austral Ecology 36:530–536
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27:325–349
Brönmark C, Edenhamn P (1994) Does the presence of fish affect the distribution of tree frogs (Hyla arborea)? Conservation Biology 8:841–845
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics 7:434–455
Casterlin ME, Reynolds WW (1977) Aspects of habitat selection in the mosquitofish Gambusia affinis. Hydrobiologia 55:125–127
Clarke KR, Gorley RN (2001) PRIMER v5: user manual/tutorial. PRIMER-E, Plymouth
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Diversity and Distributions 9:89–98
Copp GH, Wesley KJ, Vilizzi L (2005) Pathways of ornamental and aquarium fish introductions into urban ponds of Epping Forest (London, England): the human vector. Journal of Applied Ichthyology 21:263–274
Cumming G, Finch S (2005) Inference by eye: confidence intervals and how to read pictures of data. American Psychologist 60:170–180
Gamradt SC, Kats LB (1996) Effect of introduced crayfish and mosquitofish on California newts. Conservation Biology 10:1155–1162
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Statistical Science 7:457–472
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biological Conservation 141:2432–2449
Hamer AJ, Parris KM (2011) Local and landscape determinants of amphibian communities in urban ponds. Ecological Applications 21:378–390
Hamer AJ, Lane SJ, Mahony MJ (2002) The role of introduced mosquitofish (Gambusia holbrooki) in excluding the native green and golden bell frog (Litoria aurea) from original habitats in south-eastern Australia. Oecologia 132:445–452
Harris RN (1999) The anuran tadpole. Evolution and maintenance. In: McDiarmid RW, Altig R (eds) Tadpoles. The biology of anuran larvae. The University of Chicago Press, Chicago, pp 279–294
Hartel T, Nemes S, Cogălniceanu D, Öllerer K, Schweiger O, Moga CI, Demeter L (2007) The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 583:173–182
Hero JM, Littlejohn M, Marantelli G (1991) Frogwatch field guide to Victorian frogs. Department of Conservation and Environment, East Melbourne
Hero JM, Gascon C, Magnusson WE (1998) Direct and indirect effects of predation on tadpole community structure in the Amazon rainforest. Australian Journal of Ecology 23:474–482
Heyer WR, McDiarmid RW, Weigmann DL (1975) Tadpoles, predation and pond habitats in the tropics. Biotropica 7:100–111
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Diversity and Distributions 9:99–110
Kentula ME, Gwin SE, Pierson SM (2004) Tracking changes in wetlands with urbanization: sixteen years of experience in Portland, Oregon, USA. Wetlands 24:734–743
Lee SY, Dunn RJK, Young RA, Connolly RM, Dale PER, Dehayr R, Lemckert CJ, McKinnon S, Powell B, Teasdale PR, Welsh DT (2006) Impact of urbanization on coastal wetland structure and function. Austral Ecology 31:149–163
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecology Letters 8:1235–1246
McCarthy MA (2007) Bayesian methods for ecology. Cambridge University Press, Cambridge
McDonnell MJ, Holland K (2008) Biodiversity. In: Newton PW (ed) Transitions: transitioning to resilient cities. CSIRO, Melbourne, pp 253–266
Morgan LA, Buttemer WA (1996) Predation by the non-native fish Gambusia holbrooki on small Litoria aurea and L. dentata tadpoles. Australian Zoologist 30:143–149
Parris KM (2006) Urban amphibian assemblages as metacommunities. Journal of Animal Ecology 75:757–764
Pearl CA, Adams MJ, Leuthold N, Bury RB (2005) Amphibian occurrence and aquatic invaders in a changing landscape: implications for wetland mitigation in the Willamette Valley, Oregon, USA. Wetlands 25:76–88
Pechmann JHK, Scott DE, Gibbons JW, Semlitsch RD (1989) Influence of wetland hydroperiod on diversity and abundance of metamorphosing juvenile amphibians. Wetlands Ecology and Management 1:3–11
Peterson AG, Bull MC, Wheeler LM (1992) Habitat choice and predator avoidance in tadpoles. Journal of Herpetology 26:142–146
Pyke GH (2005) A review of the biology of Gambusia affinis and G. holbrooki. Reviews in Fish Biology and Fisheries 15:339–365
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annual Review of Ecology, Evolution and Systematics 39:171–191
Pyke GH, White AW (2000) Factors influencing predation on eggs and tadpoles of the endangered green and golden bell frog Litoria aurea by the introduced plague minnow Gambusia holbrooki. Australian Zoologist 31:496–505
Richter KO (1995) A simple aquatic funnel trap and its application to wetland amphibian monitoring. Herpetological Review 26:90–91
Riley SPD, Busteed GT, Kats LB, Vandergon TL, Lee LFS, Dagit RG, Kerby JL, Fisher RN, Sauvajot RM (2005) Effects of urbanization on the distribution and abundance of amphibians and invasive species in southern California streams. Conservation Biology 19:1894–1907
Rubbo MJ, Kiesecker JM (2005) Amphibian breeding distribution in an urbanized landscape. Conservation Biology 19:504–511
Semlitsch RD, Scott DE, Pechmann JHK, Gibbons JW (1996) Structure and dynamics of an amphibian community: evidence from a 16-year study of a natural pond. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic, San Diego, pp 217–248
Shaffer HB, Alford RA, Woodward BD, Richards SJ, Altig RG, Gascon C (1994) Quantitative sampling of amphibian larvae. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and monitoring biological diversity. Standard methods for amphibians. Smithsonian Institution Press, Washington, DC, pp 130–141
Shulse CD, Semlitsch RD, Trauth KM, Williams AD (2010) Influences of design and landscape placement parameters on amphibian abundance in constructed wetlands. Wetlands 30:915–928
Shulse CD, Semlitsch RD, Trauth KM, Gardner JE (2012) Testing wetland features to increase amphibian reproductive success and species richness for mitigation and restoration. Ecological Applications 22:1675–1688
Skelly DK (1996) Pond drying, predators, and the distribution of Pseudacris tadpoles. Copeia 3:599–605
Snodgrass JW, Bryan AL Jr, Burger J (2000a) Development of expectations of larval amphibian assemblage structure in southeastern depression wetlands. Ecological Applications 10:1219–1229
Snodgrass JW, Komoroski MJ, Bryan AL Jr, Burger J (2000b) Relationships among isolated wetland size, hydroperiod, and amphibian species richness: implications for wetland regulations. Conservation Biology 14:414–419
Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002) Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B 64:583–639
Spiegelhalter D, Thomas A, Best N, Lunn D (2007) OpenBUGS user manual, version 3.0.2. MRC Biostatistics Unit, Cambridge
Stern H (2005) Climate. In: Brown-May A, Swain S (eds) The encyclopedia of Melbourne. Cambridge University Press, Melbourne, pp 147–154
Tarr TL, Babbitt KJ (2002) Effects of habitat complexity and predator identity on predation of Rana clamitans larvae. Amphibia-Reptilia 23:13–20
Tyre AJ, Tenhumberg B, Field SA, Niejalke D, Parris K, Possingham HP (2003) Improving precision and reducing bias in biological surveys: estimating false-negative error rates. Ecological Applications 13:1790–1801
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27:337–363
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Welsh AH, Cunningham RB, Donnelly CF, Lindenmayer DB (1996) Modelling the abundance of rare species: statistical models for counts with extra zeros. Ecological Modelling 88:297–308
Werner EE, Skelly DK, Relyea RA, Yurewicz KL (2007) Amphibian species richness across environmental gradients. Oikos 116:1697–1712
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wintle BA, Bardos DC (2006) Modeling species-habitat relationships with spatially autocorrelated observation data. Ecological Applications 16:1945–1958
Acknowledgments
We thank Sam Davis, Terry Coates, Andrew Gay, Andrew Smith and Colin Walker for facilitating access to wetland sites. Geoff Heard and Eliza Poole assisted with fieldwork. Michael Smith provided advice on trap design. We also thank Marion Anstis for assistance with tadpole identification, and Tara Martin, Michael McCarthy and Joslin Moore for assistance with statistical modeling. The Baker Foundation and National Environmental Research Program (Research Hub for Environmental Decisions) provided generous support for this research. This study was approved by the University of Melbourne Animal Ethics Committee (register no. 0706488). Fieldwork was conducted under research permit no. 10004319 issued by the Victorian Department of Sustainability and Environment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Spearman correlation coefficients (Spearman’s rho, ρ) among predator and habitat variables (PDF 61 kb)
Online Resource 2
Summary of aquatic sampling at 64 urban wetlands in the Greater Melbourne area, Australia (PDF 9 kb)
Online Resource 3
The mean CPUE of predatory fish (mosquitofish and redfin perch) and the larvae of six frog species according to hydroperiod at 64 wetlands in the Greater Melbourne area, Australia, 2007–2008. CPUE data were log(x+1)-transformed to reduce the influence of large values and to aid in interpretation. Permanent wetlands had a hydroperiod score = 1; ephemeral wetlands had a score <1. (PDF 15 kb)
Online Resource 4
Proportion of predatory invertebrate taxa captured at the 64 wetlands (PDF 8 kb)
Rights and permissions
About this article
Cite this article
Hamer, A.J., Parris, K.M. Predation Modifies Larval Amphibian Communities in Urban Wetlands. Wetlands 33, 641–652 (2013). https://doi.org/10.1007/s13157-013-0420-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0420-2