Abstract
Previous studies have shown that naturally occurring phytochemicals, indole-3-carbinol, phenethyl isothiocyanate, protocatechuic acid, and tannic acid increased the activity and protein level of hepatic phase II enzymes in animal models. In order to further explore the mechanism of this activity, we investigated the effect of these compounds on the activation of nuclear factor erythroid-2-related factor 2 (Nrf2)-regulated transcription in human hepatocellular carcinoma HepG2 cells. Treatment with all the tested compounds resulted in the translocation from the cytosol and nuclear accumulation of active phosphorylated Nrf2. Furthermore, phenethyl isothiocyanate and indole-3-carbinol increased the transcript and protein levels of GSTA, GSTP, GSTM, GSTT, and NQO1. On the other hand, protocatechuic and tannic acids enhanced only the expression of GSTA, GSTM, and GSTT. The expression of genes encoding antioxidant enzymes CAT, SOD, GR, and GPx was increased after the treatment with all the tested phytochemicals. These results indicate that isothiocyanates/indoles and protocatechuic and tannic acids induce phase II and antioxidant gene expression in HepG2 cells through the Nrf2-Keap1-ARE signaling pathway. Moreover, the results of this study confirmed that the degradation products of glucosinolates are more effective inducers of phase II and antioxidant enzymes than protocatechuic and tannic acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The induction of genes encoding phase II enzymes that protect against damage by electrophilic and reactive oxygen intermediates is considered as an important strategy in reducing the risk of cancer and other chronic degenerative diseases linked to oxidative stress [14, 32, 40]. The expression of phase II genes is controlled by antioxidant response element (ARE) sequences which are regulated by nuclear factor erythroid-2-related factor 2 (Nrf2) signaling [26]. Inactive Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1) molecules and is retained in the cytoplasm. Under oxidative stress or in response to other stimulating factors, Nrf2 dissociates from Keap1 and undergoes translocation to the nuclear compartment, where its accumulation correlates with binding to ARE regulatory DNA sequences and stimulation of target gene transcription [30].
Overexpression of Nrf2 has been reported to upregulate ARE-mediated gene expression that was inducible by various antioxidants and chemopreventive agents such as beta-naphtoflavone, tert-butyl hydroquinone, and phenethyl isothiocyanate [7]. The critical role of Nrf2 in the expression of antioxidant genes has been confirmed by using the nrf2-disrupted mice, which not only show reduced levels of antioxidant enzymes but are also more susceptible to carcinogens compared to wild-type mice [4]. Therefore, the regulation of Nrf2 is now of great interest. Treatment of cells with antioxidants and xenobiotics can lead to the release of Nrf2 from Keap1 [5].
Many naturally occurring compounds have been found to be potent cancer chemopreventive agents in cell lines and experimental animal models [36]. Of great interest are the isothiocyanates such as phenethyl isothiocyanate and indoles such as indole-3-carbinol which are the degradation products of glucosinolates found in Brassica vegetables. Despite the significant progress in the basic understanding of carcinogenesis, the molecular and cellular targets for the effective chemoprevention by these compounds are poorly characterized. Recent studies have reported the induction of Nrf2 by several chemopreventive compounds including sulforaphane and phenethyl isothiocyanate [10, 28]. Moreover, phenolic compounds, especially protocatechuic acid also increased phase II enzymes. Our earlier studies [8, 11–13] showed the increased activity of glutathione S-transferase (GST), which correlated with an enhanced level of GSTM isoform after treatment with this phenolic acid possessing a simple chemical structure. However, the questions concerning how different classes of natural chemopreventive compounds regulate the expression and stability of Nrf2 and how the regulation of Nrf2 by these compounds influences the expression of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx) and phase II enzymes—GSTs or NAD(P)H:quinone oxidoreductase 1 (NQO1) remain largely unanswered. In addition, the relationship between Nrf2 stability and Keap1 in the presence of chemopreventive compounds remains unclear.
In the present study, we investigated the effect of different classes of naturally occurring chemopreventive agents, phenethyl isothiocyanate, indole-3-carbinol, and protocatechuic and tannic acids, on the expression of the enzymes controlled by the Nrf2/ARE pathway activation in human hepatocellular carcinoma HepG2 cells.
Materials and methods
Chemicals and antibodies
Protocatechuic acid, tannic acid, indole-3-carbinol, and phenethyl isothiocyanate were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chemical structures of the studied phytochemicals are presented in Fig. 1.
Dimethyl sulfoxide (DMSO), antibiotic solution (104 U penicillin, 10 mg streptomycin, 25 μg amphotericin B), fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), radio-immunoprecipitation assay (RIPA) buffer, and Tris were obtained from Sigma-Aldrich (St. Louis, MO, USA). HepG2 (ATCC HB 8065) cells were provided by Prof. Zofia Mazerska from the Department of Pharmaceutical Technology and Biochemistry, Gdańsk University of Technology, Poland. All the primers used in PCR reactions were obtained from oligo.pl (Warsaw, Poland). Protease inhibitor cocktail was obtained from Roche Diagnostics GmbH (Penzberg, Germany). Primary and secondary antibodies against CAT, SOD, GPx, GR, GSTA, GSTM, GSTP, GSTT, NQO1, Nrf2, Keap1, β-actin, and laminin were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-Nrf2 (phospho Ser40) antibody was obtained from Abcam (Cambridge, UK). All the other chemicals were commercial products of the highest purity available.
Cell culture and treatment
HepG2 cells were maintained in DMEM containing 10 % fetal bovine serum and antibiotics. The cells were grown at 37 °C in a humidified atmosphere containing 5 % CO2. To assess the effect of tannic acid, protocatechuic acid, indole-3-carbinol, and phenethyl isothiocyanate on the measured parameters, 5 × 105 cells were seeded per 100 mm culture dish. After 24 h of initial incubation, the cells were treated with 2 and 10 μM tannic acid, protocatechuic acid, or indole-3-carbinol and 1 and 5 μM phenethyl isothiocyanate or 0.1 % vehicle (DMSO) control. The incubation was continued for subsequent 72 h, and cells were harvested.
Viability assay
The effect of phytochemicals on cell viability was assessed by the MTT assay, according to standard protocols. Briefly, 104 HepG2 cells were seeded per well in a 96-well plate. After 24 h of preincubation in complete medium, phytochemicals were added to culture medium in various concentrations and cells were incubated for subsequent 72 h. DMSO concentration did not exceed 0.1 %. After 72 h, cells were washed twice with warm PBS buffer and fresh medium containing MTT salt (0.5 mg/ml) was added. After 4 h of incubation, formazan crystals were dissolved in acidic isopropanol and absorbance was measured at 540 and 690 nm. All the experiments were repeated three times, with at least three measurements per experiment.
In all the subsequent experiments, non-toxic concentrations of phytochemicals (viability above 80 % based on the MTT assay) were used, ranging from 2 to 10 μM (tannic acid, protocatechuic acid, indole-3-carbinol) or 1 to 5 μM (phenethyl isothiocyanate).
Nuclear, cytosolic, and total protein lysate preparation
The cytosol and nuclear extracts from HepG2 were prepared using the Nuclear/Cytosol Fractionation Kit (BioVision Research, CA USA). Whole cell lysates were prepared from HepG2 using RIPA buffer.
RNA isolation
Extraction of total RNA from cells was performed using GeneMatrix Universal DNA/RNA/Protein Purification Kit (EurX, Gdańsk, Poland) according to the manufacturer’s instructions.
Quantitative PCR
Total RNA was subjected to reverse transcription using RevertAid Kit (Fermentas, Burlington, Canada) followed by quantitative real-time PCR. For real-time analyses, the Maxima SYBR Green Kit (Fermentas) and a BioRad Chromo4 thermal cycler were used. The protocol started with 5 min enzyme activation at 95 °C, followed by 40 cycles of 95 °C for 15 s, 56 °C for 20 s, and 72 °C for 40 s and final elongation at 72 °C for 5 min. Melting curve analysis was used for product specificity verification. The estimation of the expression of TATA box binding protein (TBP) and porphobilinogen deaminase (PBGD) was used for data normalization. Primer sequences used for the analysis of Nrf2, Keap1, GSTA, GSTM, GSTP, GSTT, and NQO1 were described previously [9]. The sequences of primers used for the analysis of SOD, CAT, GR, GPx are listed in Table 1.
Western blot analysis
For the determination of the level of GSTA, GSTP, GSTM, GSTT, NQO1, Keap1, Nrf2, CAT, GR, SOD, and GPx proteins, the immunoblot assay was used. Protein content in the samples was determined by the Lowry method [20]. All the experiments were repeated three times. Whole cell lysates (GPx), nuclear extracts (Nrf2), or cytosolic proteins (Nrf2, Keap1, GSTA, GSTP, GSTM, GSTT, NQO1, SOD, CAT, GR) (100 μg) were separated on 12 or 10 % SDS-PAGE slab gels, and proteins were transferred to nitrocellulose membranes. After blocking with 10 % skimmed milk, proteins were probed with mouse monoclonal GSTA, rabbit polyclonal GSTM, goat polyclonal GSTP, mouse monoclonal GSTT, mouse monoclonal NQO1, goat polyclonal Keap1, rabbit polyclonal Nrf2, rabbit monoclonal Nrf2 (phospho Ser40), goat polyclonal SOD, rabbit polyclonal GPx, rabbit polyclonal GR, goat polyclonal CAT, rabbit polyclonal β-actin, and rabbit polyclonal laminin antibodies. Either β-actin or laminin expression was used as an internal control. As the secondary antibodies in the staining reaction, the alkaline phosphatase-labeled anti-goat IgG, anti-mouse IgG, or anti-rabbit IgG were used. Bands were visualized with BioRad AP Conjugate Substrate Kit NBT/BCIP. The amount of the immunoreactive product in each lane was determined using the Quantity One software (BioRad Laboratories, Hercules, CA, USA). Values were calculated as relative absorbance units (RQ) per mg protein.
Statistical analysis
The statistical analysis was performed by one-way ANOVA. The statistical significance between the experimental groups and their respective controls was assessed by Tukey’s post hoc test, with p < 0.05.
Results
The effect of phytochemicals on cell viability
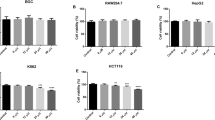
The impact of tannic acid, protocatechuic acid, indole-3-carbinol, and phenethyl isothiocyanate on the viability of HepG2 cells was evaluated using the MTT assay. Within the concentration range 0.2–150 μM, tannic acid, protocatechuic acid, phenethyl isothiocyanate, and indole-3-carbinol reduced the viability of HepG2 cell in a dose-dependent manner (Fig. 2). The IC50 values obtained for phenethyl isothiocyanate, indole-3-carbinol, tannic acid, and protocatechuic acid were 15, 93, 42, and 150 μM, respectively. Based on these findings, all the subsequent studies were conducted at concentrations of tannic acid, protocatechuic acid, and indole-3-carbinol of 2 and 10 μM while phenethyl isothiocyanate was applied at the concentrations of 1 and 5 μM.
The effect of phytochemicals on the viability of HepG2 cells. Cell viability was expressed as the percentage of the optical density of phytochemical-treated cells in comparison with that of untreated control cells (100 % viability). Results were representative of three independent experiments (mean ± SEM). PEITC phenethyl isothiocyanate, I3C indole-3-carbinol, PCA protocatechuic acid, TA tannic acid
The effect of phytochemicals on Nrf2 expression and phosphorylation
Quantitative analysis revealed a significant increase in the amount of Nrf2 transcript in HepG2 cells as the result of phenethyl isothiocyanate treatment at the concentrations of 1 and 5 μM and tannic acid, protocatechuic acid, and indole-3-carbinol at the concentrations of 2 and 10 μM (Fig. 3a). Phenethyl isothiocyanate was shown to be the most potent inducer of Nrf2 transcription leading to an increase in its transcript level by 86–140 %. Nrf2 may induce gene expression only after its translocation into the nucleus. To investigate the translocation of Nrf2 upon phytochemical treatment, levels of Nrf2 in the cytosol and nuclear fractions of the cells were evaluated by Western blot analysis.
The effect of phytochemicals on Nrf2 activation in HepG2 cells. a Nrf2 transcript levels. The values were calculated as mRNA level in comparison with control cells (expression equals 1). b Representative immunoblots for the analysis of the nuclear level of Nrf2 protein. Lane 1 control, lane 2 PEITC 1 μM, lane 3 PEITC 5 μM, lane 4 I3C 2 μM, lane 5 I3C 10 μM, lane 6 PCA 2 μM, lane 7 PCA 10 μM, lane 8 TA 2 μM, lane 9 TA 10 μM. The level of laminin was used as a normalization control. Results of Western blot analysis of the nuclear content of the Nrf2 protein. c Representative immunoblots for the analysis of the nuclear level of the p-Nrf2 protein. Results of Western blot analysis of the nuclear content of the p-Nrf2 protein. The values were calculated as protein level in comparison with control cells (expression equals 1). d Keap1 transcript levels. e Representative immunoblots for the analysis of the cytosolic level of Keap1 protein. The level of β-actin was used as a normalization control. Results of Western blot analysis of the cytosolic content of Keap1 protein. The values were calculated as protein level in comparison with control cells (expression equals 1). The data are the means ± SEM from three separate experiments. The asterisk (*) above the bar denotes statistically significant differences from the control group, p < 0.05
As shown in Fig. 3b, c, the Nrf2 protein was accumulated and phosphorylated at Ser 40 in the nucleus after exposure to all the studied phytochemicals; however, the highest level of Nrf2 phosphorylated at Ser 40 was induced by phenethyl isothiocyanate.
Since the activation of Nrf2 is generally accompanied by the degradation of Keap1, we also measured the amount of Keap1 messenger RNA (mRNA) and cytosolic protein. However, the amount of neither Keap1 mRNA nor the cytosolic protein was statistically significantly changed by phenolic acids, indoles, or isothiocyanates upon cell treatment (Fig. 3d, e).
The effect of phytochemicals on the expression and protein level of phase II enzymes
The expression of GSTs and NQO1 was measured by quantitative PCR (Fig. 4). The level of GSTA, GSTP, GSTM, and GSTT mRNA transcripts was significantly increased after the treatment of HepG2 cells with indole-3-carbinol and phenethyl isothiocyanate. Protocatechuic acid in both tested doses enhanced only the expression of GSTA, GSTM, and GSTT while tannic acid increased the expression of GSTT in both tested doses and GSTA and GSTM only in a dose of 2 μM. On the other hand, the expression of GSTP was enhanced only by protocatechuic acid in both tested doses. Moreover, all the tested compounds at higher concentrations increased the expression of NQO1 in HepG2 cells.
The effect of phytochemicals on the expression of phase II enzymes in HepG2 cells. mRNA expression of GSTA, GSTM, GSTP, GSTT, and NQO1. The values were calculated as mRNA level in comparison with control cells (expression equals 1). Data (mean ± SEM) from three separate experiments are presented; asterisk above bar denotes statistically significant differences from the control group, p < 0.05
The level of GSTA, GSTP, GSTM, GSTT, and NQO1 proteins in HepG2 cells was evaluated using Western blot assay (Fig. 5). The cytosolic content of GSTA, GSTP, GSTM, and GSTT (Fig. 5) was significantly increased after treatment with indole-3-carbinol and phenethyl isothiocyanate. The similar effect was observed for protocatechuic acid with the exception of GSTP protein. The level of GSTA, GSTM, and GSTP was not statistically changed after treatment with tannic acid whereas the protein level of GSTT was enhanced after incubation with 2 and 10 μM of tannic acid. Indole-3-carbinol and phenethyl isothiocyanate only at their highest doses enhanced the level of NQO1. The similar effect was observed for phenolic acids.
The effect of phytochemicals on the protein level of phase II enzymes in HepG2 cells. Representative immunoblots for the analysis of GSTA, GSTM, GSTP, GSTT, and NQO1. Lane 1 control, lane 2 PEITC 1 μM, lane 3 PEITC 5 μM, lane 4 I3C 2 μM, lane 5 I3C 10 μM, lane 6 PCA 2 μM, lane 7 PCA 10 μM, lane 8 TA 2 μM, lane 9 TA 10 μM. Results of Western blot analysis of the content of GSTA, GSTM, GSTP, GSTT, and NQO1. The values were calculated as protein level in comparison with control cells (expression equals 1). Data (mean ± SEM) from three separate experiments are presented; asterisk above bar denotes statistically significant differences from the control group, p < 0.05
The effect of phytochemicals on the expression and protein level of antioxidant enzymes
Figure 6 shows data on the effect of phytochemicals on antioxidant gene expression in HepG2 cells. The expression of CAT, SOD, GR, and GPx increased after treatment with indole-3-carbinol and phenethyl isothiocyanate. As shown in Fig. 6, a similar effect was observed for phenolic acids except for the expression of SOD.
The effect of phytochemicals on the expression of antioxidant enzymes in HepG2 cells. mRNA expression of SOD, CAT, GPx, and GR. The values were calculated as mRNA level in comparison with control cells (expression equals 1). Data (mean ± SEM) from three separate experiments are presented, asterisk above bar denotes statistically significant differences from the control group, p < 0.05
The results of Western blot analysis detecting the level of CAT, SOD, GR, and GPx proteins are shown in Fig. 7. The CAT, GPx, and GR protein level increased after the treatment of HepG2 cells with all the tested compounds. The level of SOD was significantly enhanced by indole-3-carbinol and phenethyl isothiocyanate. By contrast, no significant increase in the SOD protein level was found in HepG2 as a result of the exposure to phenolic acids.
The effect of phytochemicals on the protein level of antioxidant enzymes in HepG2 cells. Representative immunoblots for the analysis of SOD, CAT, GPx, and GR. Lane 1 control, lane 2 PEITC 1 μM, lane 3 PEITC 5 μM, lane 4 I3C 2 μM, lane 5 I3C 10 μM, lane 6 PCA 2 μM, lane 7 PCA 10 μM, lane 8 TA 2 μM, lane 9 TA 10 μM. Results of Western blot analysis of SOD, CAT, GPx, and GR. The values were calculated as protein level in comparison with control cells (expression equals 1). Data (mean ± SEM) from three separate experiments are presented; asterisk above bar denotes statistically significant differences from the control group, p < 0.05
Discussion
Phase II along with antioxidant enzymes are responsible for the detoxification and elimination of xenobiotics and reactive metabolites including ROS that may cause damage to cells and tissues [21, 37]. The induction of these enzymes is now recognized as an efficient approach to prevent cancer. The key role in the induction of expression of phase II detoxifying enzymes as well as other cytoprotective proteins is played by the Nrf2–ARE pathway [3, 17].
The ability to activate Nrf2–ARE pathway and/or induce phase II enzymes by the breakdown products of glucosinolates such as isothiocyanates and indoles has widely been studied in different systems including HepG2 cells [6, 10, 14, 28]. In contrast, the effect of protocatechuic and tannic acids on phase II enzyme induction was evaluated only in animal models indicating that both compounds have the ability to enhance the activity and protein levels of phase II enzymes in mouse and rat liver [8, 10, 13].
The results of our current study indicate that protocatechuic and tannic acids induce phase II enzymes both on the transcriptional and protein level in human HepG2 cells in a manner similar to isothiocyanates/indoles, i.e., through the activation of Nrf2-ARE-pathway. Although characterized by relatively low levels of cytochrome P450 enzymes, HepG2 cells possess a complete set of phase II enzymes at the levels comparable to those found in primary human hepatocytes, with the exception of UDP-glucuronosyltransferases [33, 34]. Thus, the results obtained in this study might reflect the situation in normal human hepatocytes.
All the phytochemicals tested in this study stimulated the translocation of the active form of Nrf2 into the cell nucleus in a concentration-dependent manner. However, Nrf2 activation by these compounds was not associated with any changes in the level of Keap1 expression. These observations suggests that the tested phytochemicals may react with redox reactive cysteines in Keap1 and disrupt the interaction between Nrf2 and Keap1, hence allowing its translocation [29]. This suggestion further supports the accumulation of Nrf2 phosphorylated at Ser 40 in the nuclear compartment correlating with its translocation. The latter is in agreement with the findings showing that Nrf2 phosphorylation is a critical event in the nuclear translocation of this transcription factor and is necessary for its transcriptional activity [27].
The majority of genes that encode phase II and antioxidant enzymes possess an ARE sequence in their promoter region [26]. Thus, the increased levels of the mRNA transcript and protein of phase II enzymes, GSTs, NQO1, and antioxidant enzymes, CAT, SOD, GPx, and GR as the result of treatment of HepG2 cells with the examined phytochemicals suggest that they may facilitate the binding of Nrf2 to this sequence.
Quantitative PCR analysis revealed the significant increase in the expression of all the tested isoforms of GST (GSTM, GSTT, GSTA, and GSTP) as the result of treatment of HepG2 cells with phenethyl isothiocyanate and indole-3-carbinol. Thus, the results of our present study at mRNA level confirm our earlier observations in in vivo model, showing increased protein levels of these isoforms in rat liver as the result of the administration of these Brassicacae components [10]. Moreover, similarly as in rat liver, phenethyl isothiocyanate was a stronger inducer of the expression of GST isoforms than indole-3-carbinol also in human cells. The earlier in vivo observations in rat [8] and mouse liver [13] were confirmed by the results of the present study also in the case of protocatechuic acid. This compound similarly as in animal models significantly upregulated the expression of GST isozymes, particularly GSTM, in HepG2 cells.
However, tannic acid with a more complex structure being a polymer of digallic acid and glucose increased only the transcript and protein level of GSTT. This observation is in contrast to the results obtained in animal models [8, 13], in which tannic acid decreased the protein level of GSTT and GSTA. This discrepancy might result from the differences in the bioavailability of this complex molecule in vivo and in vitro. The available data indicate that in rats following oral administration at a dose of 1.0 g/kg, ~60 % of tannic acid remained unchanged, but some was hydrolyzed to gallic acid by tannase in the intestine and further metabolized to 4-O-methylgallic acid, pyrogallol, and resorcinol [24]. In vitro bioavailability of tannic acid was evaluated in ligated rat small intestine segments showing 50 % uptake but not complete transfer through the gut wall [2]. Thus, further studies are required in order to explain the fate of tannic acid in HepG2 cells and the mechanism of GST isoform induction by this compound.
GSTT which is induced in HepG2 cells by tannic acid acts as a scavenger of electrophiles, such as epoxides; however, it may also metabolically activate halogenated compounds, thus producing a variety of intermediates that can potentially damage DNA and cells [16]. Thus, its induction in cancer cells might be considered as ambiguous.
Another important observation of this study was the increased expression of GSTP induced by phenethyl isothiocyanate and indole-3-carbinol. This GST isoform protects cells against the adverse effects of such compounds as toxins, carcinogens, and ROS [19]. An increase in GSTA and GSTM levels by the same compounds might be beneficial as protection against ROS and the products of lipid peroxidation, such as 4-hydroxynonenal [38].
Besides the influence on GSTs, the phytochemicals tested in our study enhanced both the mRNA and protein level of NQO1 in HepG2 cells. The induction of NQO1 which coincided with increased activity and protein level of NQO1 was observed in our earlier study [10] in rat liver as the result of oral administration of phenethyl isothiocyanate and indole-3-carbinol. In this regard, the results of the present in vitro study confirmed those obtained in the animal model. Again, the results of this study showed that phenethyl isothiocyanate and indole-3-carbinol were also more potent inducers of this enzyme than protocatechuic and tannic acids. These compounds possess totally different chemical structures. Isothiocyanates are characterized by the presence of sulfur containing N = C = S functional group, which is a Michael reaction acceptor [31]. This functional group plays an important role in the induction of phase II enzymes.
Nrf2 transcription factor controls also the expression of the antioxidant enzymes, GR, GPx, CAT, and SOD, playing a crucial role in the defense against oxidative stress [1]. Our study showed that isothiocyanates and indoles induced all these enzymes in HepG2 cells. A similar effect in the case of SOD expression was found in Hepa1c1c7 cells by Saw and Kong [29] for phenethyl isothiocyanate. This compound was also shown to increase the activity of the antioxidant enzymes GR and CAT in hepatocytes isolated from Wistar rats [15].
Interestingly, the results of the present study showed also that protocatechuic and tannic acids were equally potent inducers of the expression of antioxidant enzymes (except SOD) in HepG2 cells as isothiocyanates/indoles. These in vitro observations showing the ability of protocatechuic and tannic acids to induce antioxidant enzymes are also in agreement with recently published data on the effect of these compounds in animal models [35, 39]. These studies showed that treatment of rats with both protocatechuic acid and tannic acid induced the activity of GPx and CAT.
It has to be stressed that the doses used in our in vitro study were comparable to those which might be obtained in human serum after oral administration of indole-3-carbinol [23], isothiocyanate phenethyl [18], protocatechuic acid [22], or tannic acid [25]. Thus, the results of our studies might partly reflect possible in vivo effects.
In summary, we demonstrated that isothiocyanates/indoles and protocatechuic and tannic acids induce phase II and antioxidant genes in vitro through the Nrf2-Keap1-ARE signaling pathway and that the mechanism of this induction might result from their interaction with the Keap1 protein.
Moreover, the results of this study confirmed that protocatechuic and tannic acids are less effective than the degradation products of glucosinolates—phenethyl isothiocyanate or indole-3-carbinol in the induction of phase II enzymes. However, protocatechuic and tannic acids induced antioxidant enzymes to the same extent as isothiocyanates/indoles in HepG2 cells.
This activity may be a part of their chemoprotective activity against injury by toxicants and/or oxidants. Thus, our results contribute to a better understanding of the effect of these chemically diverse phytochemicals, which are common ingredients of daily human diet.
Abbreviations
- ARE:
-
Antioxidant response element
- CAT:
-
Catalase
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GST:
-
Glutathione S-transferase
- I3C:
-
Indole-3-carbinol
- Keap1:
-
Kelch-like ECH-associated protein 1
- NQO1:
-
NADPH:quinone oxidoreductase 1
- Nrf2:
-
Nuclear factor erythroid-2-related factor 2
- PCA:
-
Protocatechuic acid
- PEITC:
-
Phenethyl isothiocyanate
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TA:
-
Tannic acid
References
Alia M, Ramos S, Mateos R, Granado-Serrano AB, Bravo L, Goya L (2006) Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol Appl Pharmacol 212:110–118
Carbonaro M, Grant G, Pusztai A (2001) Evaluation of polyphenol bioavalability in rat small intestine. Eur J Nutr 40:84–90
Giudice A, Montella M (2006) Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays 28:169–181
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Jeong WS, Keum YS, Chen C, Jain MR, Shen G, Kim JH, Li W, Kong AN (2005) Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J Bioch Mol Biol 38:167–176
Keum YS, Owuor ED, Kim BR, Hu R, Kong AN (2003) Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC). Pharm Res 20:1351–1356
Krajka-Kuźniak V, Kaczmarek J, Baer-Dubowska W (2008) Effect of naturally occurring phenolic acids on the expression of glutathione S-transferase isozymes in the rat. Food Chem Toxicol 46:1097–1102
Krajka-Kuźniak V, Paluszczak J, Baer-Dubowska W (2013) Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol In Vitro 27:149–156
Krajka-Kuźniak V, Szaefer H, Bartoszek A, Baer-Dubowska W (2011) Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Br J Nutr 105:816–826
Krajka-Kuźniak V, Szaefer H, Baer-Dubowska W (2004) Modulation of 3-methylcholantrene-induced rat hepatic and renal cytochrome P450 and phase II enzymes by plant phenols: protocatechuic and tannic acids. Toxicol Lett 152:117–126
Krajka-Kuźniak V, Szaefer H, Baer-Dubowska W (2005) Modulation of cytochrome P450 and phase II enzymes by protocatechuic acid in mouse liver and kidney. Toxicology 216:24–31
Krajka-Kuźniak V, Szaefer H, Baer-Dubowska W (2008) Hepatic and extrahepatic expression of glutathione S-transferase isozymes in mice and its modulation by naturally occurring phenolic acids. Environ Toxicol Pharmacol 25:27–32
Kwak MK, Wakabayashi N, Kensler TW (2004) Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res 555:133–148
La Marca M, Beffy P, Della Croce C, Gervasi PG, Iori R, Puccinelli E, Longo V (2012) Structural influence of isothiocyanates on expression of cytochrome P450 phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem Toxicol 50:2822–2830
Landi S (2000) Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res 463:247–283
Lee JS, Surh YJ (2005) Nrf2 as a novel molecular target for chemoprevention. Cancer Lett 224:171–184
Liebes L, Conaway CC, Hochster H, Mendoza S, Hecht SS, Crowell J, Chung FL (2001) High-performance liquid chromatography-based determination of total isothiocyanate levels in human plasma: application to studies with 2-phenethyl isothiocyanate. Anal Biochem 291:279–289
Lo HW, Ali-Osman F (2007) Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr Opin Pharmacol 7:367–374
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Matés JM, Pérez-Gómez C, Núñez de Castro I (1999) Antioxidant enzymes and human diseases. Clin Biochem 32:595–603
McKay DL, Chen CY, Zampariello CA, Blumberg JB (2015) Flavonoids and phenolics from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem 168:233–240
Moiseeva EP, Almeida GA, Jones GDD, Manson MM (2007) Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther 6:3071–3079
NakamuraY TS, Tonogai Y (2003) Method for analysis of tannic acid and its metabolites in biological samples: application to tannic acid metabolism in the rat. J Agric Food Chem 51:331–339
Nam S, Smith DM, Dou QP (2001) Tannic acid potently inhibit tumor cell proteasome activity increases p27 and Bax expression, and induces G1 arrest and apoptosis. Cancer Epidemiol Biomarkers Prev 10:1083–1088
Nguyen T, Sherrat PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, Waalkes MP (2007) Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Rad Biol Med 15:1797–1806
Saw CL, Cintrón M, Wu TY, Guo Y, Huang Y, Jeong WS, Kong AN (2011) Pharmacodynamics of dietary phytochemical indoles I3C and DIM: induction of Nrf2-mediated phase II drug metabolizing and antioxidant genes and synergism with isothiocyanates. Biopharm Drug Dispos 32:289–300
Saw CLL, Kong A-NT (2011) Nuclear factor-erythroid 2-related factor 2 as a chemopreventive target in colorectal cancer. Exp Opin Therap Targets 15:281–295
Suhr Y-J, Kundu JK, Na H-K (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74:1526–1539
Talalay P (2000) Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors 12:5–11
Talalay P, Dinkova-Kostova AT, Holtzclaw WD (2003) Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Advan Enzyme Reg 43:121–134
Westerink WM, Schoonen WG (2007) Phase II enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol In Vitro 21:1592–1602
Wilkening S, Stahl F, Bader A (2003) Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation. Drug Metab Dispos 3:1035–1042
Winiarska-Mieczan A (2013) Protective effect of tannic acid on the brain of adults expose to cadmium and lead. Environ Toxicol Pharmacol 36:9–18
Yang CS, Maliakal P, Meng X (2002) Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol 42:25–54
Yang YM, Noh K, Han CY, Kim SG (2010) Transactivation of genes encoding for phase II enzymes and phase III transporters by phytochemical antioxidants. Molecules 15:6332–6348
Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC (2003) Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol 50:319–336
Yeh C-T, Yen GC (2006) Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance-associated protein 3 mRNA expression. J Nutr 136:11–15
Yu X, Hensler T (2005) Nrf2 as a target for cancer chemoprevention. Mutat Res 591:93–102
Acknowledgment
The present study was supported by research funding from Poznań University of Medical Sciences.
Conflicts of interest
All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krajka-Kuźniak, V., Paluszczak, J., Szaefer, H. et al. The activation of the Nrf2/ARE pathway in HepG2 hepatoma cells by phytochemicals and subsequent modulation of phase II and antioxidant enzyme expression. J Physiol Biochem 71, 227–238 (2015). https://doi.org/10.1007/s13105-015-0401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0401-4