Abstract
Coumarin-pi, a new coumarin derivative isolated from the mushroom Paxillus involutus, has antioxidative activity, but the underlying mechanism against intracellular oxidative stress is still unclear. This study investigated its cytoprotective effects and the antioxidative mechanism in tert-butyl hydroperoxide (t-BHP)-induced HepG2 cells. The results demonstrated that coumarin-pi suppressed t-BHP-stimulated cytotoxicity, cell apoptosis, and generation of reactive oxygen species (ROS). Additionally, coumarin-pi promoted nuclear factor erythroid 2-related factor 2 (Nrf2) expression and upregulated the protein expression of antioxidantenzymes, including heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidase (NQO1), glutamyl cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase regulatory subunit (GCLM). After coumarin-pi treatment, transcriptome sequencing and bioinformatic analysis revealed that 256 genes were differentially expressed; interestingly, only 20 genes were downregulated, and the rest of the genes were upregulated. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional annotation were used to identify changes in metabolic pathways. Collectively, the results presented in this study indicate that coumarin-pi has a protective effect against t-BHP-induced cellular damage and oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress, an imbalance between the oxidative and antioxidative systems of cells and tissues, is a result of the overproduction of oxidative-free radicals and associated reactive oxygen species (ROS) [1]. Oxidative stress plays an important role in the pathogenesis of Alzheimer's disease (AD), and antioxidants may be useful for AD treatment [2, 3]. Oxidative stress can also contribute to atherosclerosis, cancers, diabetes, etc. [4,5,6].
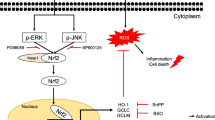
Cells have elaborated various antioxidant defense mechanisms to scavenge ROS and reduce oxidative stress [7]. The transcription factor Nrf2 which binds DNA antioxidant response elements (AREs) is needed to improve the progression of various diseases, especially those caused by oxidative stress-related transcription factors [8, 9].Under physiological conditions, Nrf2 constitutively binds the linker protein, Kelch-like ECH-associated protein 1 (Keap1, also known as a repressor of Nrf2). When exposed to ROS or electrophilic molecules, Nrf2 detaches from Keap1 and is transferred to the nucleus to activate several genes for antioxidant and detoxification [10], such as heme oxygenase-1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1), glutamate-cysteine ligase modifier (GCLM), and catalytic (GCLC) subunit. Activation of Nrf2 has been investigated as a therapeutic target for the prevention and treatment of liver diseases [11].
Interestingly, numerous drugs, including abundant phytochemicals, can activate the Nrf2 signaling pathway, which improves the antioxidative capacity and reduces apoptotic cell death [9]. A new coumarin derivative was isolated from Paxillus involutus and named coumarin-pi [12]. The coumarin derivatives were reported to have numerous biological activities, such as anticoagulant, anticancer, antioxidant, antiviral, anti-diabetics, anti-inflammatory, antibacterial, antifungal, and anti-neurodegerative [13, 14].
This molecule displayed potent antioxidant activity against DPPH with an IC50 value of 16.3 μg/mL compared with the positive control BHA (59.9 μg/mL) [12]. Consequently, we investigated the cytoprotective effect of coumarin-pi on oxidative stress in t-BHP-induced HepG2 cells.
Extraction and isolation
Paxillus involutus fruit body was isolated from the Yanshan Mountain, Hebei Province and identified by Prof. Li-an Wang. The fresh fruit body was dried in the constant temperature drying box at 42 ℃ until there was no weight change and was powdered in a grinder. 3 kg powder of dried P. involutus fruit body was stirred using 30 L of ethyl acetate for 2 h and left to stand overnight at 4 ℃. The remaining precipitate was re-extracted 3–5 times following the protocol described above. The organic layer was evaporated in vacuum yield crude extract (94 g).This residue was separated over silica gel CC by gradient elution with petroleum ether–ethyl acetate (1:0 to 0:1). The fraction eluted by ethyl acetate was evaporated in vacuum and re-separated by silica gel CC with CHCl3–MeOH (1:0 to 0:1). The fraction eluted by CHCl3–MeOH at 10:1 was separated by Sephadex LH-20 (MeOH) to obtain coumarin-pi.
Cell culture
HepG2, HCT116, BGC, and RAW 264.7 cells were cultivated in Dulbecco's modified Eagle's medium (DMEM), and K562 cells were cultivated with 1640 medium. These cells were supplemented with 10% fetal bovine serum, penicillin (100 IU/mL), and streptomycin (100 mg/mL) at 37 °C in 5% CO2. All the cells were kindly provided by professor Junxia Zhao form Hebei Medical University.
Cell viability assay
Cell viability was evaluated by CCK8 assays. HepG2, HCT116, BGC, RAW 264.7, and K562 cells were first grown in 96-well plates for 24 h. HepG2 cells were plated at 1 × 104 cells/well, and the remaining cell density was 3 × 104 cells/well. Then, the cells mentioned above were individually incubated with coumarin-pi (0–48 μM) for 6 h. Next, 10 μL of CCK8 was added to each well and incubated for 2 h. Then, the absorbance of CCK8 was measured at 450 nm using a Multiskan GO Microplate Spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, United states of America).
HepG2 cells were plated in 96-well plates (1 × 104 cells/well) and cultured for 24 h. The cells were pretreated with various concentrations of coumarin-pi (0–48 μM) for 6 h and subjected to t-BHP (400 μΜ) for 3 h. After the supernatant was discarded, 100 μL of DMEM culture medium and 10 μL of CCK8 solution were added for another 2 h. The absorbance at 450 nm was measured using a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, United states of America).
Measurement of intracellular ROS levels
HepG2 cells were plated in 96-well plates (1 × 104 cells/well) and cultured for 24 h. The cells were pretreated with various concentrations of coumarin-pi (0–48 μM) for 6 h. Then, the cells were treated with 0.4 mM t-BHP for 30 min to generate ROS and stained with 1 μM DCFH-DA for 40 min. Fluorescence was detected on a fluorescence-detecting microplate reader with excitation/emission wavelengths of 485/525 nm, and the cell morphology was observed under a inverted microscope (Leica DM IRB, Leica, Wetzlar, Germany) [15].
Quantification of apoptotic cells and necrotic cells
HepG2 cells were grown in 60 × 15 mm (1.25 × 106 cells/well) plates for 24 h, treated with various concentrations of coumarin-pi (0–48 μM) for 6 h, and subjected to t-BHP (2 mM) for 1 h. Then, the cells were washed three times with PBS, collected, and centrifuged at 300×g for 10 min at 4 °C. Subsequently, the cells were stained with Annexin V-FITC and propidium iodide (PI). The percentages of apoptosis and necrosis were determined by flow cytometry (Beckman-Coulter, Brea, CA, USA).
Western blot analysis
HepG2 cells were homogenized and solubilized using RIPA lysis buffer supplemented with 1 mM PMSF for 20 min and then centrifuged for 15 min at 4 °C and 12,000 rpm. Western blot analysis was performed as described by previously published methods [16]. An equal amount of protein was separated on SDS polyacrylamide gels and transferred to PVDF membranes (Millipore). The membranes were blocked with 10% skim milk in TBST buffer for 1 h at room temperature and probed with the appropriate primary antibodies, followed by incubation with HRP-conjugated secondary antibodies. The protein bands were detected using an enhanced chemiluminescence detection system (Bio-Rad laboratories, Hercules, California, US).
RNA-seq transcriptomic assay
HepG2 cells were grown in 100 × 20 mm (3.5 × 106 cells) plates for 24 h and treated with 24 μM coumarin-pi for 6 h. The treated cells were harvested and treated by TRIzol® Reagent according to the manufacturer’s instructions (Invitrogen) for total RNA extraction. The genomic DNA was removed using DNase I (TaKara). Then RNA quality was determined to construct sequencing library. The untreated HepG2 cells were used as control, and there were three repetitions in both groups. For the mRNA-seq assay, the samples were submitted to Shanghai Majorbio Bio-pharm Technology Corporation for RNA-seq. The method and data analysis process are the same as those reported by Rui [17]. Briefly, Poly(A) mRNA was isolated and then converted to double-stranded cDNA using random hexamer primers (Illumina) through reverse transcription. The synthesized cDNA was modified according to Illumina’s library construction protocol and PCR amplified using Phusion DNA polymerase (NEB) for 15 PCR cycles. The products were sequenced with the Illumina HiSeq xten. Functional enrichment analysis (GO and KEGG) was performed to identify which differential expression genes were significantly enriched in GO terms and metabolic pathways at Bonferroni-corrected P-value ≤ 0.05 compared with the untreated group.
Data analysis
The data were analyzed using t tests with GraphPad Prism 6.0 software, and statistical significance was defined at P < 0.05. All experiments were repeated at least three times, and the data are presented as \(\overline{X}\) ± SD.
Results
Effect of coumarin-pi on cell viability
Cells were treated with different concentrations of coumarin-pi (0, 6, 12, 24, 48 μM) for 6 h, and cell viability was determined by CCK8 assays. As shown in Fig. 1, different cells had different sensitivities to coumarin-pi. Coumarin-pi at the tested concentrations did not cause significant cytotoxicity to BGCs (Fig. 1a) or RAW 264.7 cells (Fig. 1b). At 48 μM, this compound had significant cytotoxicity to HepG2 cells (P < 0.001) (Fig. 1c). As shown in Fig. 1d and e, K562 and HCT116 cells were more sensitive to coumarin-pi than the other cells. When the concentration reached 24 μM, the activity of the two cell lines decreased significantly (P < 0.001) compared with that of the control group.
Effects of coumarin-pi on t-BHP-induced cells. a–e Represent BGC, RAW 264.7, HepG2, K562, and HCT116 cells incubated with increasing concentrations of coumarin-pi, and cell viability was determined by CCK8 assays. Values are expressed as the \(\overline{X}\) ± SD (n = 6). Different letters indicate statistically significant differences. **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. the control group
Coumarin-pi protects HepG2 cells from t-BHP-induced cytotoxicity
Chronic liver diseases are nearly always characterized by increased oxidative stress, regardless of the cause of the liver disorder [18], and HepG2 cells are often used for antioxidant tests. We chose HepG2 cells as the study object.
The cytoprotection of coumarin-pi against t-BHP-induced cell injury was tested. HepG2 cells were pretreated with increasing concentrations of coumarin-pi (6, 12, 24 and 48 μM) for 6 h and then treated with t-BHP (400 μM) for 3 h. As shown in Fig. 2, t-BHP-induced cytotoxicity was restrained by coumarin-pi in a dose-dependent manner. Among the concentrations, 48 μM coumarin-pi significantly restored cell activity to over 72.89%.
Coumarin-pi protects HepG2 cells from t-BHP-induced reductions in ROS production and apoptosis
We studied the effect of coumarin-pi on ROS induced by t-BHP. As shown in Fig. 3, t-BHP can effectively induce ROS overproduction, which increases to approximately 2.5 times compared to that of the control group. After treatment with coumarin-pi, intracellular ROS production was inhibited.
As t-BHP can induce apoptosis, we studied the effect of coumarin-pi on apoptosis in HepG2 cells by using flow cytometry. As shown in Fig. 4a, the apoptosis rate of the control group was 7.90% and that of the t-BHP group was 46.21%. With increasing coumarin-pi concentrations, the apoptosis rate gradually decreased. When the concentration of coumarin-pi was 48 μM, the apoptosis rate of the cells was close to that of the control group.
The expression of the members of the caspase family, which have important roles in apoptosis, including caspase-3, caspase-8, and caspase-9, was examined. The expression of cleaved caspase-3, caspase-8, and caspase-9 all increased markedly after treated with t-BHP (Fig. 4b), and their expression decreased gradually when the concentration of coumarin-pi increased.
Our results showed that coumarin-pi could effectively decrease the t-BHP-induced apoptosis and necrosis in HepG2 cells.
Effect of coumarin-pi on the GCLC, GCLM, HO-1, NQO1, and Nrf2 activation induced by t-BHP
Recent reports have suggested that various antioxidative enzymes, including GCLC, GCLM, HO-1, and NQO1, play essential roles in the amelioration of oxidative injury [9].Consequently, we determined whether coumarin-pi could induce GCLC, GCLM, HO-1, and NQO1 expression to enhance the resistance of cells to oxidative damage. As shown in Fig. 5, coumarin-pi could enhance the expression of these proteins to different degrees.
Nrf2, an essential transcription factor, can translocate into the nucleus and lead to the expression of several of the antioxidant and detoxification genes mentioned above. We tested whether the expression of Nrf2 was upregulated. As shown in Fig. 5, coumarin-pi enhanced the expression of Nrf2. According to the above results, we hypothesized that the Nrf2 signal may play a crucial role against oxidative stress and cytotoxicity.
Analysis of functional enrichment of differentially expressed genes
To further investigate the biological effect of coumarin-pi, we analyzed the transcriptomic changes of the HepG2 cells treated with 24 μM coumarin-pi for 6 h compared with the untreated cells. In the coumarin-pi-treated HepG2 cells, 20 genes were downregulated and 236 genes were upregulated (fold change > 2.0). The differentially expressed genes related to GO terms were enriched in response to stimulus and metabolic process regulation (response to organic substance, cellular response to stimulus, response to chemicals, regulation of cellular metabolic processes, biological regulation, regulation of cellular processes, regulation of metabolic processes, regulation of primary metabolic processes, regulation of biological processes, etc.) (Fig. 6a). The differentially expressed genes related to KEGG pathways were enriched in cancer (microRNAs in cancer, choline metabolism in cancer, non-small cell lung cancer, endometrial cancer, etc.) (Fig. 6b).
Discussion
This study demonstrated that coumarin-pi can protect HepG2 cells from oxidative stress-induced cell death. In this study, t-BHP, a short-chain analog of lipid peroxide, was chosen as an oxidative stress inducer and could increase intracellular ROS in HepG2 cell lines [19]. Coumarin-pi could alleviate t-BHP-induced cytotoxicity, ROS accumulation, and cell apoptosis. Moreover, this molecule could induce the antioxidant enzyme expression of HO-1, NQO1, GCLC, and GCLM, which are transcriptionally activated by the Nrf2 signaling pathway. It was reported that Nrf2 undergoes nuclear translocation followed by the disaggregation of Nrf2 from the Nrf2-Keap1 complex [20,21,22]. The change in Keap1 protein needs further study. It was also reported that some antioxidants were associated with Nrf2 activation through the modulation of the Akt and ERK pathways [15, 23, 24]. Whether the protective effect of coumarin-pi is also mediated through these two signaling pathways requires further study. Knockout of Nrf2 signal in HepG2 cell lines is also needed to see if Nrf2 is the key signal of coumarin-pi-induced cytoprotective and antioxidant effects in HepG2 cells.
In addition, we found that some metabolic pathways were regulated in HepG2 cell after coumarin-pi treatment, as shown by transcriptome analysis. In future studies, we will conduct in-depth research on the antioxidant mechanism of coumarin-pi and further study this compound to determine whether it can be used as a therapeutic or health care product.
References
Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt Jr PI (2016) Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 473:4527–4550. https://doi.org/10.1042/BCJ20160503C
Christen Y (2000) Oxidative stress and Alzheimer disease. Am J Clin Nutr 71:621S–629S. https://doi.org/10.1093/ajcn/71.2.621s
de la Monte SM, Neely TR, Cannon J, Wands JR (2000) Oxidative stress and hypoxia-like injury cause Alzheimer-type molecular abnormalities in central nervous system neurons. Cell Mol Life Sci 57:1471–1481. https://doi.org/10.1007/PL00000630
Narendhirakannan RT, Hannah MA (2013) Oxidative stress and skin cancer: an overview. Indian J Clin Biochem 28:110–115. https://doi.org/10.1007/s12291-012-0278-8
Palade F, Alexa ID, Azoicai D, Panaghiu L, Ungureanu G (2003) Oxidative stress in atherosclerosis. Rev Med Chir Soc Med Nat Iasi 107:502–511
Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C (2013) Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci 14:21525–21550. https://doi.org/10.3390/ijms141121525
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Lee KM, Kwon TY, Kang U, Seo EK, Yun JH, Nho CW, Kim YS (2017) Tussilagonone-induced Nrf2 pathway activation protects HepG2 cells from oxidative injury. Food Chem Toxicol 108:120–127. https://doi.org/10.1016/j.fct.2017.07.035
Lv H, Liu Q, Zhou J, Tan G, Deng X, Ci X (2017) Daphnetin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death. Free Radic Biol Med 106:38–52. https://doi.org/10.1016/j.freeradbiomed.2017.02.016
Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P (2004) Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA 101:2040–2045. https://doi.org/10.1073/pnas.0307301101
Shin SM, Yang JH, Ki SH (2013) Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev 2013:763257. https://doi.org/10.1155/2013/763257
Zhang JX, Lv JH, Zhao LQ, Shui XX, Zhang J, Wang LA (2019) Coumarin-pi, a new antioxidant coumarin derivative from Paxillus involutus. Nat Prod Res. https://doi.org/10.1080/14786419.2018.155717013
Peng XM, Damu GL, Zhou C (2013) Current developments of coumarin compounds in medicinal chemistry. Curr Pharm Des 19:3884–3930. https://doi.org/10.2174/1381612811319210013
Pereira TM, Franco DP, Vitorio F, Kummerle AE (2018) Coumarin compounds in medicinal chemistry: some important examples from the last years. Curr Top Med Chem 18:124–148. https://doi.org/10.2174/1568026618666180329115523
Qi Z, Ci X, Huang J, Liu Q, Yu Q, Zhou J, Deng X (2017) Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed Pharmacother 88:252–259. https://doi.org/10.1016/j.biopha.2017.01.067
Hwang YP, Choi JH, Choi JM, Chung YC, Jeong HG (2011) Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem Toxicol 49:2081–2089. https://doi.org/10.1016/j.fct.2011.05.021
Wang R, Tao B, Fan Q, Wang S, Chen L, Zhang J, Hao Y, Dong S, Wang Z, Wang W, Cai Y, Li X, Bao T, Wang X, Qiu X, Wang K, Mo X, Kang Y, Wang Z (2019) Fatty-acid receptor CD36 functions as a hydrogen sulfide-targeted receptor with its Cys333-Cys272 disulfide bond serving as a specific molecular switch to accelerate gastric cancer metastasis. EBioMedicine 45:108–123. https://doi.org/10.1016/j.ebiom.2019.06.037
Cicho-Lach H, Michalak A, Gastroenterology DO (2014) Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol 20(25):8082–8091. https://doi.org/10.3748/wjg.v20.i25.8082
Rush GF, Gorski JR, Ripple MG, Sowinski J, Bugelski P, Hewitt WR (1985) Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicol Appl Pharmacol 78:473–483. https://doi.org/10.1016/0041-008x(85)90255-8
Han X, Ding C, Zhang G, Pan R, Liu Y, Huang N, Hou N, Han F, Xu W, Sun X (2020) Liraglutide ameliorates obesity-related nonalcoholic fatty liver disease by regulating Sestrin2-mediated Nrf2/HO-1 pathway. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2020.03.032
Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL (2013) The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1:45–49. https://doi.org/10.1016/j.redox.2012.10.001
Gureev AP, Popov VN, Starkov AA (2020) Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp Neurol 328:113285. https://doi.org/10.1016/j.expneurol.2020.113285
Bai X, Gou X, Cai P, Xu C, Cao L, Zhao Z, Huang M, Jin J (2019) Sesamin enhances Nrf2-mediated protective defense against oxidative stress and inflammation in colitis via AKT and ERK activation. Oxid Med Cell Longev 2019:2432416. https://doi.org/10.1155/2019/2432416
Kim JH, Khalil AAK, Kim HJ, Kim SE, Ahn MJ (2019) 2-Methoxy-7-acetonyljuglone isolated from reynoutria japonica increases the activity of nuclear factor erythroid 2-Related factor-2 through inhibition of ubiquitin degradation in HeLa cells. Antioxidants (Basel) 8(9):E398. https://doi.org/10.3390/antiox8090398
Acknowledgements
This work was supported by a grant from the Science and Technology Plan Projects of Hebei Province (16237301D), the Earmarked Fund for Hebei Edible Fungi Innovation Team of Modern Agro-industry Technology Research System (HBCT2018050207), the National Natural Science Foundation of China (31900329), and the Hebei Natural Science Foundation (C2019205197).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Feng, H., Lv, J. et al. Protective effect of coumarin-pi against t-BHP-induced hepatotoxicity by upregulating antioxidant enzymes via enhanced Nrf2 signaling. Mol Cell Biochem 475, 277–283 (2020). https://doi.org/10.1007/s11010-020-03880-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03880-x