Abstract
High fat diet (HFD) is a common cause of metabolic syndrome and type 2 diabetes mellitus. Published data showed that HFD and subsequent dyslipidemia are major triggers for oxidative stress. Forty-eight male Sprague–Dawley rats, weighing 170–200 g, were divided into six groups: control, control with vitamin E (100 mg/kg/day, i.p.), control with simvastatin (SIM) (10 mg/kg of body weight/day), HFD, HFD with vitamin E, and HFD with SIM. Standard and high cholesterol diets were given for 15 weeks and SIM and vitamin E were added in the last 4 weeks. In all rats, serum vitamin E, total cholesterol (TC), triglycerides (TG), low (LDL) and high (HDL) density lipoproteins, alanine (ALT) and aspartate (AST) transaminases, alkaline phosphatase (ALP), and gamma glutamyl transpeptidase (GGT) as well as cardiac and hepatic thiobarbituric acid-reactive substances (TBARS) and antioxidants (reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT)) were measured. Also, electrocardiogram (ECG) was recorded. HFD significantly increased QTc interval, heart rate (HR), serum TC, TG, LDL, ALT, AST, ALP, GGT, liver TG, and cardiac and hepatic TBARS but decreased antioxidants and HDL, while SIM decreased HR, liver TG, serum TC, TG, and LDL and increased HDL in HFD rats. Vitamin E had no effect. Moreover, SIM and vitamin E decreased QTc interval, serum ALT, AST, ALP, GGT, and cardiac and hepatic TBARS and increased antioxidants in HFD rats. Histopathological observations confirm the biochemical parameters. SIM and vitamin E slow progression of hypercholesterolemia-induced oxidative stress in liver and heart and improve their functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic disturbances such as hyperlipidemia have been implicated in etiopathogenesis of several human diseases. Dyslipidemia plays a key role in development of atherosclerosis that is the vascular lesion responsible for most cardiovascular-related complications including myocardial infarction [46], stroke [6], and kidney injury [31]. In addition, there is a close correlation between cardiovascular diseases and lipid abnormalities, especially high level of plasma cholesterol, and blood pressure [26]. Dyslipidemia may be due to hereditary disorders such as familial hypercholesterolemia (FH) [17] and polygenic hypercholesterolemia (familial combined hypercholesterolemia) or is associated to other diseases including type 2 diabetes, cholestatic liver diseases, nephrotic syndrome, chronic renal failure, hypothyroidism, and obesity [17]. In addition, dyslipidemia can be caused by unsafe nutritional habit as continuous ingestion of high amounts of fat. Consequently, it has been tried to provoke dyslipidemia in laboratory animals in order to understand better the relationship between disorders in cholesterol metabolism and atherogenesis and to test possible treatments for reduction of circulating cholesterol level. A great number of animal models, such pigeons, chickens, swine, cats, dogs, nonhuman primates, mice, rabbits, and rats, have been tested [30]. Irrespective of etiology of dyslipidemia, one common feature is elevated serum total cholesterol (TC), low density lipoprotein (LDL), very LDL cholesterol (LDL-C), reduced high density lipoprotein (HDL), or hypertriglyceridemia [36]. The current hypothesis suggests oxidative stress as an underlying mechanism by which dyslipidemia, particularly hypercholesterolemia, induces tissue damage or provokes several human diseases [24]. It is known that the primary risk organs of hypercholesterolemia are the liver and heart [42]; therefore, it is possible that these organs may be damaged by oxidative stress in hypercholesterolemia.
Statins, a widely used group of hypocholesteremic drugs, have shown a beneficial effect in reducing cardiovascular-related morbidity and mortality in patients with or without coronary artery disease and with or without high cholesterol levels [41]. Some evidences suggest that statins exert a multiplicity of favorable effects that are not directly related to their impact on lipid metabolism such as anti-inflammatory, antithrombotic, antiangiogenic, and antihypertropic effects [7]. In addition, they exert immunosuppressive activity against pathological remodeling of the heart and blood vessels [21]. Statins are also known to decrease free radical generation in the vascular wall [44] and in the myocardium during ischemia–reperfusion injury [25] via suppression of oxygen-derived free radicals produced upon reperfusion.
Vitamin E, a nonenzymatic antioxidant, is an important lipid-soluble antioxidant placed in a special region of membranes. Its most important role is to protect the membrane polyunsaturated fatty acids from oxidation involving reactive oxygen species (ROS) by termination of free-radical chain reactions [28]. Vitamin E reduces hypercholesterolemia-induced oxidative stress in the rabbit heart [33].
Therefore, the present study aimed at investigating the effect of a commonly used statin, simvastatin (SIM), and vitamin E on serum and liver lipid levels and also on lipid peroxidation and enzymatic and nonenzymatic antioxidants in liver and heart of rats fed on a high cholesterol diet (high fat diet, HFD). In addition, electrocardiographic data, liver enzymes as well as histopathological changes of heart and liver in HFD-fed rats with or without SIM and vitamin E administration were evaluated.
Materials and methods
Animals
Forty-eight male Sprague–Dawley rats weighing 170–200 g were purchased from the Vaccine and Immunization Authority (Helwan, Cairo, Egypt) and housed (Animal House, Medical Physiology Department, Faculty of Medicine, Mansoura University, Egypt) under controlled conditions (temperature of 23 ± 1 °C and a 12 h light:12 h dark cycle). The animals were allowed free access to food and tap water. Experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85–23, revised 1996). All experimental procedures in this study were approved by the Medical Research Ethics Committee of Mansoura University, Egypt.
Experimental design
After 1 week of acclimatization to the laboratory environment, the animals were randomly divided into six groups (eight rats each). Animals in group 1 were used as control group and fed with standard laboratory chow for 15 weeks. Animals in group 2 were fed a standard laboratory chow for 15 weeks and SIM (10 mg/kg of body weight/day) [2] that was suspended in 0.5 % methylcellulose and administered by oral gavage during the last 4 weeks. Animals in group 3 were fed on standard laboratory chow for 15 weeks with vitamin E (100 mg/kg/day, i.p.) [43] that was administered in the last 4 weeks. Rats in group 4 were fed HFD. The HFD, recently described, contained 10.1 % fat (5 % coconut oil and 5.1 % linoleic acid), 17 % protein, 51.6 % carbohydrate, and 4 % cholesterol with 0.5–1 % cholic acid [4, 12]. Rats in group 5 were fed HFD for 15 weeks, and SIM (10 mg/kg of body weight/day) was given by oral gavage during the last 4 weeks. Rats in group 6 were fed HFD for 15 weeks, and vitamin E (100 mg/kg/day, i.p.) was administered in the last 4 weeks.
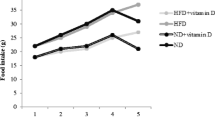
Body weight and water and food intake were recorded weekly. Feeding efficiency was expressed as weight gain (grams) / food intake (grams) × 100 [12]. At the end of the experimental period, electrocardiogram (ECG) was recorded, blood samples were obtained, and liver and heart tissues were removed from all animals.
Chemicals
SIM and vitamin E were purchased from Sigma-Aldrich (St. Louis, MO, USA). SIM was dissolved in normal saline at a dose of 10 mg/kg/day [2] by orogastric gavage, with an appropriate feeding needle and a volume of 5 ml/kg vitamin E was dissolved in mineral oil immediately before use and administered to rats at a dose of 100 mg/kg/day [40].
ECG recording
At the end of the treatment, all rats were fasted overnight but had free access to water and ECG was recorded, under the effect of light ether anesthesia, by PowerLab data acquisition system (ML866, PowerLab 4/30, AD Instruments, Australia). Touch electrodes (MLA1214, AD Instruments) were attached to the skin of the animals at position II for ECG recording. The recorded ECG was used to measure ST, and QT and QTc intervals; QRS complex; and heart rate (HR).
Sampling protocol
Blood samples
At the end of the experimental period, after ECG recording, blood samples were collected by cardiac puncture. These blood samples were collected without anticoagulant, left for 10 min and then centrifuged for 10 min at 4,000 r/min to obtain serum, which was stored at −20 °C until further biochemical analysis for determination of serum triglycerides (TG), TC, HDL cholesterol (HDL-C), LDL-C, alanine (ALT) and aspartate transaminases (AST), alkaline phosphatase (ALP), and gamma glutamyl transpeptidase (GGT).
Tissue samples
Heart and liver were removed, cleaned, and weighted to calculate the relative organs' weight to body weight. Both organs were kept at −80 °C until biochemical analysis for assessment of lipid peroxidation product (malondialdehyde and thiobarbituric acid-reactive substances (TBARS)) and antioxidants (reduced glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD)).
Biochemical investigations
Assessment of lipid peroxide and enzymatic and nonenzymatic antioxidants in tissues
Heart and liver were removed and rinsed with phosphate-buffered saline (pH 7.4) to remove any red blood cells and clots. Then, the tissues were homogenized in 5–10 ml of cold 20 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.2) containing 1 mmol/l ethylene glycol tetraacetic acid (EGTA), 210 mmol/l mannitol, and 70 mmol/l sucrose per gram tissue. Homogenates were centrifuged at 15,000 r/min (18,000g) for 15 min at 4 °C, and the supernatant was removed and stored at −80 °C until further analysis for determination of reduced GSH, CAT, SOD, and TBARS levels.
In the tissue homogenates of heart and liver, GSH (cat. no. 703002; Cayman Chemical, Ann Arbor, MI, USA), SOD (cat. no. 706002, Cayman Chemical, Ann Arbor, MI, USA), and CAT (cat. no. NWK-CAT01, Northwest Life Science Specialties, Vancouver, WA, USA) were measured according to the manufacturer's instructions. TBARS was analyzed by measuring the production of TBARS according to the method of Buege and Aust [8] using a TBARS assay kit (cat. no. 10009055, Cayman Chemical, Ann Arbor, MI, USA).
Assessment of serum lipids
Serum TG concentrations were measured by the peroxidase method using a commercial kit (Spinreact, Spain). Serum TC measurement was performed using the cholesterol oxidase method, where the pink color of quioneimine was measured at 500 nm. The used kit was supplied by Spinreact, Spain. Serum HDL-C was determined enzymatically after precipitation of apoB-containing lipoproteins by the phosphotungstic acid Mg method with the kit supplied by Spinreact, Spain. Serum LDL–C was estimated with a commercial kit (Spinreact, Spain), with a two-step technique [18]. First, chylomicrons, very low density lipoproteins (VLDL) and HDL were eliminated. Second, LDL was specifically measured through the action of peroxidase with the formation of pinkish-colored quinine. Intensity of the color formed is proportional to the LDL concentration in the sample. The atherogenic index (AI) was later calculated as the ratio of TC-HDL/HDL [42].
Assessment of serum vitamin E
Serum vitamin E was determined using enzyme-linked immuno sorbent assay (ELISA) kits delivered from MyBioSource catalogue number: MBS161162.
Liver lipids level
Lipids were extracted from tissues using chloroform–methanol (2:1 by volume) by the method of Folch et al. [16]. Dried lipid extracts were resuspended in 1 ml of saline solution (9 g/l NaCl, 1 % Triton X-100), used for the estimation of lipid profile, and assayed using reagent kits (Hospitex Diagnostics, Florence, Italy).
Evaluation of liver function
Liver function was evaluated by assessing serum ALT, AST, ALP, and GGT levels using an enzymatic kit (Randox Laboratories, UK) according to the manufacturer's instructions.
Histopathological examination
Cardiac muscle and liver samples were taken from rats in different groups, fixed in 10 % formal saline for 1 day and then washed with water. Ascending serial dilutions of ethyl alcohol were used for dehydration. Samples were cleared in xylene and then embedded in paraffin at 56° in a hot oven for 24 h. Paraffin blocks were prepared and cut at 4–6-μm thickness. Sections are mounted on glass slides, deparaffinized, and stained by hematoxylin and eosin stains for histological examinations through a light microscope.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). Data were processed and analyzed using the SPSS version 10.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was done followed by Tukey’s post hoc test. Pearson's correlation statistical analysis was done for detection of a probable significance between two different parameters. Results were considered significant if p ≤ 0.05.
Results
Changes in body weight, feeding efficiency, liver and heart to body weight ratio, and liver TG are shown in Table 1. SIM or vitamin E only did not alter body weight, feeding efficiency, liver and heart to body weight ratio, and liver TG in normal control rats (CR) (Table 1). After 15 weeks of feeding, the body weights of the HFD group were significantly increased (31 %) compared with those of the control group (CR) (p < 0.05) and daily administration of SIM or vitamin E in the last 4 weeks did not induce any significant changes in body weight (p > 0.05) (Table 1). Body weight gain was not significantly different in all groups of rats (p > 0.05) (Table 1). Feeding efficiency in the HFD, HFD + SIM, and HFD + vitamin E groups was significantly increased in comparison with those in the control group (P < 0.05). Moreover, rats fed HFD for 15 weeks had a significant increase in relative liver and heart to body weight ratio (p < 0.05) as compared to CR. Administration of SIM to HFD fed rats in the last 4 weeks significantly decrease relative liver and heart to body weight ratio in HFD rats (p < 0.05) but their values were significantly different from those of the CR (p < 0.05) (Table 1). No significant effects on liver and heart to body weight ratio were observed in HFD-fed rats given vitamin E (p > 0.05) (Table 1). The liver triglyceride and cholesterol contents were significantly increased in HFD rats in comparison with CR, whereas in HFD rats treated with SIM, hepatic lipid accumulation was significantly reduced but its value remained more than that of CR (p < 0.05) (Table 1). No significant effect on liver TG and cholesterol were observed in HFD fed rats supplemented with vitamin E (p > 0.05) (Table 1).
Table 2 showed the effects of SIM and vitamin E on serum TG, TC, HDL-C, LDL-C, and AI in rats kept on standard diet (control) or HFD. No significant changes in serum TG, TC, HDL-C, LDL-C, and AI were observed due to SIM or vitamin E treatment in normal CR (Table 2). Fifteen weeks of HFD feeding raised serum TC, triglyceride, LDL-C, and AI significantly (p < 0.05), whereas HDL-C was decreased (p < 0.05) as compared with normal CR. Administration of SIM to the HFD-fed rats in the last 4 weeks caused a significant reduction in the levels of TG, TC, LDL-C, and AI as well as elevation in HDL-C (P < 0.05) when compared with rats fed on HFD alone (P < 0.05), but all values remain significantly increased except HDL-C that is significantly decreased from those of the control group (p < 0.05) (Table 2). On the other hand, no significant changes were observed in all parameters (TG, TC, LDL-C, HDL-C, and AI) in HFD-fed rats supplemented with vitamin E (p > 0.05) as compared to rats fed on HFD alone (Table 2). Vitamin E treatment to the CR did not increase the serum vitamin E level significantly (p > 0.05). While in HFD-fed rats, vitamin E decreased significantly (p < 0.05) as compared with the control normal rats. Furthermore, vitamin E supplementation to HFD-fed rats significantly (p < 0.05) increased the serum level of vitamin E versus HFD-fed rats.
Table 3 shows the effects of SIM and vitamin E on TBARS and GSH levels as well as CAT and SOD activities in heart and liver of rats fed on standard or high cholesterol diets. SIM or vitamin E caused no significant change in TBARS, GSH levels, and CAT and SOD (antioxidant enzymes) activities in liver and heart of normal CR (Table 3). Levels of hepatic and cardiac TBARS increased, whereas GSH level and antioxidant enzymes activities were decreased in HFD-fed rats (Table 3). In contrast, the HFD-fed rats treated with SIM or vitamin E showed a significant decrease in the level of hepatic and cardiac TBARS with increase in the GSH level and antioxidant enzymes activities compared with rats fed on HFD only (p < 0.05) but their values remained significantly lower than the control normal rats (p < 0.05) (Table 3). In addition, the effects of SIM administration on TBARS and GSH levels as well as CAT activity in HFD-fed rats were more obvious than vitamin E supplementation by decreasing TBARS level and increasing CAT activity and GSH level (p < 0.05), whereas SOD increased to a similar value with administration of either SIM or vitamin E (p > 0.05). For cardiac tissue, CAT activity in HFD-fed rats treated with SIM was increased to a similar level of normal rat (p > 0.05) (Table 3). In HFD-fed rats, a significant positive correlation (p < 0.0001) was reported between the plasma cholesterol level and TBARS levels of both cardiac (r = 0.9720) and hepatic (r = 0.8504) homogenates.
Effects of SIM and vitamin E on ECG parameters of rats fed on standard diet (control group) and high cholesterol diet (HFD) are shown in Table 4. At the end of SIM or vitamin E treatment, no significant changes were observed in rats receiving SIM or vitamin E alone (p > 0.05). Meanwhile, HFD induced a significant prolongation in ST segment, and QT and QTc intervals, with no significant effect on QRS complex when compared with the control group. In addition, a significant increase in HR of HFD-fed rats was observed as compared to normal group. Treatment of HFD-fed rats with SIM or vitamin E significantly shortened the ST and, QT and QTc intervals as well as the HR as compared to untreated HFD-fed group. SIM or vitamin E significantly shortened the duration of ST segment, and QT and QTc intervals in HFD-fed rats in comparison with the untreated HFD group (p < 0.05) but SIM is more effective than vitamin E (p < 0.05) (Table 4). In addition, SIM significantly decreased the HR (p < 0.05) when administered to HFD-fed rats, while vitamin E had no effect (p > 0.05). In HFD-fed rats, a significant positive correlation (p < 0.0001) was reported between the TBARS level in the cardiac homogenate and the Q–T interval (r = 0.9363) and with the Q–Tc interval (r = 0.9606) with Pearson's correlation coefficient (Table 4).
As reported in Table 5, no significant changes were observed in ALT, AST, ALP, and GGT levels in normal CR treated with SIM or vitamin E (p > 0.05), while HFD caused a significant increase in serum levels of ALT, AST, ALP, and GGT in comparison with CR. In HFD-fed rats treated with SIM or vitamin E, serum ALT, AST, ALP, and GGT levels were significantly decreased as compared to rats fed on HFD alone (p < 0.05) but remained elevated relative to the control group (p < 0.05) (Table 5). In addition, the decrease in serum ALT, AST, ALP, and GGT levels in HFD-fed rats administered SIM was significant when compared with HFD-fed animals supplemented with vitamin E (p < 0.05) (Table 5).
Histopathological examination of hepatic and cardiac tissues
Heart
Heart sections of normal CR showed normal characteristic features of myocardium without cellular infiltration and normal vasculature. Myocardiocyte of normal rats had oval-elongate nucleus centrally and homogeneous cytoplasm (Fig. 1a). Rats received SIM or vitamin E alone also showed apparently normal myocardial features similar to that of normal CR. In HFD-fed rats, multifocal vacuolar degeneration and necrosis or apoptosis were seen in the myocardial cells (Fig. 1b). Moreover, there were congestion of cardiac blood vessel and hyalinosis of its wall (Fig. 1b), in addition to granularity of the sarcoplasm of focal cardiac myocytes in HFD-fed rats (Fig. 1b). In contrast, myocardial cell of HFD-fed rat treated with SIM and vitamin E revealed normal histological structure (Fig. 1c and d).
Liver
Histopathological examination of livers from normal CR revealed normal histological structure of hepatic lobule as shown in Fig. 2a. The normal hepatocyte had the round nucleus centrally and homogeneous cytoplasm (Fig. 2a). There are flat endothelial cells around central vein and sinusoid. The hepatic cord arrangement as a regular ray pattern was also seen in normal hepatocyte. Rats received SIM or vitamin E alone also showed apparently normal histological structure of hepatic lobule similar to that of normal CR. Liver sections of HFD-fed rats had cytoplasmic vacuolization of hepatocytes and fatty changes of hepatocytes (Fig. 2b). The hepatocytes from HFD-fed rats showed diffused vacuolar degeneration and necrosis (loss of nucleus) that indicated that the cells were severely deteriorated (Fig. 2b). Endothelial lining of the central vein exhibited more cell injury with an increased accumulation of fat vacuoles in the hepatocytes. In contrast, liver of HFD-fed rats treated with SIM and vitamin E showed fatty change of hepatocytes but less than that of HFD-fed rats (Fig. 2c and d).
Discussion
In the current study, Sprague–Dawley rats were used and fed on a HFD containing 4 % cholesterol and 1 % cholic acid for 15 weeks. Cholic acid is known to improve cholesterol absorption because of its emulsifying property, and it also has an inhibitory action on hepatic cholesterol 7-α hydroxylase activity [30]. Moreover, SIM and vitamin E were administered to rats in the last 4 weeks of the 15-week period during which the rats were fed on a HFD. Thus, this study investigated whether or not SIM and vitamin E can slow the progression of oxidative stress. The results of the present study demonstrate that SIM and vitamin E treatment markedly attenuated hypercholesterolemia-induced oxidative stress in the heart and liver of rats as confirmed by biochemical assays and microscopic examination but SIM is more effective. Moreover, SIM and vitamin E attenuated the cardiac and hepatic dysfunctions, resulting from hypercholesterolemia-induced oxidative stress, as evidenced by ECG analysis (Fig. 3) and liver enzyme assessment respectively; however, SIM is more efficient. Also, in this study, vitamin E was administered after the hypercholesterolemia-induced oxidative stress in the heart and liver in a dose of 100 mg/kg/day that is considered as safe, nontoxic dose and hepatoprotective [43]. A safe dose of vitamin E in rats is up to 600 mg/kg body weight twice weekly [20]. Previous experimental studies used different doses of vitamin E as antioxidant. Vitamin E treatment to CR did not increase its serum level significantly as compared to control, while in HFD-fed rats, vitamin E supplementation significantly increased its serum level as compared to HFD-fed rats. To be of potential clinical benefit in patients with hypercholesterolemia-induced oxidative stress, the agents used should produce regression and/or slow the progression of oxidative stress. It is not known if vitamin E would slow the progression of hypercholesterolemia-induced oxidative stress in the heart and liver.
ECG recording from the chest of rats by the ECG electrodes. The data were filtered and amplified and sent to an analog-to-digital converter (PowerLab data acquisition and analysis system: AD Instruments, Australia). Data were collected and analyzed by LabChartpro7 software (AD Instruments, Australia). a Control group, b HCD group, c HCD + SIM, and d HCD + vit. E
Vitamin E can prolong the prothrombin time (PT) in animal models by inhibiting vitamin K-dependent carboxylase that is corrected with vitamin K administration [15]. Vitamin E at dosages of 1,600 IU/day (720 mg/day) also reduces platelet thromboxane production. Vitamin E supplementation may impair the hematologic response to iron in children with iron deficiency anemia. In the Heart Protection Study, a combination of vitamin E (600 IU), vitamin C, and beta carotene did not affect mortality [9].
Results of the present study revealed that HFD significantly increased body weight, while food intake was reduced compared with normal basal diet (Table 1). No significant change in body weight gain (grams per week) was observed with HFD, while feeding efficiency was significantly increased (Table 1). The increased fat content of the diet is responsible for satiety and increased total calories. These results were in agreement with Matos et al. [27] and Rezq and El-Khamisy [37] who demonstrated that high fat cholesterol diet, which is used to induce hypercholesterolemia, leads to lower ingestion by the animals and induces malnutrition. No changes in body weight, weight gain, food intake, and feeding efficiency were reported in HFD-fed rats administered SIM or vitamin E (Table 1).
In this study, the relative liver and heart weight to body weight ratio, showed a significant increase in hypercholesterolemic group as compared to normal group due to accumulation of fat in the liver and heart cells. Moreover, liver lipids were increased in rats fed on HFD (Table 1) that was confirmed by a histopathological examination that showed fatty changes of hepatocytes and granularity of the sarcoplasm with increased intracellular lipids of focal cardiac myocytes. These results were in accordance with Matos et al. [27] and Rezq and El-Khamisy [37] who reported that the increase in liver weight could be a consequence of their higher fat content. Nonalcoholic fatty liver disease (NAFLD) is the buildup of extra fat in liver cells that is one of the characteristics of metabolic syndrome. It is normal for the liver to contain some fat. However, if more than 5–10 % of the liver's weight is fat, then it is called a fatty liver (steatosis). It is complicated with steatohepatitis and liver cirrhosis. In addition, Matos et al. [27] and Rezq and El-Khamisy [37] observed intracellular lipid accumulation in cardiomyocytes in response to cholesterol diet, while administration of SIM to rats fed on HFD significantly decreased liver and heart weight to body weight ratio and liver lipids as compared to control group (Table 1). This can be explained, as SIM but not vitamin E reduces accumulation of visceral fat in HFD-fed rats. Our results were in accord with Cui et al. [14] who demonstrated decreased liver weight in HFD-fed rats treated with SIM.
Hypercholesterolemia and hypertriglyceridemia are independent risk factors that can accelerate the development of coronary artery disease and the progression of atherosclerotic lesions [29]. Fifteen weeks of HFD, in our study, resulted in significant impairment of the lipid profile as compared with normal diet-fed rats (Table 2). These results were in accord with those reported in previous studies investigating the effects of a HFD on serum lipid levels [23, 42, 47]. In addition, the AI is significantly increased in HFD-fed rats (p < 0.05) as compared with normal CR (Table 2). A similar finding of AI [39, 42] had been reported. Administration of SIM to HFD-fed rats significantly decreased the serum TC, TG, LDL, and liver lipids, whereas it increased serum HDL level when compared with CR (Table 2). These results confirm those of others who study the effect of SIM [13, 36] and vitamin E [19, 34] on the lipid profile of HFD-fed animals. Moreover, SIM significantly decreased the AI in HFD-fed rats, while vitamin E supplementation had no effect (Table 2). Our results support previous studies [10, 39].
Oxidative stress is defined as a disturbance in the pro-oxidant and antioxidant balance within tissues [23]. Looking to oxidative stress markers, we observed significant increases in cardiac and hepatic TBARS with significant reduction in the antioxidant system in HFD-fed rats compared with CR (Table 3). Oxidative stress is implicated in the pathogenesis of NAFLD. The observed increase in cardiac and hepatic TBARS in rats fed with HFD have been reported in previous studies investigating the effects of HFD on cardiac and hepatic [23, 32, 34] TBARS. This increase in cardiac TBARS could be due to increases in the production of ROS and decreases in the antioxidants. Hypercholesterolemia increases the levels of ROS through various mechanisms. A high-cholesterol diet increases cardiac superoxide anion generation and NADPH oxidase expression [13] that utilizes the antioxidant capacity of the liver [14, 32, 35] and heart [24, 32]. In support for this, the present study showed a significant positive correlation between serum cholesterol level and TBARS level in the heart and liver (Table 3), indicating that elevated cardiac and hepatic TBARS levels could be due to hypercholesterolemia. Moreover, administration of SIM and vitamin E to HFD-fed rats significantly decreased the TBARS content of cardiac and hepatic tissues (Table 3). Similar findings with vitamin E [34] and SIM [3] had been reported. However, Al-Dosari et al. [3] used SIM concomitantly with a high cholesterol diet (preventative measure). In our study, SIM with a HFD was used for 4 weeks following 11 weeks high cholesterol diet alone to determine whether SIM slows the progression of oxidative stress in cardiac tissue or not. Additionally, administration of SIM or vitamin E to HFD-fed rats significantly increased the antioxidants in the heart and liver (Table 4) when compared with untreated rats fed with HFD only, but the effect of SIM is more obvious than vitamin E (p < 0.05) (Table 4). These results were in accord with previous studies investigating the effects of SIM [3, 14] and vitamin E [19, 34].
In the present study, the development of oxidative injury in the heart of rats fed on HFD for 15 weeks was confirmed by alteration in oxidative stress markers (significant increase in TBARS and decrease in antioxidants) as well as by histopathological examination that showed myocardial cell damage (Fig. 1). In addition, the ECG findings showed significant increase in the HR as well as significant ST segment, QT and QTc interval prolongation in HFD-fed rats in comparison to control animals (Table 4). The increased HR in HFD-fed rats suggests increased sympathetic tone. In support for this, Axelsen et al. [5] reported increased HR in high cholesterol fructose-fed rats. The increased HR of HFD-fed rats can lead to increase in oxygen consumption and can accelerate myocardial necrosis. This suggestion is in line with the deteriorated histopathological features observed in HFD-fed rats. The long QT is a disorder characterized by delayed cardiac repolarization and increased risk of developing potentially fatal ventricular arrhythmias. The delayed rectifier current (IK) is a major determinant of the phase 3 of the cardiac action potential. It comprises two independent components: one rapid (IKr) and one slow (IKs) that is responsible for prolongation of QT. The activity of some K+ channels is drastically altered by the oxidation of critical SH groups of the channel protein. [14]. On the other hand, administration of SIM to HFD-fed rats significantly decreased the HR as compared to control animals (Table 4), while vitamin E had no effect. This could be due to reduction of the exacerbated cardiac sympathetic effect and increase of the vagal effect to the heart by SIM [40].
A recent study further revealed that among the various ion currents, the rapid delayed rectifier K+ current (IKr) is the major determinant of diabetic QT prolongation. In diabetic hearts, IKr is decreased by ~80 % leading to ~30 % prolongation of action potential duration (APD), in agreement with the clinical diabetic QT prolongation. Particularly striking is the finding that oxidative stress, due to increased intracellular ROS, is the common pathway for IKr dysfunction caused by diabetic metabolic perturbations such as hyperglycemia, overproduction of tumor necrosis factor-alpha (TNF-α), and ceramide. These data suggest that oxidative stress, as a result of metabolic perturbations, is the major cause for IKr dysfunction and the consequent QT prolongation in diabetes [45]. Moreover, prolonged QTc interval can be secondary to hypercholesterolemia. Animal and human studies reported a prolongation of QT interval in hypercholesterolemic states and are considered to be due to increased oxidative stress and myocardial remodeling [11]. Consistent with these studies, this work demonstrated a significant increase in QT and QTc intervals in HFD-fed rats as compared to CR (Table 4). Moreover, a positive correlation between cardiac TBARS, and QT and QTc intervals was reported in our study, indicating that prolongation of the QT and QTc intervals could be due to cardiac oxidative stress resulting from hypercholesterolemia. In addition, data of the current study demonstrated that vitamin E supplementation to hypercholesterolemic rats significantly decreased the QT and QTc intervals (Table 4). This could be explained by the antioxidant effect of vitamin E that, therefore, can correct the prolonged QTc interval as it preserves IKr function by protecting the membrane lipids and proteins from oxidative damages that manifests in hypercholesterolemic conditions. Also, SIM significantly decreased QT and QTc intervals in rats fed on HFD when compared with control animals (Table 4), suggesting the preventive and beneficial role of SIM in hypercholesterolemia-induced repolarization characteristics. This effect could be explained by the antioxidant and the cholesterol-lowering effects of SIM, thus protecting the IKr function and correcting the prolonged QTc interval. Moreover, Adameová et al. (2009) [2] demonstrated that in the diseased diabetic hypercholesterolemic animals, both electrical and mechanical myocardial function may be influenced by statins independently of cholesterol lowering.
The cardioprotective effect of SIM and vitamin E, in this study, was further confirmed by the significant decrease in oxidative stress, (Table 3) and the shortening of prolonged QT and QTc intervals in hypercholesterolemic rats (Table 4). However, the protective effect of SIM is more efficient than vitamin E (Tables 3, 4). In a recent study, the oxidative myocardial injury caused by doxorubicin treatment was attenuated by SIM that reflects the direct antioxidant activity of the drug that is independent of its cholesterol-lowering activity [1]. Another study demonstrated that SIM significantly attenuates the increase in plasma levels of F2-isoprostanes (8-epi-PGF2α) and TBARS, both markers of increased oxidative stress in vivo, associated with experimental hypercholesterolemia in the absence of any lipid-lowering effect [45]. Moreover, Prasad et al. (2012) [34] demonstrated that vitamin E slows the progression of hypercholesterolemia-induced oxidative stress in the heart.
In the present study, the development of oxidative injury in the liver of rats fed on HFD for 15 weeks was confirmed by the alteration in oxidative stress markers as well as by histopathological examination that showed cytoplasmic vacuolization, degeneration, necrosis, and fatty changes of hepatocytes that indicated that the cells were severely deteriorated (Fig. 2). In addition, the enzyme activities shown in Table 5 indicated that all four enzymes were elevated in the serum of the HFD-fed rats. These results were in accord with those of previous studies [42]. The elevation of serum levels of these enzymes could be due to their leakage into the serum as a result of damage to the integrity of the liver [37]. Elevated serum ALT levels in the absence of viral hepatitis and alcoholism have been reported to lead to higher risk of cardiovascular disease with the risk greater in women [22]. The heart also has high AST content that becomes elevated in myocardial infarction. GGT has been reported to be a very strong risk factor, taking third place, for all forms of heart diseases and a possible indicator for early development of atherosclerosis. Ruttmann et al. [38] reported a correlation between GGT and cardiovascular mortality, indicating that the higher the elevation of GGT, the greater the risk of death. These reports agree with the present study that showed raised levels of liver enzymes with hepatic injury and cardiovascular distress in the rats fed with HFD (Table 5).
Administration of SIM or vitamin E to HFD-fed rats significantly decreased serum levels of liver enzymes (Table 5) and improves hepatocyte degeneration (Fig. 2), but SIM is more effective than vitamin E. However, their effect was not powerful enough to completely protect the liver function as shown by the serum levels of liver enzymes that remained significantly higher than control animals (Table 3). The protective effect of SIM or vitamin E on the liver, in this study, was further confirmed by the significant decrease in oxidative stress improvement of liver structure and function. These results are in line with the previously confirmed data of Cui et al. [14] who demonstrated decreased liver enzymes in HFD-fed rats treated with SIM that decreases the hepatic oxidative stress.
Conclusion
Hypercholesterolemia increases the oxidative stress in the heart and liver. SIM and vitamin E are efficient in slowing the progression of hypercholesterolemia-induced oxidative stress in these organs and improving their functions but SIM is more effective.
References
Abd Elbaky NA, Ali AA, Ahmed RA (2010) Cardioprotective effect of simvastatin on doxorubicin induced oxidative cardiotoxicity in rats. J Basic Appl Sci 6(1):29–38
Adameová A, Harčárová A, Matejíková J, Pancza D, Kuželová M, Čarnická S, Švec P, Barteková M, Styk V, Ravingerová T (2009) Simvastatin alleviates myocardial contractile dysfunction and lethal ischemic injury in rat heart independent of cholesterol-lowering effects. Physiol Res 58:449–454
Al-Dosari MS (2011) Hypolipidemic and antioxidant activities of avocado fruit pulp on high cholesterol fed diet in rats. Afr J Pharm Pharmacol 5(12):1475–1483
Aragno M, Tomasinelli CE, Vercellinatto I, Catalano MG, Collino M, Fantozzi R et al (2009) SREBP-1c in nonalcoholic fatty liver disease induced by Western-type high-fat diet plus fructose in rats. Free Radic Biol Med 47:1067–1074
Axelsen LN, Pedersen HD, Petersen JS, Holstein-Rathlou N-H, Kjølbye AL (2010) Metabolic and cardiac changes in high cholesterol–fructose-fed rats. J Pharmacol Toxicol Methods 61:292–296
Balakumar P, Jindal S, Shah DI, Singh M (2007) Experimental models for vascular endothelial dysfunction. Trends Med Res 2:12–20
Bonetti PO, Lerman LO, Napoli C, Lerman A (2003) Statin effects beyond lipid lowering—are they clinically relevant? Eur Heart J 24:225–248
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cheung MC, Zhao XQ, Chait A et al (2001) Antioxidant supplements block the response of HDL to simvastatin–niacin therapy in patients with coronary artery disease and low HDL. Arterioscler Thromb Vasc Biol 21(8):1320–1326
Chia BL (1991) Cholesterol and coronary artery disease—issues in the 1990s. Singapore Med J 32:291–294
Chih-Sheng C, Kun-Tai L, Shuo-Tsan L et al (2007) Effects of atorvastatin on ventricular late potentials and repolarization dispersion in patients with hypercholesterolemia. Kaohsiung J Med Sci 23:217–224
Collino M, Aragno M, Castiglia S, Miglio G, Tomasinelli C, Boccuzzi G, Thiemermann C, Fantozzi R (2010) Pioglitazone improves lipid and insulin levels in overweight rats on a high cholesterol and fructose diet by decreasing hepatic inflammation. Br J Pharmacol 160:1892–1902
Csont T, Bereczki E, Bencsik P et al (2007) Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc Res 76:100–109
Cui B, Liu S, Lin X, Wang J, Li S, Wang Q, Li S (2011) Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules 16:9116–9128
Diplock AT (1995) Safety of antioxidant vitamins and beta-carotene. Am J Clin Nutr 62(6 Suppl):1510S–1516S
Folch J, Less M, Solane SGH (1957) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 26:497–509
Frederick JR (2009) Hypercholesterolaemia in children and young adults—current management. JEMDSA 14:9–12
Friedwald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Gokkusu C, Mostafazadeh T (2003) Changes of oxidative stress in various tissues by long-term administration of vitamin E in hypercholesterolemic rats. Clin Chim Acta 328:155–161
Hajiani M, Golestani A, Shariftabrizi A, Rastegar R, Payabvash S, Salmasi AH, Dehpour AR, Pasalar P (2008) Dose-dependent modulation of systemic lipid peroxidation and activity of anti-oxidant enzymes by vitamin E in the rat. Redox Rep 13(2):60–66
Harst P, Voors AA, Gilst WH, Bohm M, Veldhuisen DJ (2006) Statins in the treatment of chronic heart failure: biological and clinical considerations. Cardiovasc Res 71:443–454
Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP (2006) Elevated serum Alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepathology 43(5):1145–1151
Küçükgergin C, Fatih Aydin A, Özdemirler-Erata G, Mehmetçik G, Koçak-Toker N, Uysal M (2010) Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol Trace Elem Res 135:264–274
Ma J, Qiao Z, Xiang X (2011) Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rats models. J Med Plants Res 5:855–858
Maack C, Kartes T, Kilter H, Schäfers HJ, Nickenig G, Böhm M (2003) Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 108:1567–1574
Mahan LK, Scott-Stump (1998) Krause -Alimentos, Nutrição e Dietoterapia. ROCA, São Paulo
Matos SL, de Paula H, Pedrosa ML, dos Santos RC, de Oliveira EL, Júnior DAC, Silva ME (2005) Dietary models for inducing hypercholesterolemia in rats. Braz Arch Biol Tech 48:203–209
Mazor D, Brill G, Shorer Z, Moses S, Meyerstein N (1997) Oxidative damage in red cells of vitamin E deficient patients. Clin Chim Acta 265:131–137
McKenney JM (2001) Pharmacotherapy of dyslipidemia. Cardiovasc Drugs Ther 15:413–422
Moghadasian MH (2002) Experimental atherosclerosis: a historical overview. Life Sci 70:855–865
Mune M, Meydani M, Gong J, Fotouhi N, Ohtani H, Smith D, Blumberg JB (1999) Effect of dietary fish oil, vitamin E, and probucol on renal injury in the rat. J Nutr Biochem 10:539–546
Olorunnisola OS, Bradley G, Afolayan AJ (2012) Protective effect of T. violacea rhizome extract against hypercholesterolemia-induced oxidative stress in Wistar rats. Molecules 17:6033–6045
Prasad K, Mantha SV, Kalra J, Lee P (1997) Hypercholesterolemia-induced oxidative stress in heart and its prevention by vitamin E. Int J Angiol 6:13–17
Prasad K, McNair ED, Qureshi AM, Casper-Bell G (2012) Vitamin E slows the progression of hypercholesterolemia-induced oxidative stress in heart, liver and kidney. Mol Cell Biochem 20 June
Prasanna GS, Purnima A (2011) Protective effects of leaf extract of Trichilia connaroides on hypercholesterolemia induced oxidative stress. Int J Pharmacol 7:106–112
Rader DJ, Hobbs HH (2005) Disorders of lipoprotein metabolism. In: Harrison’s principles of internal medicine 16th Edition. Chap 335, Page 2286. McGraw–Hill. New York 2005
Rezq AA, El-Khamisy AE (2011) Hypolipideimic and hypocholestermic effect of pine nuts in rats fed high fat, cholesterol-diet. World Appl Sci J 15(12):1667–1677
Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H (2005) Gamma glutamyl transferase as a risk factor for cardiovascular mortality: an epidemiologic investigation in a Cohort of 163,944 Austrian adults. Circulation 112:2130–2137
Saikia H, Lama A (2011) Effect of Bougainvillea spectabilis leaves on serum lipids in albino rats fed with high fat diet. IJPSDR 3(2):141–145
Silva RJ, Bernardes N, Brito JO, Sanches IC, Irigoyen MC, De Angelis K (2011) Simvastatin-induced cardiac autonomic control improvement in fructose-fed female rats. Clinics 66(10):1793–1796
Simes J, Furberg CD, Braunwald E, Davis BR, Ford I, Tonkin A, Shepherd J (2002) Effects of pravastatin on mortality on patients with and without coronary heart disease across a broad range of cholesterol levels. Eur Heart 23:207–215
Suanarunsawat T, Ayutthaya WDN, Songsak T, Thirawarapan S, Poungshompoo S (2010) Antioxidant activity and lipid-lowering effect of essential oils extracted from Ocimum sanctum leaves in rats fed with a high cholesterol diet. J Clin Biochem Nutr 46:52–59
Ulas M, Cay M (2010) The effects of 17b-estradiol and vitamin E treatments on oxidative stress and antioxidant levels in brain cortex of diabetic ovariectomized rats. Acta Physiol Hung 97(2):208–215
Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M (2000) Improvement of nitric oxide dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol 20:61–69
Wilson SH, Simari RD, Best PJM et al (2001) Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol 21:122–128
Xu Y, Wei Y, Zhang Y, Gu J, Ma J, Zheng L, Hu D (2007) The characteristics of living and behaviour factors in Chinese patients metabolic syndrome. J Health Sci 53:84–91
Yakubu MT, Akanji MA, Oladiji AT (2008) Alterations in serum lipid profile of male rats by oral administration of aqueous extract of Fadogia agrestis stem. Res J Med Plant 2:66–73
Acknowledgments
The authors thank Dr. Asem Shalaby, Lecturer of Pathology, Pathology Department, College of Medicine, Mansoura University, Mansoura, Egypt for his assistance in histopathological studies.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbas, A.M., Sakr, H.F. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J Physiol Biochem 69, 737–750 (2013). https://doi.org/10.1007/s13105-013-0250-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0250-y