Abstract
Considering the well-known antioxidant properties of statins, it seems important to assess their impact on major markers of oxidative stress (superoxide anion radical, nitric oxide, and index of lipid peroxidation) to compare the antioxidative potentials of atorvastatin and simvastatin during the different degrees of hyperhomocysteinemia (HHcy) in rats. This study was conducted on adult male Wistar albino rats (n = 90; 4 weeks old; 100 ± 15 g body mass) in which HHcy was achieved by dietary manipulation. For 4 weeks, the animals were fed with one of the following diets: standard rodent chow, diet enriched in methionine with no deficiency in B vitamins (folic acid, B6, and B12), or diet enriched in methionine and deficient in B vitamins (folic acid, B6, and B12). At the same time, animals were treated with atorvastatin at doses of 3 mg/kg/day i.p. or simvastatin at doses of 5 mg/kg/day i.p. Levels of superoxide anion radical and TBARS were significantly decreased by administration of simvastatin in normal and high-homocysteine (Hcy) groups (p < 0.05). At 4 weeks after feeding with purified diets, the concentrations of the GSH, CAT, and SOD antioxidants were significantly affected among all groups (p < 0.05). Our results indicated that statin therapy had variable effects on the redox status in hyperhomocysteinemic rats, and simvastatin demonstrated stronger antioxidant effects than did atorvastatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathophysiological mechanism of cardiovascular disease includes both functional and structural impairment [1, 2]. Endothelial dysfunction, coordination disorder of the relaxation and contraction of corporal smooth muscles via the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway and RhoA/Rho-kinase (ROCK) pathway, autonomic neuropathy, and apoptosis in corporal smooth muscles simultaneously occurred. Therefore, to maintain the endothelial function, it is important to reduce risk factors, such as oxidative stress, HHcy, and dyslipidaemia [3, 4].
Excess prevalence of HHcy, an independent risk factor of cardiovascular diseases (CVD), has been observed in patients with dyslipidaemia [5].
HHcy may promote atherosclerotic plaque development and instability [6]. Hcy dose-dependently induced the apoptosis of the cells via increasing NADPH (nicotinamide adenine dinucleotide phosphate), oxidase (Nox) 1 and Nox2, which are oxidative stress inducing enzymes, suggesting that cell apoptosis by oxidative stress may be one of the underlying mechanisms of Hcy-induced vascular fragility [7,8,9].
Conversely, statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which acts as a rate-limiting enzyme for endogenous cholesterol synthesis and is considered a key enzyme in the mevalonate pathway. Statins are widely used to prevent cardiovascular events [10]. Statins improve the prognosis in patients with coronary heart diseases by decreasing the incidence of vascular events and ameliorating oxidative stress by regulating oxidant and antioxidant enzymes. Many studies have reported the cholesterol-lowering effects of statins, which exert pleiotropic effects, including cardioprotection [11, 12]. Clinical studies have also shown that statins can reduce the risk of heart attack, stroke, and death in patients with heart disease, such as coronary artery disease and cardiovascular disease [13, 14]. The efficacy of statins is related to the ‘common soil’ hypothesis, which proposes oxidative stress and inflammation as the primary pathophysiological processes in the disease group of diabetes and endothelial dysfunction [15].
Nevertheless, other studies have reported conflicting results, which indicated that statin therapy does not decrease the incidence of cardiovascular events, and it could be a risk for CVD, especially in the presence of one risk factor. One of the potential factors contributing to this discrepancy is the variability in the pleiotropic effects of different statins. Chemical properties vary among different statins, influencing the solubility and drug distribution, and the bioavailability and pleiotropic effects [16].
Therefore, we hypothesized that different statins might different prevent Hcy-induced oxidative stress and apoptosis. To date, however, there are few comparative studies investigating the effects of atorvastatin and simvastatin on the redox status in the presence of intermediate or severe HHcy [17].
Moreover, considering the well-known antioxidant properties of statins, it seems important to assess their impact on major markers of oxidative stress (superoxide anion radical, nitric oxide, and index of lipid peroxidation) to compare the antioxidative potentials of atorvastatin and simvastatin based on the degree of HHcy in rats.
Materials and methods
Ethical approval
All research procedures were performed in accordance with European Directive for welfare of laboratory animals No: 2010/63/EU and principles of good laboratory practice (GLP). The protocol of the current study was approved by the ethics committee for experimental animal well-being of the Faculty of Medical Sciences of the University of Kragujevac, Serbia (No: 01-11794). The investigators understand the ethical principles under which the journal operates, and that their work complies with the animal ethics checklist.
Animals and dietary manipulation
The animals were acclimatized for two weeks in the animal house of Faculty of Medical Sciences in Kragujevac before dietary manipulation. Two rats were housed per wire floored cage in an air-conditioned room (22 ± 2 °C) with a 12-h light/dark cycle and had free access to standard or special laboratory chow diet and water ad libitum.
The study was conducted on adult male Wistar albino rats (n = 90; 4 weeks old; 100 ± 15 g body mass) in which HHcy was achieved by dietary manipulation [18].
Age-matched littermates were selected for each experimental group. For 4 weeks, the animals were fed one of the following diets (Mucedola SRL., Milan, Italy): standard rodent chow, diet enriched in methionine with no deficiency in B vitamins (folic acid, B6 and B12), or diet enriched in methionine and deficient in B vitamins (folic acid, B6 and B12) (Table 1). At the same time, animals were exposed to pharmacology treatment with atorvastatin at a dose of 3 mg/kg/day i.p or simvastatin at a dose of 5 mg/kg/day i.p at the same time every day, according to equivalent therapeutic doses of these statins (10 mg atorvastatin = 20 mg simvastatin). Animals were divided into the following nine [9] different groups as follows:
-
(1)
Animals fed a standard rodent chow without statins (S);
-
(2)
Animals fed a diet enriched in methionine with no deficient in B vitamins (folic acid, B6, and B12) without statins (M);
-
(3)
Animals fed with diet enriched in methionine and deficient in B vitamins (folic acid, B6, and B12) without statins (MD);
-
(4)
Animals fed a standard rodent chow with atorvastatin at a dose of 3 mg/kg/day i.p. (S + Atorva);
-
(5)
Animals fed a standard rodent chow with administration of simvastatin at a dose of 5 mg/kg/day i.p. (S + Simva);
-
(6)
Animals fed a diet enriched in methionine with no deficiency in B vitamins (folic acid, B6, and B12) with atorvastatin at a dose of 3 mg/kg/day i.p. (M + Atorva);
-
(7)
Animals fed a diet enriched in methionine with no deficient in B vitamins (folic acid, B6, and B12) with administration of simvastatin at a dose of 5 mg/kg/day i.p. (M + Simva);
-
(8)
Animals fed a diet enriched in methionine and deficient in B vitamins (folic acid, B6, and B12) with administration of atorvastatin at a dose of 3 mg/kg/day i.p. (MD + Atorva);
-
(9)
Animals fed a diet enriched in methionine and deficient in B vitamins (folic acid, B6, and B12) with administration of simvastatin at a dose of 5 mg/kg/day i.p. (MD + Simva).
Biochemical assay in blood
After week-dietary manipulation, animals were sacrificed, and rat blood samples were collected by exsanguination. In serum samples, we determined the concentration of Hcy and lipids, such as the total cholesterol (tChol), high-density lipoprotein (HDL) and triglycerides (Try). In plasma samples, we measured the concentration of pro-oxidative markers, such as superoxide anion radical (O2 −), nitric oxide (NO−), and index of lipid peroxidation measured as TBARS (TBARS). In lysate, we determined the activity of nonenzymatic antioxidant, such as reduced glutathione (GSH), and activity of the enzymatic defence system, by evaluating of catalase (CAT) and superoxide dismutase (SOD) concentrations.
Determination of homocysteine (Hcy)
Total serum Hcy concentrations were measured with a high-performance liquid chromatography (HPLC) procedure with reverse phase separation and fluorescence detection, as previously described [19]. The total serum concentrations were determined using commercial kits from SIEMENS, at automatic analyser CENTAUR XP SIEMENS, with a sensitivity assay range <0.5–65 μmol/L.
Determination of lipid profile
Prior to blood collection to measure plasma parameters, animals were fasted for 12 h to minimize the interference of food intake in the lipid profile results. Blood was collected during sacrifice of animals into micro-tubes. Total cholesterol (tChol), high-density lipoprotein (HDL), and triglycerides (Try) were determined using commercial kits from SIEMENS Diagnostica, an automatic analyser Dimension R × L Max.
Determination of superoxide anion radical (O2 −)
The concentration of the superoxide anion radical (O2 −) was measured after the reaction of nitro blue tetrazolium in TRIS buffer with plasma at 530 nm. Distilled water solution served as a blank [20].
An indirect method for monitoring nitric oxide (NO) by determining nitrate (NO3 −) and nitrite (NO2 −)
The nitrate (NO3 −) and nitrite (NO2 −) levels are measured and NO levels calculated as previously described [21]. A total of 0.5 mL of plasma was precipitated with 200 μl of 30% sulfosalicylic acid, vortexed for 30 min, and centrifuged at 3000×g. Equal volumes of the supernatant and Griess reagent, containing 1% sulphanilamide in 5% phosphoric acid/0.1% naphthalene ethylenediamine dihydrochloride, were added and incubated for 10 min in the dark and measured at 543 nm [21].
TBARS determination (index of lipid peroxidation)
The degree of lipid peroxidation in the plasma was estimated by measuring the TBARS using 1% thiobarbituric acid in 0.05 NaOH, incubated with plasma at 100 °C for 15 min, and measured at 530 nm. Distilled water served as a blank [22].
Determination of reduced glutathione (GSH)
The level of reduced glutathione (GSH) was determined based on GSH oxidation with 5.5- dithio-bis-6.2-nitrobenzoic acid using the method reported by Beutler [23].
Determination of catalase (CAT)
CAT activity was determined according to Aebi. Lysates were diluted with distilled water (1:7 v/v) and treated with chloroform-ethanol (0.6:1 v/v) to remove haemoglobin; then 50 μl of CAT buffer, 100 μl of ample, and 1 mL of 10 mM H2O2 were added to the samples. Detection was performed at 360 nm [24].
Determination of superoxide dismutase (SOD)
SOD activity was determined by the epinephrine method of Beutler. One hundred μl lysate and 1 mL of carbonate buffer were mixed; then 100 μl of epinephrine was added. Detection was performed at 470 nm [25].
Reagents and diets
All reagents and substances were purchased from Sigma-Aldrich (Sigma–Aldrich Chemie GmbH, Germany): Atorvastatin calcium salt trihydrate ≥98% (C33H34FN2O5 × 0.5 Ca × 1.5·H2O) MW: 604.69, (product number: PZ0001-25MG) and Simvastatin ≥97% (C25H38O5), MW: 418.35 (product number: S6196-25MG). All diets for animals were purchased from Mucedola Corporation (Milan, Italy).
Statistical analysis
Data are presented as the mean ± SD. The effects of dietary methionine and folate intake on oxidative parameters and antioxidant status between groups were analysed by one-way ANOVA (Scheffé’s F test) and the nonparametric analogue test of ANOVA (Kruskall–Wallis test). Differences were considered to be significant at p < 0.05.
Results
Homocysteine concentration
In MD and M groups, the average serum concentrations of Hcy were 63.89 ± 0.1 and 22.43 ± 0.3 μmol/L, while it was 8.11 ± 0.1 μmol/L in the control group. In the S + Atorva and S + Simva groups, the average serum concentrations of Hcy were less than 15 μmol/L; in the M + Atorva and M + Simva groups, they were in range from 15 to 30 μmol/L, and in the MD + Atorva and MD + Simva groups, they were above 30 μmol/L. According to these results, all groups were divided into normal (S, S + Atorva, and S + Simva), high (M, M + Atorva, and M + Simva), and very high (MD, MD + Atorva, and MD + Simva) homocysteine groups (Table 2).
Lipid profile
The values of tChol, HDL, and Try were significantly decreased after the SIM administration in the Meth-purified groups (p < 0.05). The values of tChol and Try were significantly decreased after the ATO administration in the Meth-B deficient groups, while the levels of HDL were not. Interestingly, blood concentrations of Chol, HDL, and Try were not significantly affected by atorvastatin or simvastatin in the groups fed a standard diet (p > 0.05) (Table 3).
Concentration of pro-oxidant markers
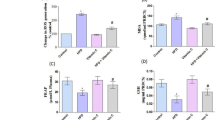
The levels of superoxide anion radical were significantly decreased by the administration of simvastatin in the normal and high-homocysteine groups (p < 0.05); however, in group with Hcy levels above 30 μmol/L, the level of O2 − was not significantly changed (p > 0.05) (Fig. 1). Simvastatin significantly decreased the level of O2 − in the M groups compared to atorvastatin, while the atorvastatin decreased the level of this pro-oxidative marker in MD groups more than simvastatin without reaching statistical significance. Compared to the control groups, SIM and atorvastatin significantly decreased O2 − in the hyperhomocisteinemic groups (Fig. 1).
The level of NO (nitrites+nitrates) was not significantly changed in the groups with hyperhomocysteinemia compared to control groups (p < 0.05) (Fig. 2). Compared to control groups (S, M, and MD groups), both statins had similar effects on the NO levels after 30 days of administration (Fig. 2).
The index of lipid peroxidation was significantly altered (Fig. 3). Compared to the control groups without drug administration, atorvastatin increased the levels of TBARS in the S and M groups, while the levels of this marker were decreased in the MD groups. Conversely, simvastatin administration decreased the levels of TBARS in the M+SIM group compared to all other groups and compared to control conditions (Fig. 3). Simvastatin, compared to atorvastatin, significantly decreased the levels of TBARS in the M group of animals fed with meth-purified food.
Concentration of antioxidant markers
At 4 weeks after feeding with purified diets, the blood concentrations of the GSH antioxidant in blood were significantly affected among all groups (Fig. 4). Interestingly, at a higher concentration of Hcy in blood, simvastatin induced increasing levels of GSH; in normal conditions (Hcy < 15 μmol/L), simvastatin reduced the levels of GSH compared to the Atorva-groups. Additionally, compared to groups without drug administration, simvastatin and atorvastatin alone affected this parameters in the same manner, simvastatin had higher potency (Fig. 4).
Furthermore, simvastatin administration significantly increased the CAT values among all groups that were given this statin compared to atorvastatin (p < 0.05) (Fig. 5). Separately, simvastatin induced increased CAT levels compared to control groups without drug administration, as well as atorvastatin. Additionally, by comparing their separate effects, simvastatin exerts stronger antioxidant activity (Fig. 5).
Finally, atorvastatin and simvastatin had similar actions on the levels of superoxide dismutase in groups with high-homocysteine concentrations. However, in extreme conditions, such as very high or very low Hcy concentrations, simvastatin significantly increased the SOD levels compared to atorvastatin (p < 0.05) (Fig. 6). Compared to groups without drug treatment, simvastatin alone induced increased levels of SOD, but there was no difference in relation to the Hcy levels. However, compared to groups without drug treatment, atorvastatin alone was increased during the normal and very high Hcy levels, while it was decreased compared to simvastatin for the normal and very high Hcy groups (Fig. 6).
Discussion
Several clinical and epidemiological studies have shown that HHcy was independently associated with vascular disorders, and it has recently been considered a co-morbid risk factor for cardiovascular disease [26,27,28,29,30]. Nevertheless, the oxidant potential of HHcy in the heart is still unclear.
In the present study, we used a rat model of diet-induced HHcy with the high intake of methionine to mimic, as much as possible, the clinical condition of HHcy. This type of experimental design updated the current knowledge combining elevated Hcy levels with and without deficiency vitamin B12, B6 and folic acid. At the termination of treatment, we diagnosed mild and moderate HHcy by biochemical and pathological evaluation, confirming the reliability of the model.
Prior studies have reported that elevated serum levels of Hcy have various mechanisms of influence on the cardiovascular system, such as a role in endothelial dysfunction, proliferation, inflammation, and oxidative processes [31, 32].
Hcy can be viewed as a first risk factor for atherosclerosis that is thought to exert its effects through a mechanism involving oxidative damage [39], inducing endothelial dysfunction and leading to atherosclerosis and other cardiovascular diseases. Clinical in vivo studies have confirmed the occurrence of endothelial dysfunction in HHcy [17]. The basis of the Hcy-induced oxidative stress hypothesis relies on the chemical auto-oxidation of Hcy. In our study, it can be clearly observed that HHcy can be associated with increased release of O2 − and TBARS (Figs. 1, 3). Well, our results show that both control groups with different levels of HHcy had higher values O2 − and TBARS than control group fed with standard diet (Figs. 1, 3).
Therefore, understanding the mechanisms by which HHcy affects the signalling pathways linked with oxidative damage could form the basis for developing new treatment strategies. It is known that statins prolong the lifespan of lower organisms [33] and reduce the total human mortality, even in people who have normal lipid levels, and statins are the first-line drugs in the primary and secondary prevention of cardiovascular diseases [30, 31].
Due to pleiotropic effects of statins, which are not associated with lipid-lowering effects, we examined the effects of widely used statins, atorvastatin, and simvastatin, on the redox status of rats with normal and high levels of Hcy and normal levels of lipoproteins in the blood.
First, we tested the well-known observation that the statins are a class of drugs used for decades to treat hypercholesterolaemia. During the experimental period, groups of animals that fed a methionine-purified diet with high levels of B vitamins had significantly higher growth and body mass indexes compared to the other groups. In agreement, the blood of these animals had higher levels of tChol and Try (Table 3), and atorvastatin and simvastatin had similar effects on the lipid profiles in the same state of Hhcy. Our results agree with previous results [40, 41], which suggested that equivalent doses of atorvastatin and simvastatin have equivalent lipid-lowering effects.
In the second part of our study, we evaluated the effects of atorva/simvastatin on pro-oxidant markers in intermediate and severe HHcy compared to physiological conditions.
Simvastatin exerts more potential effects in reducing the O2− level at lower Hcy levels compared to atorvastatin (Fig. 1). Hcy is readily oxidized when added to plasma, principally because of auto-oxidation, leading to the formation of Hcy, homocysteine-mixed disulphides, and homocysteine thiolactone. During oxidation of the sulfhydryl group, superoxide anion radical (O2 −) and hydrogen peroxide (H2O2) are generated, and these oxygen-derived molecules are believed to account for the endothelial cytotoxicity of homocyst(e)ine. In the same conditions of oxidation Hcy in plasma, simvastatin strongly decreased the level of superoxide anion radical. Additionally, TBARS were changed in the same manner by simvastatin (Fig. 3). By contrast, nitric oxide (measured in form of nitrites), as a potent vasodilator, was not altered by long-term simvastatin or atorvastatin at different Hcy levels (Fig. 2). Compared with control groups without drug administration, we can confirm the dominant effects of simvastatin (Figs. 1, 2, 3). Our findings indicate that statins exhibit differential effects in preventing oxidative stress when administered at equivalent lipid-lowering doses.
Crespo et al. compared the effects of daily administration over a four-week period at low doses (10 mg/kg per day) of atorvastatin (AV), simvastatin (SV), and pravastatin (PV) on cardiac performance and oxidative stress in diabetic rats. These researchers determined that statins decrease the malondialdehyde (MDA) content without affecting the eNOS and iNOS protein levels [34], which correlates with our results. A clinical study also suggested that atorvastatin had slight effects on inflammation and oxidative stress, while rosuvastatin had the best anti-inflammatory effect. Simvastatin again achieved the best antioxidant effects [35]. Previous reports noted that all three statins decreased the media thickness, perivascular fibrosis, and both MDA and 4-HAE in the aortas of diabetic rats and observed that the haemodynamic benefits were cholesterol-independent. These benefits appear to be secondary to the improved endothelial function and to the reduced vascular tone and remodelling that result from decreased oxidative stress. However, both literature and our data indicate no change in the nitrite dynamics after statin treatment.
In every previous study and in ours, nitrite was not altered with any statin treatment compared to the control conditions [13,14,15,16]. This result can be a positive, considering that a decrease in the NO bioavailability is related to impaired endothelial function. Also, pro-oxidant effects of HHcy are less evident under drug treatment (Figs. 1-3). It seems that statins failed to diminish pro-oxidant effects of hyperhomocysteinemia via still unknown mechanisms.
Although anti-inflammatory and antioxidant properties of statins are well-known, the antioxidant properties of atorvastatin and simvastatin during HHcy have not been thoroughly elucidated. To assess one potential mechanism through which statins interfere with ROS during HHcy, we measured the activity of antioxidant enzyme system. After 4-wk feeding rats with purified diets, concentrations of the antioxidant GSH in blood were significantly affected among all groups. In the Hcy-control groups without drug administration, all antioxidants were significantly reduced before statin treatments, and there was stronger antioxidant activity with simvastatin (Figs. 4, 5, 6). Interestingly, at a higher concentration of Hcy in blood, simvastatin induced increasing levels of GSH, but in normal conditions (Hcy < 15 μmol/L), simvastatin reduced the levels of GSH compared to the Atorva-groups (Fig. 4). CAT values were less increased. Finally, atorvastatin and simvastatin had similar effects on the levels of superoxide dismutase in groups with high-homocysteine concentrations; however, in extreme conditions, such as very high or very low Hcy concentrations, simvastatin significantly increased the levels of SOD compared to atorvastatin (Figs. 5, 6). Simvastatin generally exerts more antioxidant properties than atorvastatin, especially with severe HHcy, and only the groups treated with atorvastatin exhibited decreased GSH and CAT activity compared to the simvastatin groups.
Few clinical studies suggest that atorvastatin and pravastatin, but not simvastatin, exhibit anti-inflammatory and antioxidant activity in different diseases, i.e., endotoxin-induced acute lung injury [36] and acute nephropathy [33], but the duration of pretreatment and comorbidity can also affect each drug’s antioxidant properties.
However, our data are not consistent with the results of Wang et al., who observed that atorvastatin and rosuvastatin had similar effectiveness against contrast media-induced oxidative stress, while simvastatin was less effective. These researchers showed that atorvastatin was the most effective against NO system dysfunction and cell apoptosis, while rosuvastatin was most effective against inflammation [37]. Li and co-workers concluded that atorvastatin reduces oxidative stress more effectively than simvastatin [38]. The diversity of experimental models could be a reason for the inconsistent results because these authors examined the effects of widely prescribed statins in the absence of a strong generator of ROS, HHcy. HHcy is likely an important interfering co-factor in lowering pro-oxidants with any potential antioxidants, as a producer of reactive oxygen species.
One of the potential explanations for the more potent impact of simvastatin on reducing of oxidative stress by elevating of GSH and CAT activity compared to atorvastatin is the difference in their pharmacological properties, which may result in different antioxidant activities [27,28,29,30,31,32]. During metabolism, simvastatin is given as lactone prodrug and converted to the active beta-hydroxy form, simvastatin hydroxy acid (SVA), which is the most potent competitive inhibitor of HMG-CoA reductase. Conversely, atorvastatin metabolism primarily occurs through cytochrome P4503A4 hydroxylation to form active ortho- and parahydroxylated metabolites, as well as various beta-oxidation metabolites which are responsible for 70% of systemic HMG-CoA reductase activity [39].
Furthermore, it is speculated that the strong antioxidant effects of simvastatin are related to its ability to inhibit the isoprenoid compounds produced by the mevalonate pathway and their ability to inhibit the activation of nicotinamide adenine dinucleotide phosphate [30]. Additionally, reduction in leukocytes by simvastatin could directly reduce redox markers. Other antioxidant mechanisms of atorvastatin and simvastatin are inhibition of platelet Nox275, reducing the circulating concentration of the catalytic subunit of Nox247; increasing adiponectin, which inhibits Nox activation; increasing catalase and SOD by phosphorylation of Akt41 and by S-nitrosylation27; and increasing of the activity of PON1, which is responsible for the antioxidant properties of HDL cholesterol [26,27,28,29,30,31,32,33,34,35,36,37].
These various mechanisms of action in statins can be different because of differences between pharmacokinetic characteristics of atorvastatin and simvastatin, such as hydrophobicity, bioavailability, metabolism in the liver, and half-life associated with the dose and duration of drug use. These features should be considered.
Conclusions
To the best of our knowledge, few studies have compared the effects of atorvastatin and simvastatin on markers of oxidative stress in rats with Hhcy. Our results have indicated that statin therapy resulted in variable effects on the redox status in hyperhomocysteinemic rats, and simvastatin demonstrated stronger antioxidant effects than atorvastatin, independent of its effects on the lipid profile and dependent on the homocysteine concentration. Sufficiently powered prospective clinical and intervention studies are needed to investigate whether simvastatin lowers oxidative stress more effectively than other statins.
Abbreviations
- Hcy:
-
Homocysteine
- NOS:
-
Nitric oxide synthase
- –SH:
-
Sulfhydryl group
- CVD:
-
Cerebrovascular disease
- ROS:
-
Reactive oxygen species
- ONOO–:
-
Peroxynitrite
- NO:
-
Nitric oxide
- GSH:
-
Reduced gluthatione
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
- NO2 :
-
Nitrites
- O2 :
-
Superoxide anion radical
- EDTA:
-
Ethylenediaminetetraacetic acid
- HRPO:
-
Horseradish peroxidase
- TRIS:
-
2-Amino-2-hydroxymethyl- propane-1,3-diol buffer
References
AlJaroudi WA, Hage FG (2017) Cardiovascular disease in the literature: a selection of recent original research papers. J Nucl Cardiol. doi:10.1007/s12350-017-0854-7
Fujisue K, Tsujita K (2017) Current status of lipid management in acute coronary syndrome. J Cardiol 914:30036–30039. doi:10.1016/j.jjcc.2017.02.004
Katsiki N, Doumas M, Mikhailidis DP (2016) Lipids, statins and heart failure: an update. Curr Pharm Des 22:4796–4806
Costa S, Reina-Couto M, Albino-Teixeira A, Sousa T (2016) Statins and oxidative stress in chronic heart failure. Rev Port Cardiol 35:41–57
Adameova A, Xu YJ, Duhamel TA, Tappia PS, Shan L, Dhalla NS (2009) Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des 15:3094–3107
Chaturvedi P, Kamat PK, Kalani A, Familtseva A, Tyagi SC (2016) High methionine diet poses cardiac threat: a molecular insight. J Cell Physiol 231:1554–1561
Bełtowski J, Jamroz-Wiśniewska A (2016) Hydrogen sulfide in the adipose tissue-physiology, pathology and a target for pharmacotherapy. Molecules. doi:10.3390/molecules22010063
Park J, Kwon OS, Cho SY, Paick JS, Kim SW (2017) Chronic administration of atorvastatin could partially ameliorate erectile function in streptozotocin-induced diabetic rats. PLoS ONE 12:e0172751. doi:10.1371/journal.pone.0172751
Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J. doi:10.1186/1475-2891-14-6
Stewart J, Manmathan G, Wilkinson P (2017) Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis. doi:10.1177/2048004016687211
Banfi C, Baetta R, Gianazza E, Tremoli E (2017) Technological advances and proteomia applications in drug discovery and target deconvolution: identification of the pleiotropic effects of statins. Drug Discov Today. 22(6):848–869. doi:10.1016/j.drudis.2017.03.001
Sandhu K, Mamas M, Butler R (2017) Endothelial progenitor cells: exploring the pleiotropic effects of statins. World J Cardiol 9(1):1–13. doi:10.4330/wjc.v9.i1.1
Baigent C, Blackwell L, Emberson J et al (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. The Lancet 376:1670–1681
Smith SC, Allen J, Blair SN et al (2006) AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 47:2130–2139
Takeno A, Kanazawa I, Tanaka K, Notsu M, Yokomoto-Umakoshi M, Sugimoto T (2016) Simvastatin rescues homocysteine-induced apoptosis of osteocytic MLO-Y4 cells by decreasing the expressions of NADPH oxidase 1 and 2. Endocr J 63(4):389–395. doi:10.1507/endocrj.EJ15-0480
Bhandari U, Pathan RA, Kumar V, Khanna N (2011) Ameliorative role of atorvastatin on methionine-induced hyperhomocysteinemia and hematological changes in albino rats. Indian J Exp Biol 49(2):132–139
Rohilla A, Ahmad A, Khan MU, Khanam R (2015) A comparative study on the cardioprotective potential of atorvastatin and simvastatin in hyperhomocysteinemic rat hearts. Eur J Pharmacol 764:48–54
Zhang R, Ma J, Xia M, Zhu H, Ling WH (2004) Mild hyperhomocysteinemia induced by feeding rats diets rich in methionine or deficient in folate promotes early atherosclerotic inflamammatory processes. J Nutr 134(4):825–830
Ulbink JB, Vermak WJH, Bissbort S (1991) Rapid high-performance liquid chromatographic assay for total homocysteine levels in humans serum. J Chromatogr B 565:441–446
Auclair C, Voisin E (1985) Nitroblue tetrazolium reduction. In: Greenvvald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 123–132
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood. Glutathione. J Lab Clin Med. 61:882–888
Aebi H (1984) Catalase in vitro. Methods Enzymol. 105:121–126
Beutler E (1984) Superoxide dismutase. In: Beutler E (ed) Red cell metabolism a manual of biochemical methods. Grune & Stratton, Philadelphia, pp 83–85
Nigwekar SU, Kang A, Zoungas S et al (2016) Interventions for lowering plasma homocysteine levels in dialysis patients. Cochrane Database Syst Rev 5:468–473
Baggott JE, Tamura T (2015) Homocysteine, iron and cardiovascular disease: a hypothesis. Nutrients. 7:1108–1118
Kang SS, Wong PW, Malinow MR (1992) Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr 12:279–298
McCully KS (2015) Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol. 8:211–219
Zhou J, Austin RC (2009) Contributions of hyperhomocysteinemia to atherosclerosis: causal relationship and potential mechanisms. BioFactors 35:120–129
Faeh D, Chiolero A, Paccaud F (2006) Homocysteine as a risk factor for cardiovascular disease: should we (still) worry about it? Swiss Med Wkly 136:745–756
Loscalzo J (1996) The oxidant stress of hyperhomocyst(e)inemia. J Clin Investig 98:5–7
Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R et al (2012) Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS ONE 7:e39581. doi:10.1371/journal.pone.0039581
Crespo MJ, Quidgley J (2015) Simvastatin, atorvastatin, and pravastatin equally improve the hemodynamic status of diabetic rats. World J Diabetes 6:1168–1178
Ferreira TS, Lanzetti M, Barroso MV et al (2014) Oxidative stress and inflammation are differentially affected by atorvastatin, pravastatin, rosuvastatin, and simvastatin on lungs from mice exposed to cigarette smoke. Inflammation 37:1355–1365
Melo AC, Valença SS, Gitirana LB et al (2013) Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int Immunopharmacol 17(1):57–64
Wang XL, Zhang T, Hu LH, Sun SQ, Zhang WF, Sun Z, Shen LH, He B (2017) Comparison of effects of different statins on contrast-induced acute kidney injury in rats: histopathological and biochemical findings. Oxid Med Cell Longev. doi:10.1155/2017/6282486
Li J, Sun YM, Wang LF, Li ZQ, Pan W, Cao HY (2010) Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clin Cardiol 33:222–227
Whirl-Carrillo M, McDonagh EM, Hebert JM et al (2012) Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92(4):414–417
Variya BC, Patel SS, Trivedi JI et al (2015) Comparative evaluation of HMG CoA reductase inhibitors in experimentally-induced myocardial necrosis: biochemical, morphological and histological studies. Eur J Pharmacol 764:283–291
Tunceli K, Sajjan SG, Ramey DR et al (2010) Switching from high-efficacy lipid-lowering therapies to simvastatin and low-density lipoprotein cholesterol goal attainment in coronary heart disease/coronary heart disease-equivalent patients J Clin Lipidol 4(6):491–500
Funding
This work was supported by the Ministry of Science and Technical Development of the Republic of Serbia (Grant No. 175043) and the Faculty of Medical Sciences, University of Kragujevac (Junior Project No. 09/2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial, or otherwise, are declared by the authors.
Rights and permissions
About this article
Cite this article
Nikolic, T., Zivkovic, V., Srejovic, I. et al. Effects of atorvastatin and simvastatin on oxidative stress in diet-induced hyperhomocysteinemia in Wistar albino rats: a comparative study. Mol Cell Biochem 437, 109–118 (2018). https://doi.org/10.1007/s11010-017-3099-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3099-5