Abstract
Ninjurin-1 is a novel adhesion molecule which is involved in many inflammatory diseases. Functional blockage of Ninjurin-1 has exerted an atheroprotective effect. The aim of the study is to explore the association between serum Ninjurin-1 and the risk of large artery atherosclerotic acute ischemic stroke. From August 2020 through December 2021, patients with large artery atherosclerotic acute ischemic stroke (LAA-AIS) admitted to the First Hospital Affiliated to Soochow University, and age- and sex-matched controls free of ischemic stroke were recruited. Serum Ninj1 was measured with an enzyme-linked immunosorbent assay. Multivariable logistic regression models were used to calculate the odds ratios and 95% confidence intervals of LAA-AIS associated with serum Ninj1 levels, and receiver operating characteristic (ROC) curves were performed to assess the improvement value of Ninj1 for the prediction of LAA-AIS after adding Ninj1 to established risk factors. Of the 110 patients and 110 age- and sex-matched controls free of ischemic stroke enrolled, serum Ninj1 levels in LAA-AIS patients were significantly higher than that in control group (142.70 ng/ml [IQR: 110.41–163.44] vs 101.62 ng/ml [IQR: 86.63–120.86], p < 0.001). In multivariable analysis, Ninj1 levels were expressed as continuous variable and ordinal variable (tertiles), and it turned out that Ninj1 levels were positively associated with increased risk of LAA-AIS, especially in tertile3 compared with tertile1 (adjusted OR = 12.567, 95%CI: 5.148–30.678, p < 0.001), and the adjusted odds OR per 10 ng/ml increment was 1.541, 95%CI: 1.348–1.763, p < 0.001. Furthermore, adding Ninj1 to a multivariate logistic model including conventional risk factors associated LAA-AIS improved the area under ROC curves from 0.787 to 0.874. Elevated circulating levels of Ninj1 were associated with increased risk of LAA-AIS, indicating that serum Ninj1 may act as a predictor independent of established conventional risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke (AIS) remains a major cause of disability and mortality worldwide, atherosclerosis is a chronic inflammatory disease, and represents the primary cause of atherosclerotic cardiovascular disease (ASCVD) including AIS [1, 2]. For the past few years, clinical trials indicated that anti-inflammatory therapy is emerging as an effective option beside anti-platelet and lipid-lowering treatment for ASCVD [3]. Ninjurin-1 (nerve injury-induced protein 1, Ninj1) is a novel molecule with two-pass transmembrane proteins, initially identified in axons and Schwann cells following nerve injury [4]; subsequent studies found that Ninj1 was involved in a variety of inflammatory diseases, such as rheumatoid arthritis, asthma, inflammatory bowel disease, multiple sclerosis, pulmonary fibrosis, malignant tumors, and severe infection [5,6,7,8,9]. Also, Ninj1 is reported to be upregulated in inflammatory cells, particularly in macrophages, endothelial cells, and neutrophils that play key roles in the development of atherosclerosis [10]. Recently, it has re-attracted considerable attention because of its involvement in membrane rupture which is essential for stimulating pro-inflammatory responses and accelerating inflammatory diseases like atherosclerosis [11, 12]. In addition, a recent study by Jeon et al. revealed that Ninj1 was abundantly expressed in atherosclerotic aortas compared with normal vessels, and its soluble form in serum was significantly higher in patients with coronary artery disease (CAD) patients in comparison with healthy ones [13]. However, the relevance of Ninj1 in large artery atherosclerotic acute ischemic stroke (LAA-AIS) remains to be elucidated. Thus, we conducted the current study to investigate the association between serum Ninjurin-1 and the risk of LAA-AIS.
Methods
Study Population
The present study was conducted in the Stroke Center of the First Hospital Affiliated to Soochow University during the period August 2020 to December 2021. Patients with LAA-AIS were enrolled within 48 h after symptom onset. AIS was diagnosed by the World Health Organization criteria and confirmed by magnetic resonance imaging or computed Tomography scans. Stroke subtype was determined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria1. Age- and sex-matched controls free of ischemic stroke were from the Chinese Stroke Screening and Prevention Project (CNSSPP), which is an ongoing community-based, long-term follow-up study, and details on its design and rationale have been described previously [14]. Briefly, adults of 40 years or above were recruited in this study, with at least one of the following risk factors: hypertension, diabetes mellitus, hyperlipidemia, or smoking, accompanied with carotid intima thickness < 1.5 mm, and no stenosis was observed by carotid ultrasound. Subjects with known conditions to affect Ninjurin-1 levels were excluded, including rheumatoid arthritis, asthma, inflammatory bowel disease, multiple sclerosis, pulmonary fibrosis, malignant tumors, or severe infection. The protocol of the study conforms to the ethical guidelines mentioned in the Declaration of Helsinki and was approved by the Ethics Committee of the First Hospital Affiliated to Soochow University (no. 2021–07-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Baseline Data Collection
Baseline data on demographic characteristics, risk factors, and medical history were collected at admission, including age, sex, body mass index (BMI), medical history of hypertension, diabetes and smoking, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), homocysteine (HCY), and glycated hemoglobin (HbA1C).
Ninjurin-1 Measurement
Blood specimens were collected on the second day morning after 8 h of fasting, stored immediately at 4 °C after blood withdrawal. Serum samples were separated after 4 °C overnight and frozen at − 80 until laboratory testing. Serum Ninjurin-1 levels were assessed using an enzyme-linked immunosorbent assay kit (Jiangsu enzyme label Biotechnology Co. Jiangsu, China) according to the manufacturer’s instructions.
Statistical Analysis
Continuous variables are expressed as means (standard deviation [SD]) or medians (interquartile range [IQR]), and categorical variables as percentages. Normality of distributions was assessed graphically and with the Kolmogorov–Smirnov test. T-test or Mann–Whitney test was used for comparisons of continuous variables and chi-square test for categorical variables. The association between Ninj1 and LAA-AIS risk was measured by calculating crude odds ratios (ORs) and 95% CI using logistic regression models. In multiple logistic analyses, adjustment variables included age, sex, hypertension, diabetes, systolic blood pressure, fasting plasma glucose, total cholesterol, HDL-C, LDL-C, homocysteine, and glycated hemoglobin. Besides, we assessed the predictive value of Ninj1 adding to the conventional risk models by computing the area under receiver operating characteristic curves (AUC). Two-tailed p < 0.05 was considered to be statistically significant. The above statistical analyses were conducted using SPSS software (IBM SPSS Statistics for Mac, version 26.0; IBM Corp, Armonk, NY, USA).
Results
In brief, a total of 156 consecutive acute ischemic stroke (TOAST classification of large artery atherosclerosis) patients within 48 h of symptom onset were recruited in this study from August 2020 to December 2021. According to the exclusion criteria, 28 patients were excluded: 5 with malignant tumors, 12 with severe infection, 11 with rheumatoid arthritis, asthma, multiple sclerosis or pulmonary fibrosis; after further exclusion of 18 patients for lack of clinical or laboratory data, 110 patients and 110 age- and sex-matched controls were enrolled in the study (shown in Fig. 1).
Baseline Characteristics
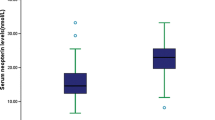
Baseline clinical characteristics are showed in Table 1. No significant imbalance was observed for the main clinical data including age, sex, BMI, hypertension, diabetes, and smoking. As for laboratory inspection, AIS patients tend to have higher SBP, FPG and glycated hemoglobin, and lower HDL-C compared with controls. Furthermore, serum Ninj1 levels in LAA-AIS patients were significantly higher than that in control group (142.70 ng/ml [IQR: 110.41–163.44] vs 101.62 ng/ml [IQR: 86.63–120.86], p < 0.001).
The ROC curve was applied to find the cut-off value of serum Ninj1 levels in predicting LAA-AIS (Fig. 2). As a result, the cut-off value of Ninj1 to diagnose LAA-AIS was suggested to be over 129.48 ng/ml, with the AUC of 0.785 (95% CI: 0.725, 0.846). The sensitivity and specificity at this concentration were 62.7% and 84.5%, respectively. The proportion of cases with Ninj1 > 129.48 ng/ml was significantly higher in AIS (69, 62.7%) than the control group (17, 15.5%).
Serum Ninj1 Levels and the Risk of LAA-AIS
In logistic regression analysis, elevated serum Ninj1 levels were associated with an increased risk of LAA-AIS. As a continuous variable, every increment of 10 ng/ml in Ninj1 was associated with an increased risk of LAA-AIS (multivariate OR = 1.541, 95% CI: 1.348–1.763; p < 0.001). A similar relationship was also observed when treating Ninj1 as an ordinal variable, the risk of LAA-AIS was significantly higher in tertile3 than that in tertile1 (OR = 10.234, 95% CI: 4.711–22.232; p < 0.001), while no significant difference was observed in tertile2 compared with tertile1 (OR = 1.302, 95% CI: 0.657–2.580; p = 0.45), the p-value for trend was < 0.001. After adjustment for age, sex, hypertension, diabetes, systolic blood pressure, fasting plasma glucose, total cholesterol, HDL-C, LDL-C, homocysteine, and glycated hemoglobin, patients with Ninj1 index of tertile3 were still independently associated with increased risk of LAA-AIS compared with patients with a Ninj1 index of tertile1 (adjusted OR = 12.567, 95% CI: 5.148–30.678; p < 0.001) (Table 2).
As displayed in Fig. 3, the AUC for the risk ratio model containing Ninj1 and other established risk factors, including age, sex, hypertension, diabetes, systolic blood pressure, fasting plasma glucose, total cholesterol, HDL-C, LDL-C, homocysteine, and glycated hemoglobin was considerably greater than the one containing only conventional risk factors (0.787 vs 0.874), suggesting that incorporation of Ninj1 into the basic model significantly improved the prediction value of LAA-AIS (p < 0.001).
Receiver operating characteristic curves (ROC) of the logistic regression model including Ninj1 and other conventional risk factors. Basic model: age, sex, hypertension, diabetes, systolic blood pressure, fasting plasma glucose, total cholesterol, HDL-C, LDL-C, homocysteine, and glycated hemoglobin. AUC, area under curve
Discussion
Atherosclerosis, as the main underlying cause of ischemic stroke, is a chronic inflammatory disease involved in endothelial dysfunction, macrophage infiltration, and foam cell formation in the vasculature [15]. Inflammation is considered to be implicated in the whole pathogenesis process of atherosclerosis. In addition, recent large-scale clinical trials have established the therapeutic potential of anti-inflammatory therapies targeting atherosclerosis [16,17,18].
Previous studies have shown that Ninj1 was significantly upregulated in atherosclerotic aortas compared with normal vessels, and its soluble form in serum was significantly higher in CAD patients than healthy controls [13]. To the best of our knowledge, the present study was the first case–control study on the correlation between serum Ninj1 levels and the morbidity risk of LAA-AIS. Our study suggested that elevated serum Ninj1 levels were correlated with higher risk of LAA-AIS patients, this positive association was independent of established risk factors including age, sex, hypertension, diabetes, systolic blood pressure, fasting plasma glucose, total cholesterol, HDL-C, LDL-C, homocysteine, and glycated hemoglobin.
Ninj1 is a two-pass transmembrane protein originally identified as a small adhesion molecule via homophilic interaction of amino acid residues from Pro26-Asn37 [19]. It is reported to be upregulated in many inflammatory diseases such as rheumatoid arthritis, asthma, inflammatory bowel disease, multiple sclerosis, pulmonary fibrosis, malignant tumors, or severe infection. Yet, its role in inflammation is still controversial, with both pro- and anti-inflammatory activity described before. In the present study, we found that serum Ninj1 levels in LAA-AIS patients were significantly higher than that in control group, so we speculated that Ninj1 may act as a pro-inflammatory agent, which is consistent with that of Seungho et al., who found that Ninj1 enhanced inflammatory response of macrophages in pulmonary fibrosis [9]. Similarly, another study on early ocular development discovered that Ninj1 increased macrophage-induced cell–cell and cell–matrix adhesion [20]. In addition, Ninj1 is also reported to promote toll-like receptors 4 signaling to aggravate systemic inflammation and the adhesion of leukocytes onto endothelial cells in experimental autoimmune encephalomyelitis model [5, 7]. However, there were a few studies showed that Ninj1 might exert anti-inflammatory effects. Hoon Choi et al. reported that Ninj1 limited colon inflammation by activating M2 macrophage polarization [21]; another study showed that Ninj1 overexpression on macrophages suppress macrophage infiltration repression of FAK signaling in colon cancer [22]. The difference may be explained by different effects of Ninj1 in various organs, and different roles between length Ninj1 and soluble Ninj1 [23]. Recently, Ninj1 has rekindled scholars’ interest as a regulator of plasma membrane rupture (PMR), which is the final event in lytic cell death to augment inflammatory response [11, 12]. All the evidences above indicate that targeting Ninj1 may be of potential therapeutic benefit in inflammatory diseases. Therefore, studies targeting Ninj1 using peptide or antibodies in inflammatory diseases were conducted, and as expected, they found that functional blocking of Ninj1 inhibits inflammatory responses on macrophages and endothelial cells [24, 25]. In atherosclerosis model, a recent study revealed that synthetic Ninj1 peptides can attenuate monocyte recruitment and reduce macrophage-mediated inflammation to alleviate atherosclerosis [13]. Taken together, these findings suggest that Ninj1 plays an important role in the development of atherosclerosis; thus, we conducted the current study and observed a positive association between Ninj1 and LAA-AIS, and we speculated that Ninj1 might be an independent predictor of LAA-AIS and might be a promising target for atherosclerosis.
Some limitations of the study should be considered. First, given this study a relatively small-sample research in a single center, a larger-scale study should replicate these conclusions. Second, the current study aimed to investigate the association between serum Ninj1 and LAA-AIS, while its correlation with other inflammatory factors such as interleukin-6 was not elucidated. Third, we did not determine the association of serum Ninj1 with other subtypes of stroke. Finally, we did not assess the consistent changes of serum Ninj1 pre- or post-stroke, together with that this is an observational study, and casual relationships cannot be elucidated. Hence, whether Ninj1 offered potential as a target for anti-inflammatory therapy in LAA-AIS warranted further verification.
Conclusions
The present study demonstrated a positive correlation between elevated serum Ninjurin-1 and a higher risk of LAA-AIS, suggesting that serum Ninj1 may act as an independent predictor for LAA-AIS; further studies are needed to evaluate whether Ninj1 could be a potential therapeutic target against atherosclerotic diseases.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
Acute ischemic stroke
- LAA-AIS:
-
Large artery atherosclerotic acute ischemic stroke
- ROC:
-
Receiver operating characteristic curve
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CAD:
-
Coronary artery disease
- TOAST criteria:
-
Trial of Org 10172 in Acute Stroke Treatment criteria
- AUC:
-
Area under receiver operating characteristic curve
References
Björkegren JLM, Lusis AJ. Atherosclerosis: Recent developments. Cell. 2022;185(10):1630–45. https://doi.org/10.1016/j.cell.2022.04.004.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V, GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, Kelly P, Tong DC, Layland J, Nidorf SM, Thompson PL, Budgeon C, Tijssen JGP, Cornel JH. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42(28):2765–75. https://doi.org/10.1093/eurheartj/ehab115.
Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17(2):353–61. https://doi.org/10.1016/s0896-6273(00)80166-x.
Ahn BJ, Le H, Shin MW, Bae SJ, Lee EJ, Wee HJ, Cha JH, Lee HJ, Lee HS, Kim JH, Kim CY, Seo JH, Lo EH, Jeon S, Lee MN, Oh GT, Yin GN, Ryu JK, Suh JK, Kim KW. Ninjurin1 deficiency attenuates susceptibility of experimental autoimmune encephalomyelitis in mice. J Biol Chem. 2014;289(6):3328–38. https://doi.org/10.1074/jbc.M113.498212.
Ifergan I, Kebir H, Terouz S, Alvarez JI, Lécuyer MA, Gendron S, Bourbonnière L, Dunay IR, Bouthillier A, Moumdjian R, Fontana A, Haqqani A, Klopstein A, Prinz M, López-Vales R, Birchler T, Prat A. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70(5):751–63. https://doi.org/10.1002/ana.22519.
Jennewein C, Sowa R, Faber AC, Dildey M, von Knethen A, Meybohm P, Scheller B, Dröse S, Zacharowski K. Contribution of Ninjurin1 to Toll-like receptor 4 signaling and systemic inflammation. Am J Respir Cell Mol Biol. 2015;53(5):656–63. https://doi.org/10.1165/rcmb.2014-0354OC.
Jang YS, Kang JH, Woo JK, Kim HM, Hwang JI, Lee SJ, Lee HY, Oh SH. Ninjurin1 suppresses metastatic property of lung cancer cells through inhibition of interleukin 6 signaling pathway. Int J Cancer. 2016;139(2):383–95. https://doi.org/10.1002/ijc.30021.
Choi S, Woo JK, Jang YS, Kang JH, Hwang JI, Seong JK, Yoon YS, Oh SH. Ninjurin1 plays a crucial role in pulmonary fibrosis by promoting interaction between macrophages and alveolar epithelial cells. Sci Rep. 2018;8(1):17542. https://doi.org/10.1038/s41598-018-35997-x.
Kang JH, Woo JK, Jang YS, Oh SH. Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells. Cells. 2020;9(5):1086. https://doi.org/10.3390/cells9051086.
Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, Zhang J, Lee WP, McKenzie BS, Ulas G, Payandeh J, Roose-Girma M, Modrusan Z, Reja R, Sagolla M, Webster JD, Cho V, Andrews TD, Morris LX, Miosge LA, Goodnow CC, Bertram EM, Dixit VM. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591(7848):131–6. https://doi.org/10.1038/s41586-021-03218-7.
Wang Y, Shao F. NINJ1, rupturing swollen membranes for cataclysmic cell lysis. Mol Cell. 2021;81(7):1370–1. https://doi.org/10.1016/j.molcel.2021.03.005.
Jeon S, Kim TK, Jeong SJ, Jung IH, Kim N, Lee MN, Sonn SK, Seo S, Jin J, Kweon HY, Kim S, Shim D, Park YM, Lee SH, Kim KW, Cybulsky MI, Shim H, Roh TY, Park WY, Lee HO, Choi JH, Park SH, Oh GT. Anti-inflammatory actions of soluble Ninjurin-1 ameliorate atherosclerosis. Circulation. 2020;142(18):1736–51. https://doi.org/10.1161/CIRCULATIONAHA.120.046907.
Tan X, Zhang Y, Shao H. Healthy China 2030, a breakthrough for improving health. Glob Health Promot. 2019;26(4):96–9. https://doi.org/10.1177/1757975917743533.
Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–36. https://doi.org/10.1161/CIRCRESAHA.115.306301.
Ridker PM. From CANTOS to CIRT to COLCOT to Clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation. 2020;141(10):787–9. https://doi.org/10.1161/CIRCULATIONAHA.119.045256.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Deftereos SG, Beerkens FJ, Shah B, Giannopoulos G, Vrachatis DA, Giotaki SG, Siasos G, Nicolas J, Arnott C, Patel S, Parsons M, Tardif JC, Kovacic JC, Dangas GD. Colchicine in cardiovascular disease: In-Depth Review. Circulation. 2022;145(1):61–78. https://doi.org/10.1161/CIRCULATIONAHA.121.056171.
Araki T, Zimonjic DB, Popescu NC, Milbrandt J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J Biol Chem. 1997;272(34):21373–80. https://doi.org/10.1074/jbc.272.34.21373.
Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009;16(10):1395–407. https://doi.org/10.1038/cdd.2009.78.
Choi H, Bae SJ, Choi G, Lee H, Son T, Kim JG, An S, Lee HS, Seo JH, Kwon HB, Jeon S, Oh GT, Surh YJ, Kim KW. Ninjurin1 deficiency aggravates colitis development by promoting M1 macrophage polarization and inducing microbial imbalance. FASEB J. 2020;34(6):8702–20. https://doi.org/10.1096/fj.201902753R.
Woo JK, Jang YS, Kang JH, Hwang JI, Seong JK, Lee SJ, Jeon S, Oh GT, Lee HY, Oh SH. Ninjurin1 inhibits colitis-mediated colon cancer development and growth by suppression of macrophage infiltration through repression of FAK signaling. Oncotarget. 2016;7(20):29592–604. https://doi.org/10.18632/oncotarget.9020.
Jeon S, Oh GT. Response by Jeon and Oh to Letter Regarding Article. Anti-inflammatory actions of soluble Ninjurin-1 ameliorate atherosclerosis. Circulation. 2021;143(19):e921–2. https://doi.org/10.1161/CIRCULATIONAHA.121.053671.
Lee HK, Kim ID, Lee H, Luo L, Kim SW, Lee JK. Neuroprotective and anti-inflammatory effects of a dodecamer peptide harboring Ninjurin 1 cell adhesion motif in the postischemic brain. Mol Neurobiol. 2018;55(7):6094–111. https://doi.org/10.1007/s12035-017-0810-1.
Wang X, Qin J, Zhang X, Peng Z, Ye K, Wu X, Yang X, Shi H, Zhao Z, Guo X, Liu X, Yin M, Lu X. Functional blocking of Ninjurin1 as a strategy for protecting endothelial cells in diabetes mellitus. Clin Sci (Lond). 2018;132(2):213–29. https://doi.org/10.1042/CS20171273.
Funding
This study was supported by National Natural Science Foundation of China (82071300), Suzhou Science and Technology Development Plan (SYSD2020073), The Stroke Team of Professor Fang Qi from the First Affiliated Hospital of Soochow University (SZYQTD202106); a follow-up study of cognitive impairment combined with depression in stroke patients (SYSD2020073); Suzhou Industrial Park Jinji Lake Health Talents (202110).
Author information
Authors and Affiliations
Contributions
QF conceived and designed the research. ND analyzed the data and drafted the manuscript. ND, XW, TH, XS, XG, HW, LY, and HZ collected the data and performed the research. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
The protocol of the study conforms to the ethical guidelines mentioned in the Declaration of Helsinki and was approved by the Ethics Committee of the First Hospital Affiliated to Soochow University (No. 2021–07-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, N., Wu, X., Hong, T. et al. Elevated Serum Ninjurin-1 Is Associated with a High Risk of Large Artery Atherosclerotic Acute Ischemic Stroke. Transl. Stroke Res. 14, 465–471 (2023). https://doi.org/10.1007/s12975-022-01077-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01077-6