Abstract
Percutaneous coronary intervention (PCI) for heavily calcified lesions is challenging because these lesions are resistant to balloon dilatation and stenting. Lacrosse non-slip element (NSE) may have the potential to dilate heavily calcified lesions. We aimed to investigate predictors of successful lesion modification using Lacrosse NSE angioplasty via optical coherence tomography (OCT)-guided PCI. We investigated 32 patients with severe target lesion calcification treated with OCT-guided PCI. Successful lesion modification was defined as the complete fracture of calcification after Lacrosse NSE angioplasty. Before PCI, 172 segments with calcification were identified. After pre-dilatation using Lacrosse NSE, successful lesion modification was achieved in 117 segments (68.0%). Calcification was significantly thinner in successfully disrupted segments than in non-disrupted segments (p < 0.001). Calcification angle tended to be larger in disrupted than in non-disrupted segments (p = 0.08). Convex types were less frequently observed in disrupted than in non-disrupted segments (p < 0.001). At minimal lumen area sites, 26 segments (81.3%) were successfully modified. Similar to the overall results, the disrupted group had significantly thinner calcification than the non-disrupted group (p < 0.001). The angle of the calcified plaque was similar between the 2 groups (p = 0.39). Convex-type calcifications were less frequently observed in the disrupted group than in the non-disrupted group (p = 0.05). Receiver-operating characteristic curve analysis showed that calcification thickness < 565 μm was the best predictor of completely disrupted calcification. The thickness and shape of calcifications were predictors of successful lesion modification after Lacrosse NSE angioplasty.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavily calcified coronary lesions are one of the most challenging subsets of lesions to manage during percutaneous coronary intervention (PCI) because they interfere with the ability of the balloon or stent to cross and may exhibit strong resistance to balloon dilatation. Even with the use of second- or third-generation drug-eluting stents (DES), stent under-expansion has been consistently reported as a strong predictor for stent restenosis as well as stent thrombosis [1, 2]. Therefore, to expand the coronary stent appropriately, effective lesion modification, prior to stent implantation, is required.
“Scoring balloons” have 2 or 3 scoring elements that prevent balloon slippage and create a crack in the calcified plaque [3, 4]. Although pre-dilatation via the Lacrosse non-slip element (NSE; Goodman. Co., Ltd., Nagoya, Japan) scoring balloon may be effective for modifying heavily calcified lesions [5], the rates and predictors of effective lesion modification are still uncertain.
Optical coherence tomography (OCT) is a near-infrared light-based intracoronary imaging modality that provides high-resolution images of the vascular wall and can detect different characteristics of atherosclerotic plaques [6,7,8,9]. OCT provides more accurate morphological and quantitative information of the calcified plaque than intravascular ultrasound [8, 10].
Accordingly, this study was conducted to determine if OCT imaging could identify the predictors of successful modification of heavily calcified lesions after pre-dilatation using a Lacrosse NSE balloon.
Methods
Study population
Between January 2013 and December 2014, 32 consecutive patients who underwent OCT-guided PCI for heavily calcified coronary lesions were enrolled. Heavily calcified coronary lesions were defined based on fluoroscopic appearance as lesions with readily apparent radio-opacities within the vascular walls in more than 1 projection on cine images before contrast medium injection.
The inclusion criteria were as follows: (1) symptomatic angina pectoris with significant coronary stenosis (> 75% diameter), and (2) severely calcified lesions treated using a scoring balloon followed by second- or third-generation DES implantation.
The following cases were excluded: (1) restenotic lesions, (2) totally occluded lesions, (3) cases where pre- and/or post-angiography OCT images could not be obtained because the OCT catheter could not cross the target lesions, (4) cases with poor or incomplete OCT image quality because of vessel tortuosity or incomplete removal of blood during OCT imaging, and (5) lesions treated with rotational atherectomy.
Successful lesion modification was defined as the creation of 1 or more complete fracture of the calcification after pre-dilatation.
After PCI, we performed a 3-year follow-up investigation and examined the number of target lesion revascularizations (TLRs). TLR was defined as PCI or bypass surgery because of restenosis or thrombotic events at the target lesion, which includes the proximal and distal edge segments.
PCI and OCT procedures
Coronary angiography and PCI were performed using the standard technique via a radial or femoral approach using a 6- or 7-Fr guiding catheter. After diagnostic coronary angiography, a commercially available frequency-domain OCT catheter (C7/C8 OCT imaging system; St. Jude Medical, MN, USA or OFDI system; Terumo Corporation, Tokyo, Japan) was inserted to obtain cross-sectional images of the target lesion prior to any interventional procedures. After intracoronary nitrate injection to achieve maximal vasodilatation, OCT images were acquired using an automated pullback (20 mm/s). Because calcified plaques may be present throughout the target lesion, we measured and analyzed multiple cross-sectional images of the calcified plaque, including the minimal lumen area (MLA) sites at 1-mm intervals. In addition, a similar analysis was also performed using cross-sectional images from the MLA site only. After OCT imaging, pre-dilatation of the target lesion was performed using a scoring balloon (Lacrosse NSE ALPHA; Goodman, Nagoya, Japan). The size of the balloon was selected at each operator’s discretion based on the angiography and OCT findings. After pre-dilatation, OCT imaging was repeated to assess lesion modification and the DES was implanted with subsequent high-pressure post-dilatation.
Analysis of OCT imaging
Calcified plaques were defined as well-delineated, signal-poor regions with sharp borders. We mainly examined the calcified plaque around the MLA. The longitudinal length, thickness of the calcified plaque, calcification angle, and shape of calcification were examined at every 1-mm increment. We classified the shape of the calcification as either convex type or non-convex type. Convex-type calcifications were defined as those that curve outward from the vessel lumen. Otherwise, the other calcifications were defined as non-convex type.
After pre-dilatation using scoring balloon, disruption of the calcified plaque was determined via OCT.
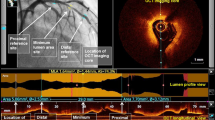
OCT showed circumferential calcification before (Fig. 1a) and after (Fig. 1b) PCI. Figure 1b shows 3 calcium fractures. The thickness of the calcification in each fracture was 520, 470, and 440 µm, respectively. In this case, the coronary calcification angle was 360° and the shape was non-convex.
Segments with disrupted calcified plaque were compared to those without disruption to investigate predictors of calcification disruption.
Angiographic follow-up
Angiographic follow-ups were scheduled at 9 months following the index procedure. TLR was defined as ischemia-driven repeated revascularization of the target lesions including stented segments and stent edges (within 5 mm from both proximal and distal stent edges).
Statistical analysis
All analyses were performed using SPSS version 19 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation, and categorical variables as frequencies and percentages. Continuous variables were compared using an unpaired Student’s t test or Wilcoxon rank-sum test based on their distribution. Categorical data were evaluated using the Chi-square test or Fisher’s exact test, as appropriate. A receiver-operating characteristic curve (ROC) was used to determine the best cutoff values for predicting the optimal coronary calcification thickness for disruption by scoring balloon angioplasty. The best cutoff values were defined as those with the highest sum of Youden’s index (sensitivity + specificity − 1). A p value of < 0.05 was considered to indicate statistical significance.
Results
A total of 172 cross-sections with calcification were selected from 32 lesions.
Table 1 shows the patient population and baseline characteristics. The angiography and procedure characteristics are presented in Table 2. Most PCIs were performed on the left anterior descending arteries. The mean longitudinal length of the calcified plaque was 17.1 ± 12.9 mm. The MLA and the minimum and maximum diameter at the culprit site after PCI were significantly larger than before PCI. The mean scoring balloon diameter was 2.61 ± 0.30 mm, and the mean balloon pressure before lesion modification was 11.9 ± 3.2 atm. We deployed the stents in all except for 1 patient. The mean stent diameter was 2.81 ± 0.28 mm, mean total stent length was 36.7 ± 18.4 mm, mean number of stents was 1.4 ± 0.61, and post-dilatation pressure was 17.2 ± 4.5 atm. Coronary perforation was not observed. Everolimus-eluting stents were mainly used. Table 3 shows a comparison of the disrupted and non-disrupted sites. Before PCI, 172 cross-sectional segments with calcified plaque were identified by OCT. After pre-dilatation using the scoring balloon, successful lesion modification (= complete disruption of the calcified plaque) was achieved in 117 segments (68%, disrupted group). The remaining 55 segments (32%) were not successfully modified (non-disrupted group). In the disrupted group, the calcified plaque was significantly thinner than in the non-disrupted group (0.35 ± 0.14 mm vs. 0.79 ± 0.13 mm, p < 0.001). Conversely, the angle of the calcified plaque tended to be larger in the segments with calcification disruption than in those with disruption (256.2° ± 84.4 vs. 232.7° ± 73.9, p = 0.08). However, there was no statistical significance between the groups. The convex type of angle was less frequently observed in the disrupted group than in the non-disrupted group (4.3% vs. 21.8%, p < 0.001). Table 4 shows the results of OCT analyses for the MLA sites. Successful lesion modification was achieved in 26 segments (81.3%, disrupted group) but not in 6 segments (non-disrupted group). Similar to the overall results, the disrupted group had significantly thinner calcification than the non-disrupted group (0.38 ± 0.17 mm vs. 0.79 ± 0.19 mm, p < 0.001). There were no significant differences in the longitudinal length of calcification between the 2 groups. The angle of the calcified plaque was similar between the 2 groups (274.6 ± 74.0° vs. 243.3 ± 99.9°, p = 0.39). The convex type of calcification was less frequently observed in the disrupted group than in the non-disrupted group (3.85% vs. 33.3%, p = 0.05). Stent delivery was successfully completed in all patients (100%) in the disrupted group and in 5 patients (83%) in the non-disrupted group. Incomplete stent apposition was present in 19 patients (73%) in the disrupted group and in 6 patients (100%) in the non-disrupted group.
The ROC curve analysis showed that a calcification thickness of < 565 μm (area under the curve [AUC] 0.928, sensitivity 85.3%, specificity 91.4%) was the best cutoff value for predicting calcification disruption (Fig. 2). In a subset of 101 segments with a calcification angle of > 270°, the ROC curve showed that a calcification thickness of < 535 μm (AUC 0.930; 95% confidence interval 0.873–0.987, sensitivity 86.7%, specificity 85.9%) was the best cutoff value for predicting calcification disruption (Fig. 3).
Angiographic follow-up was performed in 29 of 32 patients (90.6%). During follow-up (median 295 days), TLR was documented in 3 of 29 patients (10.3%) (2 [7.7%] in the disrupted group and 1 [16.7%] in the non-disrupted group).
Discussion
The principal findings of this study were that lesion modification could be achieved in 68% of the heavily calcified coronary arterial segments after pre-dilatation using a scoring balloon, and that the thickness and morphology of the calcified plaque were successful predictors of lesion modification.
Coronary artery stenosis with heavily calcified lesions can be challenging for coronary interventionists because of the strong resistance to balloon dilatation or stent implantation [11,12,13]. A previous OCT study demonstrated that the amount (area) and distribution (angle) of the calcified plaque may be associated with stent expansion [10]. In addition, incomplete stent apposition may also occur after stent deployment to calcified lesions with irregular surfaces and convex shapes [14]. Stent under-expansion, a small MLA, and the length of the stent are often reported as major factors for stent restenosis and thrombosis [15,16,17,18]. As a result, heavily calcified lesions may be associated with a higher risk for stent restenosis and thrombosis [19, 20]. As mentioned above, calcified plaque modification before stent deployment might be associated with adequate stent expansion and favorable late outcomes [21].
In our study, the thickness of the calcification was a key measure for predicting lesion modification. In patients with circumferential calcification (i.e., calcification angle > 270°) and a calcification thickness of < 535 µm, a scoring balloon angioplasty may be effective. Furthermore, the morphology of the calcification is another predictor for fracture of calcification. As expected, the non-convex type of calcified lesion could be more easily disrupted after scoring balloon angioplasty.
The so-called “scoring balloon” has been recognized as an alternative interventional tool to pre-dilate non-calcified, as well as calcified, lesions prior to stent implantation. A scoring balloon is easier to use than rotational ablation, and the usefulness of the scoring balloon for heavily calcified coronary lesions is well recognized [22, 23]. During balloon inflation, the scoring elements cut or crack the calcified portion more effectively than standard balloons without scoring elements. Lesion modification by scoring balloon can be more easily performed. On the other hand, use of a scoring balloon may cause coronary complications [24]. In a previous study, coronary calcification and use of a cutting balloon were associated with coronary perforation [25]. Furthermore, in vessels having an eccentric plaque, there might also be a risk of perforation. However, fortunately, coronary perforation did not occur in our study. Similar to a previous study [26], only 7 cases (21.8%) showed complete stent apposition. Moreover, we failed to deploy a coronary stent in 1 case. In this case, rotational ablation should have been performed. Furthermore, 3 TLRs were documented in this study. Severe calcification is still associated with an increased risk of adverse cardiovascular events following treatment with DES [27].
In our study, OCT was used to assess calcification and lesion modification. OCT provides higher-resolution imaging (10–20 μm) than intravascular ultrasound (IVUS, 150–200 μm) [8] and detects calcification contours more clearly. A previous study comparing OCT, IVUS, and histological analysis showed that OCT more accurately quantified the amount of calcification than IVUS. OCT-guided PCI is effective for heavily calcified coronary lesions.
Limitations
There are several limitations to this study. First, it was a single-center, retrospective study with a small sample size. To address the impact of balloon size and maximal inflation pressure, far more cases are needed. Second, only 1 type of scoring balloon was used for pre-dilatation. This makes the predictive value of the calcification thickness and angle for lesion modification by other scoring balloons, as well as standard balloons, uncertain.
Conclusions
Both the thickness and the shape of the calcification are predictive of successful lesion modification after scoring balloon angioplasty. A coronary plaque calcification with a thickness of < 565 μm and a non-convex shape may be disrupted better by scoring balloon angioplasty prior to stent implantation than other types of calcifications.
References
Park DW, Park SW, Park KH, Lee BK, Kim YH, Lee CW, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006;98:352–6.
Song HG, Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2014;83:873–8.
Barath P, Fishbein MC, Vari S, Forrester JS. Cutting balloon: a novel approach to percutaneous angioplasty. Am J Cardiol. 1991;68:1249–52.
de Ribamar Costa J, Jr Mintz GS, Carlier SG, Mehran R, Teirstein P, Sano K, et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007;100:812–7.
Ashida K, Hayase T, Shinmura T. Efficacy of Lacrosse NSE using the “Leopard-Crawl” technique on severely calcified lesions. J Invasive Cardiol. 2013;25:555–64.
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–5.
Kume T, Okura H, Kawamoto T, Akasaka T, Toyota E, Watanabe N, et al. Relationship between coronary remodeling and plaque characterization in patients without clinical evidence of coronary artery disease. Atherosclerosis. 2008;197:799–805.
Kume T, Okura H, Kawamoto T, Yamada R, Miyamoto Y, Hayashida A, et al. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention. 2011;6:768–72.
Miyamoto Y, Okura H, Kume T, Kawamoto T, Neishi Y, Hayashida A, et al. Plaque characteristics of thin-cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovasc Imaging. 2011;4:638–46.
Kobayashi Y, Okura H, Kume T, Yamada R, Kobayashi Y, Fukuhara K, et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209–14.
von Birgelen C, Mintz GS, Böse D, Baumgart D, Haude M, Wieneke H, et al. Impact of moderate lesion calcium on mechanisms of coronary stenting as assessed with three-dimensional intravascular ultrasound in vivo. Am J Cardiol. 2003;92:5–10.
Hoffmann R, Mintz GS, Popma JJ, Satler LF, Kent KM, Pichard AD, et al. Treatment of calcified coronary lesions with Palmaz–Schatz stents. An intravascular ultrasound study. Eur Heart J. 1998;19:1224–31.
Vavuranakis M, Toutouzas K, Stefanadis C, Chrishou C, Markou D, Toutouzas P. Stent deployment in calcified lesions: can we overcome calcific restraint with high-pressure balloon inflations? Catheter Cardiovasc Interv. 2001;52:164–72.
Kume T, Waseda K, Ako J, Sakata K, Yamasaki M, Shimohama T, et al. Intravascular ultrasound assessment of postprocedural incomplete stent apposition. J Invasive Cardiol. 2012;24:13–6.
Sonoda S, Morino Y, Ako J. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the SIRIUS trial. J Am Coll Cardiol. 2004;43:1959–63.
Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109:1085–8.
Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, VI and TAXUS ATLAS workhorse, long lesion, and direct stent trials. JACC Cardiovasc Interv. 2009;2:269–75.
Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23:124–32.
Fujimoto H, Nakamura M, Yokoi H. Impact of calcification on the long-term outcomes of sirolimus-eluting stent implantation: subanalysis of the Cypher Post-Marketing Surveillance Registry. Circ J. 2012;76:57–64.
Huang BT, Huang FY, Zuo ZL, Liu W, Huang KS, Liao YB, et al. Target lesion calcification and risk of adverse outcomes in patients with drug-eluting stents: a meta-analysis. Herz. 2015;40:1097–106.
Kubo T, Shimamura K, Ino Y, Yamaguchi T, Matsuo Y, Shiono Y, et al. Superficial calcium fracture after PCI as assessed by OCT. JACC Cardiovasc Imaging. 2015;8:1228–9.
Kawase Y, Saito N, Watanabe S, Bao B, Yamamoto E, Watanabe H, et al. Utility of a scoring balloon for a severely calcified lesion: bench test and finite element analysis. Cardiovasc Interv Ther. 2014;29:134–9.
Kang WC, Ahn TH, Han SH, Shin EK. Successful management of a resistant, focal calcified lesion following direct coronary stenting with a cutting balloon. J Invasive Cardiol. 2004;16:725–6.
Auer J, Maurer E, Berent R, Mayr H, Punzengruber C, Weber T, et al. Clinical and angiographic outcome after cutting balloon angioplasty. J Interv Cardiol. 2003;16:15–21.
Hendry C, Fraser D, Eichhofer J, Mamas MA, Fath-Ordoubadi F, El-Omar M, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. EuroIntervention. 2012;8:79–86.
Im E, Kim BK, Ko YG, Shin DH, Kim JS, Choi D, et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014;7:88–96.
Huisman J, van der Heijden LC, Kok MM, Danse PW, Jessurun GA, Stoel MG, et al. Impact of severe lesion calcification on clinical outcome of patients with stable angina, treated with newer generation permanent polymer-coated drug-eluting stents: a patient-level pooled analysis from TWENTE and DUTCH PEERS (TWENTE II). Am Heart J. 2016;175:121–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sugawara, Y., Ueda, T., Soeda, T. et al. Plaque modification of severely calcified coronary lesions by scoring balloon angioplasty using Lacrosse non-slip element: insights from an optical coherence tomography evaluation. Cardiovasc Interv and Ther 34, 242–248 (2019). https://doi.org/10.1007/s12928-018-0553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-018-0553-6