Abstract
Several reports have shown contrary results regarding the efficacy of sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) in diabetic patients. The association between hemoglobin A1c (A1c) before coronary intervention and the midterm clinical outcomes of patients treated with these stents is unclear. The enrolled population was 415 patients with diabetes or impaired glucose tolerance (IGT) who underwent follow-up angiography after being implanted with a SES (n = 282) or PES (n = 133). The enrolled population was classified into the optimal glycemic control group (n = 213) and suboptimal glycemic control group (n = 202), and the predictors of restenosis were examined in each group. In the optimal glycemic control group, the use of PES was an independent predictor of restenosis [odds ratio (OR) 9.98, 95% confidence interval (CI) 3.08–38.9, p < 0.0001]; on the other hand, the use of SES was a positive independent predictor of restenosis prevention (OR 0.10, 95% CI 0.03–0.32, p < 0.0001). In the suboptimal glycemic control group, neither stent was predictive of restenosis. In a subanalysis, preprocedural A1c (≥7.0%) was found to be an independent predictor of restenosis in the SES group (OR 3.61, 95% CI 1.14–12.8, p = 0.03), but not the PES group. Postprocedural A1c (≥7.0%) was not an independent predictor of restenosis in either stent group. This study showed that the superiority of SES compared to PES was attenuated in the suboptimal glycemic control group. Preprocedural A1c (≥7.0%) was found to be an independent predictor of restenosis in the SES group, but not in the PES group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is an important factor in the prognosis of patients who undergo percutaneous coronary intervention (PCI) [1, 2]. Drug-eluting stents (DES) display a reduced incidence of stent restenosis compared with bare metal stents (BMS) in diabetic patients [3–5]. There have been various reports about the efficacy of sirolimus-eluting stents (SES) (Cordis, Johnson & Johnson, Miami, FL, USA) and paclitaxel-eluting stents (PES) (Boston Scientific, Natick, MA, USA) in diabetic patients, and it is still disputed whether SES and PES are equally effective at preventing these patients’ conditions from worsening. A recent prospective randomized trial demonstrated that SES implantation is associated with a reduced risk of restenosis compared with PES implantation in diabetic patients [6]. On the other hand, recent meta analyses [7], retrospective studies [8], and a large registry [9] revealed no difference between these stents in terms of the frequencies of restenosis and target lesion revascularization (TLR). As the late loss was lower during treatment with SES than PES [10, 11], it could be hypothesized that diabetic patients undergoing PCI with SES would achieve better clinical outcomes than those treated with PES. However, the attenuation of the antimigratory effect of sirolimus observed under hyperglycemic conditions could reduce the efficacy of SES in diabetic patients [12]. Thus, we considered that it was important to evaluate the midterm outcomes of patients treated with each stent according to the degree of glycemic control that they achieved. In this study, we investigated the associations between the degree of glycemic control and midterm outcomes after PCI, as well as the predictors of restenosis, in patients with type 2 DM or impaired glucose tolerance (IGT) that had been treated with SES or PES.
Methods
Study design
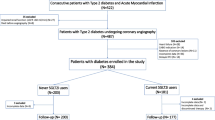
From May 2004 to July 2010, 817 patients who underwent SES or PES implantation at Mie University Hospital had their clinical, angiographic, procedural, and subsequent follow-up data prospectively entered into our database. Among these patients, 352 patients without type 2 DM or IGT and 50 patients who did not undergo repeat coronary angiography were excluded. Finally, we extracted 415 patients with type 2 DM or IGT who had undergone repeat coronary angiography after approximately 7 months (210 ± 53 days). Two hundred and eighty-two patients who were treated with SES and 133 patients who were treated with PES were retrospectively analyzed in this study. At first, we classified the enrolled population (n = 415) into the optimal glycemic control group (n = 213) and suboptimal glycemic control group (n = 202) and examined the predictors of restenosis in each group by multivariate analysis. Secondly, we performed a subanalysis of the predictors of restenosis in the patients treated with each stent. In this way, we examined whether the degree of glycemic control was associated with restenosis. All patients gave their informed consent for the procedure and angiographic follow-up treatment, which were approved by the hospital’s institutional ethics committee.
PCI
Before PCI, all patients underwent baseline laboratory measurement, including measurements of their A1c and lipid levels. All patients received 100 mg/day aspirin indefinitely. Additional antiplatelet therapy involving either 75 mg/day clopidogrel (after a loading dose of 300 mg) or 200 mg ticlopidine twice daily was administered to all patients and continued for at least 6 months in both stent groups. Other adjunctive pharmacotherapies were administered at the discretion of the responsible physician. At the beginning of the intervention, a heparin bolus of 5000 units was administered after sheath insertion, and supplementary doses were given to maintain an activated clotting time of >250 s. In all cases, the interventional strategy, including the choice of DES and the use of intravascular ultrasound (40-MHz IVUS catheter, Boston Scientific Corp, Natick, MA, USA), was at the discretion of the operator. All patients were asked to return to the hospital to undergo a coronary angiography at approximately 7 months after the procedure (or earlier if symptoms occurred).
Angiographic analysis
Quantitative coronary angiography (QCA) was performed using an automated edge-detection system (QAngioXA, MEDIS medical imaging systems, Leiden, the Netherlands) by a single observer, who was blinded to the patients’ clinical details and outcomes. Calibration was performed based on the dimensions of a contrast medium-filled non-tapered catheter tip. The following parameters were measured in two orthogonal projections before and after coronary intervention: lesion length, reference vessel diameter (RVD), minimum lumen diameter (MLD), and diameter stenosis (DS). All lesions were classified in accordance with the American College of Cardiology/American Heart Association (ACC/AHA) classification scheme [13].
Definitions
The diabetic and IGT patients were identified as patients who were undergoing treatment with diet therapy, oral hypoglycemic medication, or insulin. Seventy-five gram oral glucose tolerance tests (OGTT) were performed in the patients who had not been diagnosed with DM or IGT. DM was diagnosed as a fasting plasma glucose level of ≥126 mg/dl or a plasma glucose level at 2 h after the OGTT of ≥200 mg/dl. IGT was diagnosed as a fasting plasma glucose level of ≥110 mg/dl and <126 mg/dl or a plasma glucose level at 2 h after the OGTT of ≥140 mg/dl and <200 mg/dl. The diabetic and IGT patients were stratified into two groups based on their preprocedural glycemic control. Optimal glycemic control was defined as a preprocedural A1c value of <7.0%, and suboptimal control was defined as a preprocedural A1c value of ≥7.0%. In elective cases, the preprocedural A1c value was measured on the day before coronary intervention, and in urgent cases, it was measured on the day after coronary intervention. Postprocedural glycemic control was defined according to the A1c value at the 7-month follow-up angiography, and the postprocedural A1c value was measured on the day before angiography. The A1c values used in this study were calculated using the internationally recognized definition of A1c outlined by the National Glycohemoglobin Standardization Program; i.e., by adding 0.4% to the A1c value defined by the Japan Diabetes Society [14].
Angiographic success was defined as the achievement of residual stenosis of <50% with a Thrombolysis in Myocardial Infarction flow grade of at least 3. Procedural success was defined as the achievement of angiographic success in the absence of major in-hospital complications [death, myocardial infarction, TLR, or urgent coronary artery bypass graft surgery (CABG)]. Acute gain (AG) was defined as the difference between the MLD before and after coronary intervention, and late loss was defined as the difference between the MLD after coronary intervention and the MLD at the follow-up examination. Myocardial infarction (MI) was defined as the presence of new Q waves on the follow-up electrocardiogram or the elevation of the creatine kinase level to greater than three times the normal concentration. Cardiac death was defined as death attributable to MI, heart failure, or arrhythmia. Binary restenosis was defined as lumen diameter stenosis of 50% or more in the target lesion on follow-up angiography. TLR was considered to be clinically driven if prompted by symptoms consistent with myocardial ischemia, if preceded by an abnormal stress test result consistent with myocardial ischemia, if there were other electrocardiographic changes consistent with myocardial ischemia, or if the lesion stenosis diameter was more than 75% at the follow-up examination and was related to a PCI performed to revascularize a restenotic lesion [15]. Major adverse cardiac events (MACE) were defined as a composite of cardiac death, Q-wave MI, TLR, and CABG. Stent thrombosis was defined according the recommendations of the Academic Research Consortium and divided into acute (0–1 day), subacute (2–30 days), and late (31 days to 1 year) thrombosis [16].
Statistical analysis
All statistical analyses were performed using JMP 9.0 (SAS Institute, Cary, NC, USA). Categorical variables are presented as numbers or percentages and were compared with the Chi-square test (or Fisher’s exact test). Continuous variables are presented as the mean ± standard deviation and were compared with the unpaired t test. Univariate and multivariate logistic regression analyses were performed to investigate predictors of restenosis after PCI. Univariate variables associated with p < 0.20 were entered into the multivariate logistic models. The analysis results are shown as odds ratios (OR) together with their 95% confidence intervals (CI) and p values. A p value of <0.05 was considered to indicate a significant difference.
Results
Optimal glycemic control group versus suboptimal glycemic control group
Baseline characteristics
Table 1 shows the baseline clinical characteristics of the optimal glycemic control group and suboptimal glycemic control group. The mean age and the incidences of male sex, previous coronary bypass, the use of renin-angiotensin system blockers, and the use of calcium channel blockers were significantly higher in the optimal glycemic control group than in the suboptimal glycemic control group. Body mass index, low density lipoprotein cholesterol levels, the estimated glomerular filtration rate, and the incidences of dyslipidemia and being a current smoker were significantly higher in the suboptimal glycemic control group than in the optimal glycemic control group. Naturally, the pre- and post-procedural A1c, and the frequencies of oral hypoglycemic agent use and insulin use were significantly higher in the suboptimal glycemic control group than in the optimal glycemic control group. Table 2 shows the angiographic characteristics of the study groups. The stent diameter, RVD, and post-PCI MLD were significantly lower in the suboptimal glycemic control group than in the optimal glycemic control group.
In-hospital and 7-month outcomes
Table 3 shows the in-hospital and 7-month outcomes. The procedure success rate and in-hospital outcomes of the two groups were similar. Angiographic analysis at 7 months revealed that the incidences of binary restenosis, TLR, and MACE were similar in both groups. No subacute or late stent thrombosis occurred in either group. According to the QCA results, the follow-up MLD was significantly lower in the suboptimal glycemic control group than in the optimal glycemic control group.
Analysis for predicting restenosis in the optimal glycemic control group and the suboptimal glycemic control group
Table 4 shows univariate and multivariate logistic regression analyses for predicting restenosis in the optimal glycemic control group. Univariate analysis showed that hemodialysis, the use of insulin, stent implantation in the right coronary artery, the use of PES, and lesion length (≥25 mm) were predictors of restenosis. Multivariate logistic regression analysis showed that the use of PES (OR 9.98, 95% CI 3.08–38.9, p < 0.0001) and lesion length (≥25 mm) (OR 5.01, 95% CI 1.61–16.5, p = 0.006) were independent predictors of restenosis in the optimal glycemic control group. Stent implantation in the left circumflex artery (OR 0.13, 95% CI 0.01–0.91, p = 0.04) and the use of SES (OR 0.10, 95% CI 0.03–0.32, p < 0.0001) were independent positive predictors of restenosis prevention in the optimal glycemic control group.
Table 5 shows univariate and multivariate logistic regression analyses for predicting restenosis in the suboptimal glycemic control group. Univariate analysis showed that hemodialysis, stent implantation for an aorto-ostial lesion, and overlap stenting were predictors of restenosis. Multivariate logistic regression analysis showed that hemodialysis (OR 10.6, 95% CI 3.78–31.1, p < 0.0001), stent implantation for an aorto-ostial lesion (OR 6.38, 95% CI 1.16–36.5, p = 0.03), and overlap stenting (OR 4.09, 95% CI 1.24–13.7, p = 0.02) were independent predictors of restenosis in the suboptimal glycemic control group. PES use was not an independent predictor of restenosis in the suboptimal glycemic control group.
Subanalysis for predicting restenosis in the SES and PES groups
Figure 1 shows the incidences of binary restenosis (A) and TLR (B), and the degree of late loss (C) associated with SES and PES in the optimal and suboptimal glycemic control groups. In the PES group, the incidences of restenosis and TLR, and the degree of late loss were similar between the optimal and suboptimal glycemic control groups, but in the SES group, they were significantly higher in the suboptimal glycemic control group than in the optimal glycemic control group. Therefore, we investigated the predictors of restenosis in the SES and PES groups.
Table 6 shows univariate and multivariate logistic regression analyses for predicting restenosis in the SES group. Univariate analysis showed that hemodialysis, preprocedural A1c (≥7.0%), postprocedural A1c (≥7.0%), the use of insulin, stent implantation for an aorto-ostial lesion, and overlap stenting were predictors of restenosis. Multivariate logistic regression analysis showed that hemodialysis (OR 18.3, 95% CI 5.51–67.7, p < 0.0001) and preprocedural A1c (≥7.0%) (OR 3.61, 95% CI 1.14–12.8, p = 0.03) were independent predictors of restenosis in the SES group.
Table 7 shows univariate and multivariate logistic regression analyses for predicting restenosis in the PES group. Univariate analysis showed that stent implantation for an aorto-ostial lesion was a predictor of restenosis. Multivariate logistic regression analysis showed that stent implantation for an aorto-ostial lesion (OR 12.3, 95% CI 1.28–162.2, p = 0.03) was an independent predictor of restenosis in the PES group. Unlike the SES group, neither pre- nor post-procedural A1c (≥7.0%) was a predictor of restenosis in the PES group.
Discussion
The main findings of this study are: (1) There were several differences in the background characteristics of the optimal and suboptimal glycemic control groups, but their midterm clinical outcomes were similar; (2) In the optimal glycemic control group, the use of PES was an independent predictor of restenosis, whereas the use of SES was a positive independent predictor of restenosis prevention; (3) In the suboptimal glycemic control group, neither stent was a predictor of restenosis; (4) In our subanalysis, preprocedural A1c (≥7.0%) was found to be an independent predictor of restenosis in the SES group, but not in the PES group; (5) Postprocedural A1c (≥7.0%) was not an independent predictor of restenosis in either stent group; (6) As for predictors of restenosis except preprocedural A1c (≥7.0%), hemodialysis and stent implantation for an aorto-ostial lesion were extracted in the SES and PES group, respectively.
Efficacy of DES in diabetic patients
Coronary artery stenting in diabetic patients is associated with higher rates of stent restenosis and repeat revascularization compared with those seen in non-diabetic patients [2, 17]. With the introduction of DES, the incidence of angiographically detected stent restenosis was markedly reduced [10, 11, 18–22]. In this study, there were several differences in the clinical and angiographic characteristics of the optimal and suboptimal glycemic control groups (Tables 1, 2), but the incidences of restenosis, TLR, 7-month MACE, and late loss were similar in both groups (Table 3). Our results agree with those of previous reports that compared diabetic patients with non-diabetic patients treated with DES. However, in the present conditions, in which we can use various kinds of DES for patients with coronary artery disease, it is necessary to evaluate which DES is most effective and safe for diabetic patients.
Differences in the restenosis predictors of SES and PES
Recently, the relative efficacies of SES and PES in diabetic patients have been evaluated in several studies [6, 7, 15, 23–25]. Late loss is less common in patients treated with SES than in those treated with PES [6, 10, 11], and some studies have found that diabetic patients undergoing PCI with SES have better clinical outcomes than those undergoing PCI with PES [6, 23], whereas other studies have shown that in hyperglycemic conditions attenuation of the antimigratory effect of sirolimus can reduce the clinical efficacy of SES [12, 25]. The REALITY-diabetic subgroup analysis found that diabetic patients undergoing PCI with PES had better clinical outcomes than those undergoing PCI with SES [15]. As these previous reports did not consider the degree of glycemic control, this might have been the cause of the different results regarding the efficacy of SES and PES in diabetic patients. Therefore, in this study, we evaluated the efficacy of DES in patients with DM or IGT according to their degree of pre- and post-procedural glycemic control. As the incidence of coronary artery disease in patients with IGT is similar to that in patients with overt diabetes [26–28], we included patients with IGT in our study population as well as patients with overt diabetes. At first, multivariate analysis showed that PES use and lesion length (≥25 mm) were independent predictors of restenosis in the optimal glycemic control group (Table 4) and hemodialysis, stent implantation for an aorto-ostial lesion, and overlap stenting were independent predictors of restenosis in the suboptimal glycemic control group (Table 5). Namely, these results showed that the superiority of SES compared with PES was attenuated by a preprocedural hyperglycemic state. Next, we performed further analyses to evaluate the independent predictors of restenosis for each stent in detail. In our subanalysis, preprocedural A1c (≥7.0%) was found to be an independent predictor of restenosis in the SES group (Table 6), but not in the PES group (Table 7).
Differences in the mechanisms of action of sirolimus and paclitaxel
Sirolimus and paclitaxel prevent neointimal hyperplasia and stent restenosis by inhibiting the cell cycle and reducing smooth muscle cell proliferation [25, 29]. Several experimental studies have reported the neointimal hyperplasia mechanisms responsible for the vascular response to stent-induced injury in diabetes and the differences in the insulin intracellular signaling pathways between the normoglycemic and hyperglycemic state [30, 31]. Therefore, the differences in the clinical outcomes of patients treated with the two stents might have been caused by differences in the mechanisms of action of sirolimus and paclitaxel under hyperglycemic conditions. Patterson et al. [12] reported a similar difference in their study, in which they used smooth muscle cell cultures to compare the activities of sirolimus and paclitaxel in a diabetic animal model. Although both drugs activate mitogen-activated protein kinase pathways similarly, sirolimus potently activates AKT-dependent signaling, overriding the downregulation of this pathway by insulin resistance. This effect is associated with attenuation of the antimigratory effects of sirolimus in the hyperglycemic state, which might account for its decreased efficacy in patients with suboptimal glycemic control [12, 25].
The importance of active medical treatment from preintervention
If this mechanism affects midterm outcomes after stent implantation, improvement of the patient’s hyperglycemic state and depression of their inflammatory reactions by active medical treatment from preintervention might be important. Previous studies have demonstrated the critical role of inflammation in the mechanisms of atherosclerosis and restenosis [32, 33], and preprocedural systemic inflammatory markers such as C-reactive protein (CRP) are independent prognostic indicators of restenosis and cardiac events [32]. Paiva et al. [34] reported differences in the inflammatory response between patients with and without diabetes mellitus after coronary stenting. In their report, the diabetic and non-diabetic patients exhibited a temporary inflammatory response after stenting, and the diabetic patients presented higher preprocedural levels of inflammatory markers such as CRP and revealed an exacerbated inflammatory response after stenting. DES implantation induced a significantly reduced increase in CRP levels compared with BMS [35] and was associated with reduced stent restenosis compared with BMS in diabetic patients [3–5]. However, diabetic patients have smaller vessels and longer lesions than non-diabetic patients, and even when using DES, the incidences of stent restenosis and cardiac events are higher in diabetic patients than in non-diabetic patients [36]. Previous studies have shown that anti-inflammatory agents such as thiazolidinediones and statins have preventative effects on in-stent neointimal hyperplasia [37, 38]. This study detected attenuation of the effects of sirolimus under a preprocedural hyperglycemic state; therefore, in patients undergoing SES implantation, pretreatment with thiazolidinediones and statins, and intensive glucose control might be beneficial for stent restenosis and TLR, as well as patients treated with PES and next generation drug-eluting stents. In this study, no inflammatory markers such as CRP were measured; therefore, prospective studies that assess inflammatory marker levels, medical treatment, and outcomes after PCI are required. In this study, thiazolidinediones and statins were administered to about 20 and 78% patients, respectively; however, about half of the patients were administered these drugs from postintervention, and they did not necessarily receive enough medication from preintervention.
An interpretation of other predictors of restenosis
In this study, regarding predictors of restenosis except preprocedural A1c (≥7.0%), hemodialysis and stent implantation for an aorto-ostial lesion were found to predictors in the SES group and the PES group, respectively. These results were similar to those of several previous reports [39–42]. In hemodialysis patients, the major causes of restenosis are chronic vascular inflammation and underexpansion of the stent due to severe calcification [43, 44]. SES has a stainless steel closed-cell design, which displays low conformability and flexibility; therefore, non-uniform drug distribution and disruption of polymers in calcified lesions are associated with restenosis. Aorto-ostial lesions are distinct from other lesions because of their specific histopathological characteristics such as their markedly increased fibrous cellularity and calcification [40, 41]. These factors are associated with excessive neointimal proliferation and stent recoil. In this study, the presence of aorto-ostial lesions was not an independent predictor of restenosis in the SES group; however, there were not enough data to conclude whether SES or PES are most effective for patients with these lesions.
To the best of our knowledge, this is the first study to evaluate the impact of the degree of preprocedural glycemic control on the outcomes of diabetic and IGT patients that were treated with DES. Corpus et al. [45] reported that optimal glycemic control was associated with a lower rate of midterm events after PCI, but their report was written before DES had been developed. Ueda et al. [46] reported that A1c was a predictor of MACE after DES implantation in diabetic patients, but they did not mention any association between the type of DES and A1c levels. We consider that this study provides important information about the selection of DES according to preprocedural A1c, and the necessity of preprocedural glycemic control and anti-inflammatory treatment before coronary intervention in diabetic and IGT patients. Further evaluations are required to investigate whether more aggressive risk factor management before coronary intervention would provide benefits for the long-term outcomes of diabetic and IGT patients treated with DES and whether the degree of preprocedural glycemic control influences the outcome of patients treated with next-generation DES.
Study limitations
The present study had several limitations. (1) The study was a single-center, retrospective study, not a randomized controlled trial. (2) The number of study participants was too small to allow us to clarify why the restenosis and TLR rates of the PES subgroup of the optimal glycemic control group were higher than those in the suboptimal glycemic control group (Fig. 1), and why smoking was a positive predictor of restenosis prevention in the PES group (Table 7). (3) PCI was performed using a similar procedure for both stents; however, there were several differences in the clinical and angiographic characteristics of the two stent groups. (4) We did not consider the diabetic morbidity period or the medication being taken by the patients before the intervention.
Conclusion
In the optimal glycemic control group, PES use was an independent predictor of restenosis; on the other hand, in the suboptimal glycemic control group, neither stent was a predictor of restenosis; i.e., it was shown that the superiority of SES compared with PES was attenuated in the hyperglycemic state. Preprocedural A1c (≥7.0%) was an independent predictor of restenosis in the SES group, but not in the PES group. This study will facilitate the selection of DES and highlights the necessity of intensive risk management from preintervention, even in the DES era.
References
Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: Part II: Recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49:643–56.
Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–73.
Kirtane AJ, Ellis SG, Dawkins KD, Colombo A, Grube E, Popma JJ, et al. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008;51:708–15.
Sabate M, Jimenez-Quevedo P, Angiolillo DJ, Gomez-Hospital JA, Alfonso F, Hernandez-Antolin R, et al. Randomized comparison of sirolimus-eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus-eluting stent (DIABETES) trial. Circulation. 2005;112:2175–83.
Ortolani P, Balducelli M, Marzaroli P, Piovaccari G, Menozzi A, Guiducci V, et al. Two-year clinical outcomes with drug-eluting stents for diabetic patients with de novo coronary lesions: results from a real-world multicenter registry. Circulation. 2008;117:923–30.
Dibra A, Kastrati A, Mehilli J, Pache J, Schuhlen H, von Beckerath N, et al. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–70.
Stettler C, Allemann S, Egger M, Windecker S, Meier B, Diem P. Efficacy of drug eluting stents in patients with and without diabetes mellitus: indirect comparison of controlled trials. Heart. 2006;92:650–7.
Kuchulakanti PK, Chu WW, Torguson R, Clavijo L, Wolfram R, Mishra S, et al. Sirolimus-eluting stents versus paclitaxel-eluting stents in the treatment of coronary artery disease in patients with diabetes mellitus. Am J Cardiol. 2006;98:187–92.
Saia F, Piovaccari G, Manari A, Santarelli A, Benassi A, Aurier E, et al. Clinical outcomes for sirolimus-eluting stents and polymer-coated paclitaxel-eluting stents in daily practice: Results from a large multicenter registry. J Am Coll Cardiol. 2006;48:1312–8.
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23.
Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31.
Patterson C, Mapera S, Li HH, Madamanchi N, Hilliard E, Lineberger R, et al. Comparative effects of paclitaxel and rapamycin on smooth muscle migration and survival: role of AKT-dependent signaling. Arterioscler Thromb Vasc Biol. 2006;26:1473–80.
Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193–202.
Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol Int. 2010;1:2–20.
Morice MC, Colombo A, Meier B, Serruys P, Tamburino C, Guagliumi G, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295:895–904.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
Feld S, Almagor Y, Vaughn WK, Leon MB, Serruys PW. Predictors of clinical outcome following NIR stent implantation for coronary artery disease: analysis of the results of the First International New Intravascular Rigid-Flex Endovascular Stent Study (FINESS trial). J Interv Cardiol. 2002;15:1–6.
Schofer J, Schluter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. 2003;362:1093–9.
Schampaert E, Cohen EA, Schluter M, Reeves F, Traboulsi M, Title LM, et al. The canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol. 2004;43:1110–5.
Fajadet J, Morice MC, Bode C, Barragan P, Serruys PW, Wijns W, et al. Maintenance of long-term clinical benefit with sirolimus-eluting coronary stents: three-year results of the RAVEL trial. Circulation. 2005;111:1040–4.
Colombo A, Drzewiecki J, Banning A, Grube E, Hauptmann K, Silber S, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–94.
Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–7.
Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, et al. A randomized comparison of sirolimus- versus paclitaxel-eluting stent implantation in patients with diabetes mellitus. J Am Coll Cardiol. 2008;52:727–33.
Buch AN, Javaid A, Steinberg DH, Roy P, Pinto Slottow TL, Xue Z, et al. Outcomes after sirolimus- and paclitaxel-eluting stent implantation in patients with insulin-treated diabetes mellitus. Am J Cardiol. 2008;101:1253–8.
Mahmud E, Bromberg-Marin G, Palakodeti V, Ang L, Creanga D, Demaria AN. Clinical efficacy of drug-eluting stents in diabetic patients: a meta-analysis. J Am Coll Cardiol. 2008;51:2385–95.
The DECODE study group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–21.
Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577–83.
Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–4.
Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–45.
Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56.
Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–33.
Hong SJ, Kim MH, Ahn TH, Ahn YK, Bae JH, Shim WJ, et al. Multiple predictors of coronary restenosis after drug-eluting stent implantation in patients with diabetes. Heart. 2006;92:1119–24.
Buffon A, Liuzzo G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi AG, et al. Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999;34:1512–21.
Paiva MS, Serrano CV Jr, Nicolau JC, Jalbut BO, Fernandes JL, De Lemos JA, et al. Differences in the inflammatory response between patients with and those without diabetes mellitus after coronary stenting. J Interv Cardiol. 2008;21:403–9.
Kim JY, Ko YG, Shim CY, Park S, Hwang KC, Choi D, et al. Comparison of effects of drug-eluting stents versus bare metal stents on plasma C-reactive protein levels. Am J Cardiol. 2005;96:1384–8.
Billinger M, Beutler J, Taghetchian KR, Remondino A, Wenaweser P, Cook S, et al. Two-year clinical outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents in diabetic patients. Eur Heart J. 2008;29:718–25.
Takagi T, Okura H, Kobayashi Y, Kataoka T, Taguchi H, Toda I, et al. A prospective, multicenter, randomized trial to assess efficacy of pioglitazone on in-stent neointimal suppression in type 2 diabetes: POPPS (Prevention of In-Stent Neointimal Proliferation by Pioglitazone Study). JACC Cardiovasc Interv. 2009;2:524–31.
Miyauchi K, Kasai T, Yokayama T, Aihara K, Kurata T, Kajimoto K, et al. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ J. 2008;72:832–8.
Otsuka M, Toyofuku M, Watanabe N, Motoda C, Kawase T, Takeda R, et al. Clinical usefulness of drug-eluting stents in the treatment of dialysis patients with coronary artery disease. EuroIntervention. 2011;6:754–9.
Tsuchida K, Daemen J, Tanimoto S, Garcia–Garcia HM, Kukreja N, Vaina S, et al. Two-year outcome of the use of paclitaxel-eluting stents in aorto-ostial lesions. Int J Cardiol. 2008;129:348–53.
Applegate RJ, Draughn T, Yarbrough WD, Little WC. Restenosis of a sirolimus drug-eluting stent: aorto-ostial involvement of the proximal right coronary artery. Catheter cardiovasc interv. 2006;67:391–5.
Nakagawa Y, Kimura T, Morimoto T, Nomura M, Saku K, Haruta S, et al. Incidence and risk factors of late target lesion revascularization after sirolimus-eluting stent implantation (3-year follow-up of the j-Cypher Registry). Am J Cardiol. 2010;106:329–36.
Bocksch W, Fateh-Moghadam S, Mueller E, Huehns S, Waigand J, Dietz R. Percutaneous coronary intervention in patients with end-stage renal disease. Kidney Blood Press Res. 2005;28:275–9.
Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109:1085–8.
Corpus RA, George PB, House JA, Dixon SR, Ajluni SC, Devlin WH, et al. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol. 2004;43:8–14.
Ueda H, Mitsusada N, Harimoto K, Miyawaki M, Yasuga Y, Hiraoka H. Glycosylated hemoglobin is a predictor of major adverse cardiac events after drug-eluting stent implantation in patients with diabetes mellitus. Cardiology. 2010;116:51–7.
Acknowledgments
The authors gratefully acknowledge all the nursing staff, clinical technologists, and radiologists in our catheter laboratory room for their contributions to the study and Tomomi Yamada, PhD (Department of Translational Medical Science, Mie University Graduate School of Medicine), for performing the statistical analysis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawai, T., Miyahara, M., Nakajima, H. et al. The impact of preprocedural hemoglobin A1c on the efficacy of sirolimus-eluting and paclitaxel-eluting stents. Cardiovasc Interv and Ther 27, 72–83 (2012). https://doi.org/10.1007/s12928-012-0095-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-012-0095-2