Abstract
Pioglitazone has superior antiatherosclerotic effects compared with other classes of antidiabetic agents, and there is substantial evidence that pioglitazone improves cardiovascular (CV) outcomes. However, there is also a potential risk of worsening heart failure (HF). Therefore, it is clinically important to determine whether pioglitazone is safe in patients with type 2 diabetes mellitus (T2DM) who require treatment for secondary prevention of CV disease, since they have an intrinsically higher risk of HF. This prospective, multicenter, open-label, randomized study investigated the effects of pioglitazone on cardiometabolic profiles and CV safety in T2DM patients undergoing elective percutaneous coronary intervention (PCI) using bare-metal stents or first-generation drug-eluting stents. A total of 94 eligible patients were randomly assigned to either a pioglitazone or conventional (control) group, and pioglitazone was started the day before PCI. Cardiometabolic profiles were evaluated before PCI and at primary follow-up coronary angiography (5–8 months). Pioglitazone treatment reduced HbA1c levels to a similar degree as conventional treatment (pioglitazone group 6.5 to 6.0%, P < 0.01; control group 6.5 to 5.9%, P < 0.001), without body weight gain. Levels of high-molecular weight adiponectin increased more in the pioglitazone group than the control group (P < 0.001), and the changes were irrespective of baseline glycemic control. Furthermore, pioglitazone significantly reduced plasma levels of natriuretic peptides and preserved cardiac systolic and diastolic function (assessed by echocardiography) without incident hospitalization for worsening HF. The incidence of clinical adverse events was also comparable between the groups. These results indicate that pioglitazone treatment before and after elective PCI may be tolerable and clinically safe and may improve cardiometabolic profiles in T2DM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic cardiovascular (CV) complications are a major cause of mortality and morbidity in patients with type 2 diabetes mellitus (T2DM) [1, 2]. Despite a decreased risk of stent-related adverse events (in-stent restenosis (ISR) and thrombosis) due to recent technical progress in percutaneous coronary intervention (PCI) and the use of drug-eluting stents (DESs) rather than bare-metal stents (BMSs), T2DM is still a major determinant of short- and long-term adverse clinical outcomes in patients with T2DM who undergo coronary stent implantation [3,4,5,6].
Recent studies showed that insulin resistance was associated with ISR and the late “catch-up” phenomenon after DES implantation [7, 8]. Furthermore, Uetani et al. [9] reported that insulin resistance was also associated with post-procedural myocardial injury and an increased risk of adverse clinical outcomes in patients who underwent elective PCI with DES implantation. These results suggest that insulin resistance is surely a therapeutic target to improve clinical outcomes, especially in patients with T2DM who require secondary prevention of CV disease.
Pioglitazone, one of the thiazolidinediones, is an antidiabetic agent that acts as an agonist of peroxisome proliferator-activated receptor-gamma (PPARγ) and enhances insulin sensitivity [10, 11]. Previous studies showed that pioglitazone exerted clinically important antiinflammatory and antiatherogenic effects that were partially independent of its glucose-lowering effect [12,13,14,15,16]. Subsequent studies also showed that pioglitazone could reduce coronary plaque burden [17, 18], arterial inflammation [19], neointimal hyperplasia after stent (mainly BMS) implantation, and ISR [20,21,22]. These results suggest that pioglitazone treatment has a beneficial role in patients with T2DM undergoing elective PCI. However, little is known about the immediate and chronic effects of treatment with pioglitazone prior to elective PCI on cardiometabolic biomarkers and cardiac function. Our goal was to investigate whether pioglitazone treatment before and after elective PCI in Japanese patients with T2DM is safe and can improve cardiometabolic profiles.
Methods
Study design and population
This study was a prospective, multicenter, open-label, randomized study to investigate the effect of pioglitazone pretreatment on cardiometabolic biomarkers and long-term safety in patients with T2DM undertaking elective PCI.
Between February 2006 and October 2008, patients with T2DM, who undertook elective PCI for coronary artery disease (CAD) and had a medical history of documented T2DM with HbA1c < 10%, were enrolled in the study. Patients who were diagnosed with T2DM according to the local guideline prior to PCI, were also recruited. Exclusion criteria were the presence of symptomatic heart failure (HF) and serious liver or renal dysfunction at the time of elective PCI. In addition, patients were also excluded if they were on insulin therapy, or had a contraindication to pioglitazone according to the label.
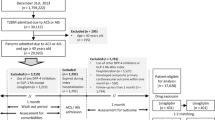
The study design is shown in Fig. 1. Prior to elective PCI, eligible patients were randomly assigned to either a pioglitazone (15–30 mg daily) or a conventional therapy (control) group (1:1) using a non-biased table of random numbers. In the pioglitazone group, pioglitazone was initiated on the evening before the PCI procedure and was continued thereafter with morning administration. If the patients could tolerate pioglitazone treatment, the dose was increased to 30 mg daily. In contrast, when any adverse side effect was documented, a decrease in dose or discontinuance of pioglitazone was permitted. In patients who were assigned to the control group, the doses of other antidiabetic agents could be increased, or new agents added if glycemic goal (HbA1c < 7.0%) could not be achieved. In both treatment groups, the background medical treatments for diabetes, hypertension, dyslipidemia or other conditions were continued during the study period, if their medical condition was not compromised by such an approach. However, if participants could not achieve their goals of risk factors, such as blood pressure, glycemic or lipid profiles, it was allowed to newly add agents or increase the doses of background drugs according to the relevant treatment guidelines by study investigators. In both treatment groups, the background medical treatments for diabetes, hypertension, dyslipidemia or other conditions were continued during the study period. The study protocol was approved by the local institutional review boards and independent ethics committees, and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to their participation in the study.
Measurements
At study entry, the following demographic variables were recorded: age, sex, body mass index (BMI), blood pressure, smoking status, previous medical history, and the use of medications. Blood and/or urine samples were obtained prior to the administration of pioglitazone on the day before elective PCI (Fig. 1). They were also obtained 48 h after PCI and at follow-up coronary angiography (CAG) (5–8 months). The specific biomarkers that were evaluated included high-sensitive C-reactive protein (hs-CRP), pentraxin3 (PTX3), monocyte chemotactic protein-1 (MCP-1), regulated on activation normal T cell expressed and secreted (RANTES), interleukin-8 (IL-8), and high-molecular weight (HMW)-adiponectin. Where appropriate, these biomarkers were evaluated at the central laboratories (Roche Diagnostics Ltd., Tokyo, Japan; Perseus Proteomics Inc., Tokyo, Japan; Department of Cardiovascular Medicine, Saga University, Saga Japan). The cardiac function biomarkers, brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), were analyzed at baseline, 48 h, and 5–8 months. Left ventricular (LV) systolic and diastolic function parameters were evaluated by echocardiography at baseline and follow-up CAG.
Study endpoints
The primary endpoints of the study were changes in specific cardiometabolic profiles, evaluated at 48 h after PCI and at follow-up CAG. Secondary endpoints were changes from baseline in the following parameters at follow-up CAG: (1) blood pressure; (2) body weight; (3) glycemic control (HbA1c and fasting plasma glucose); (4) biochemical tests (lipid profiles); (5) renal function parameters (estimated glomerular filtration rate (eGFR)), and creatinine-corrected urinary albumin excretion; and (6) echocardiographic cardiac function parameters. Other endpoints included the following: major adverse cardiac events (MACE: CV death and non-fatal myocardial infarction); coronary revascularization; HF requiring hospitalization; and major adverse effects of pioglitazone treatment, such as peripheral edema and hypoglycemia.
Statistical analysis
Categorical variables are shown as numbers (percentage) and were compared using a Chi-square test or Fisher’s exact test, where appropriate. Continuous variables are expressed as the median [IQR] and were compared between the pioglitazone and control groups using a Mann–Whitney U test for skewed distributions. A Wilcoxon signed rank test was used to compare changes from baseline to 48 h or follow-up CAG in each group. All tests were two-tailed, and P values of < 0.05 were considered statistically significant. All the analyses were conducted using the R Statistical Software (version 3.3.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics at baseline
Among 94 patients (pioglitazone group, N = 48; control group, N = 46) registered into the study, 80 patients (pioglitazone group, N = 42; control group, N = 38) received an initial check-up and underwent elective PCI at baseline. There were 71 patients (pioglitazone group, N = 39; control group, N = 32) who had follow-up CAG and completed the study (Fig. 2). In the pioglitazone group, 6 patients were treated with 30 mg of pioglitazone at follow-up CAG, and most of patients received 15 mg of pioglitazone during the study period.
The demographic characteristics of the 80 patients at baseline are shown in Table 1. The prevalence of male sex and current smoking in the pioglitazone group showed a tendency to be higher than those values in the control group, although there was no significant difference between the groups. In both groups, approximately 40% of the patients were receiving treatment for secondary prevention of CV disease prior to the elective PCI. The majority of patients in both groups were on antiplatelet therapy, and two-thirds of the patients were on renin–angiotensin system inhibitors and statin therapy at baseline. Sulfonylureas were more often administered in the pioglitazone group, whereas the use of other antidiabetic agents was comparable between the groups.
The clinical characteristics of elective PCI are shown in Table 2. Twenty-five patients (59.5%) in the pioglitazone group and 21 patients (55.3%) in the control group underwent elective PCI due to stable angina pectoris. The frequency of patients who had multivessel coronary disease was higher in the pioglitazone group than in the control group. Stents were implanted in all the patients during elective PCI (BMSs: 7 [16.7%] in the pioglitazone group, 8 [21.1%] in the control group; first-generation DESs: 35 [83.3%] in the pioglitazone group, 35 [92.1%] in the control group).
Clinical and laboratory results
Table 3 shows baseline data and the changes from baseline in the clinical and laboratory parameters at 48 h and at follow-up CAG. At follow-up CAG, BMI was unchanged in the pioglitazone group and reduced in the control group. Systolic and diastolic blood pressures were comparable at baseline between the groups, and the changes in these two variables from baseline to follow-up CAG were also similar. The median levels of HbA1c were decreased significantly at follow-up CAG in both groups (pioglitazone group, 6.5 to 6.0%, P < 0.01; control group, 6.5 to 5.9%, P < 0.001). In the pioglitazone group, lipid profiles improved, and liver enzymes decreased significantly. Interestingly, although creatinine-corrected urinary albumin excretion increased significantly in the control group, such increase was less evident in the pioglitazone group.
Table 4 shows the baseline values of the specific biomarkers and changes from baseline to 48 h and follow-up CAG. The trend of serial changes in the levels of hs-CRP, PTX3, MCP-1, and RANTES were comparable between the groups. IL-8 decreased significantly at follow-up CAG in the pioglitazone group, but not in the control group. The levels of HMW-adiponectin at baseline were comparable between the treatment groups. Although HMW-adiponectin decreased significantly 48 h after primary elective PCI in the control group, it was unchanged at 48 h in the pioglitazone group. At follow-up CAG, the level of HMW-adiponectin was significantly higher in the pioglitazone group than in the control group, and the magnitude of the increase in HMW-adiponectin from baseline was also significantly larger in the pioglitazone group than in the control group (Table 4; Fig. 3a). When patients were divided into two subgroups according to the median value of HbA1c at baseline [HbA1c < 6.5%: N = 20 (pioglitazone), N = 18 (control); HbA1c ≥ 6.5%: N = 22 (pioglitazone), N = 20 (control)], both subgroups showed a significant increase in HMW-adiponectin at follow-up CAG, and the increase was greater in the pioglitazone group than in the control group (Fig. 3b, c). Furthermore, in the subgroup with baseline HbA1c ≥ 6.5%, HMW-adiponectin at 48 h increased significantly in the pioglitazone group, whereas it decreased significantly in the control group (Fig. 3c).
CV function
Table 5 shows baseline data and changes from baseline in the CV functional parameters. The median values of BNP and NT-proBNP at 48 h were higher than those at baseline; however, the changes from baseline in these parameters were not significant in either group. At follow-up CAG, the plasma levels of BNP and NT-proBNP were comparable between the groups; however, only the levels in the pioglitazone group were significantly reduced from baseline, but not increased. The systolic (LV ejection fraction) and diastolic (peak early diastolic LV velocity/peak atrial velocity ratio and deceleration time) function parameters as assessed by echocardiography also showed no significant changes from baseline at follow-up CAG, and there were no significant differences between the groups.
Clinical safety
In both groups, all the patients received successful PCI procedures and exhibited no procedural complications at elective PCI. However, a patient in the pioglitazone group developed subacute stent thrombosis 3 days after elective PCI and dropped out of the study (Fig. 2). No other CV complications during the acute phase after elective PCI were observed in either group. Long-term clinical events at follow-up CAG were judged by each local investigator, and the result is summarized in Table 6. There were no deaths or MACE in either group during the study interval. Five patients (12.8%) in the pioglitazone group and 5 patients (15.6%) in the control group needed coronary revascularization, including target lesion revascularization (TLR) at follow-up CAG. There was one case of HF that required hospitalization in the control group, but none in the pioglitazone group. In the pioglitazone group, one patient suffered from peripheral leg edema, and another patient demonstrated an episode of hypoglycemia; therefore, pioglitazone was stopped in these two patients at 1 month and 4 months, respectively.
Discussion
The major findings of this study are as follows: (1) pioglitazone treatment reduced HbA1c levels to a similar degree as conventional treatment, without body weight gain; (2) pioglitazone treatment increased HMW-adiponectin levels more than conventional treatment, irrespective of glycemic control; (3) pioglitazone treatment did not increase plasma levels of natriuretic peptides rather significantly reduced them, and preserved cardiac performance as assessed by echocardiography without incident hospitalization for worsening HF; (4) pioglitazone treatment starting prior to elective PCI and continuing until primary follow-up CAG may be safe, and the incidence of clinical adverse events was comparable between the groups. To our knowledge, the present study was the first to demonstrate the CV safety, including cardiac function, and efficacy of pioglitazone in Japanese patients with T2DM undergoing elective PCI with first-generation DESs.
It is well known that diabetes is a strong predictor of ISR and worse clinical outcomes in patients with CAD who undergo stent implantation [23, 24]. Previous meta-analysis clearly showed the efficacy of pioglitazone treatment in decreasing ISR and revascularization after BMS implantation in patients with T2DM [25]. Hong et al. [26] also performed intravascular ultrasound and reported that pioglitazone reduced neointimal hyperplasia at the site of stented lesions and plaque burden in the stented segment at 8 months after DES implantation. This suggests that inhibition of unfavorable cellular and molecular actions during the acute phase after stent implantation should be, at least in part, critical for CV safety during the chronic phase. Therefore, in the present study, we administered pioglitazone 1 day prior to stent implantation and then assessed its immediate and chronic effects on cardiometabolic profiles and cardiac function.
Among antidiabetic agents, pioglitazone has the most established evidence of an antiatherosclerotic effect in experimental and human studies. Even short-term (4 weeks) pioglitazone treatment improved vascular endothelial function as assessed by flow-mediated dilation in patients with T2DM, and this was independent of changes in metabolic factors [27]. Pioglitazone significantly attenuated carotid intima–media thickness progression compared with standard therapy, and this effect was also independent of cardiometabolic risk factors [13, 15]. In the coronary arteries, the PERISCOPE trial showed significant attenuation of plaque volume progression by pioglitazone in patients with uncontrolled T2DM [17]. Furthermore, Nitta et al. [19] reported that pioglitazone, compared with glimepiride, decreased coronary artery inflammation depicted by (18)F-fluorodeoxyglucose-positron emission tomography combined with computed tomography angiography, and this was accompanied by a decrease in serum levels of hs-CRP. Also in our study, pioglitazone significantly reduced hs-CRP levels at follow-up CAG by the same amount as conventional treatment. Hong et al. [26] reported that pioglitazone immediately regulated immunological and inflammatory responses via suppression of interleukin 6 and MCP-1-C-C chemokine receptor type 2. The present study also showed that pioglitazone significantly decreased MCP-1 levels at 48 h after stent implantation, but not at follow-up CAG. Furthermore, serum levels of IL-8, which acts as an inflammatory-related mediator to recruit neutrophils into inflammatory sites [28], was significantly decreased by pioglitazone at follow-up CAG. In addition, Igarashi et al. [29] reported that Japanese patients with uncontrolled T2DM treated with pioglitazone for 4 months had reduced serum levels of inflammatory and atherogenic biomarkers, such as including remnant-like particle-cholesterol and tumor necrosis factor-alpha. Given these findings, it appears that pioglitazone can favorably alter vascular remodeling and attenuate local and systemic inflammatory responses in both the early and chronic phase after stent implantation, leading to CV protection in addition to amelioration of metabolic condition.
Adiponectin, which is a major adipocyte-secreted protein, plays a protective role in regulating fat and glucose metabolism [30]. Several studies demonstrated that pioglitazone increases adiponectin levels via PPARγ-mediated AMP-activated protein kinase and STAT3 phosphorylation, and subsequently enhances insulin sensitivity [31,32,33,34]. An increase in adiponectin levels by pioglitazone treatment was also found in our study, irrespective of glycemic control, and this result was similar to the findings of Otto et al. [35]. Recent animal studies demonstrated that pioglitazone suppressed neointimal formation and vascular smooth muscle cell proliferation via both adiponectin-dependent and adiponectin-independent mechanisms [36]. Furthermore, pioglitazone decreased coronary neointimal hyperplasia, accompanied by increases in circulating microRNA-24 in patients with T2DM [37]. In addition, Pistrosch et al. [38] reported that rosiglitazone, another thiazolidinediones, lessened renal damages and reduced urinary albumin excretion via alterations in intrarenal endothelial function and renal hemodynamics in patients with T2DM and microalbuminuria, although no significant reduction in UACR was observed in our study subjects who were largely normoalbuminuric. Thus, further studies are warranted to elucidate the precise molecular mechanisms underlying the adiponectin-independent pathways of pioglitazone.
In multiple observational studies, the use of pioglitazone in primary care was associated with a reduction in the risk of all-cause mortality and CV events [39,40,41]. In patients who require secondary prevention of CV disease, previous studies have shown that pioglitazone improved atherosclerotic CV outcomes [42, 43]. A recent meta-analysis to assess the clinical efficacy of pioglitazone on secondary prevention of CV disease also demonstrated similar results, although pioglitazone did not lower the risk of all-cause mortality, and it increased the risk of HF [44]. However, there seems to be controversy on the clinical impact of pioglitazone on incident HF [44,45,46,47,48]. Previous literature clearly demonstrated that pioglitazone was not associated with unfavorable effects on cardiac structure and function, but there were increases in renal sodium reabsorption with subsequent systemic fluid retention and manifestations of edema [49, 50]. In a mouse model of myocardial infarction, pioglitazone treatment significantly reduced cardiac injury, and this effect was partially mediated by a PPARγ-independent pathway [51, 52]. Furthermore, some studies showed that pioglitazone improved cardiac function [53,54,55]. It is well recognized that patients with both diabetes and CAD have a substantially higher risk of HF compared with those who have either CAD or diabetes alone [56]. Accordingly, pioglitazone treatment in patients who needed secondary prevention of CV disease had a 33% increased risk of HF, whereas there was no increased risk of HF in patients treated for primary prevention of CV disease [44, 57]. In our study, where all participants were treated for secondary prevention of CV disease, pioglitazone treatment did not exacerbate cardiac dysfunction and did not increase the natriuretic peptides and development of HF. In particular, it would be clinically meaningful to prove that pioglitazone treatment is safe before and after elective PCI in patients with T2DM.
This study has several limitations that may impact the interpretation of the results. First, there were considerable problems with the methodology, such as small sample size, simple randomization method, and lack of formal trial registration and independent event assessment committee. Therefore, it may be difficult to draw a strong clinical conclusion. Second, the present study was performed by a PROBE design, but it was not a placebo-controlled study. Therefore, unexpected bias might be introduced in the assessment of results. In addition, there were substantial dropouts from the study and many missing data especially at follow-up CAG, as shown in figure and each table. Third, we could not obtain clinical information, such as stent diameter/length, maximum inflation pressure, lesion length, plaque volume, and the degree of stenosis, at elective PCI or follow-up CAG. Hence, the effect of pioglitazone on in-stent neointimal formation was not evaluated in our study. Fourth, we could not obtain the data on serum insulin concentration and insulin resistance index, such as the homeostasis model assessment-insulin resistance. Lastly, the participants in our study were recruited from February 2006 to October 2008. Although second-generation DESs are currently used in most PCI procedures in Japan, our study included BMSs and first-generation DESs. Furthermore, newer antidiabetic agents, such as incretin-based agents, were not used. Finally, because our study was designed to evaluate the effects of pioglitazone on CV safety for a limited period from elective PCI to primary follow-up CAG in a small number of participants, further studies are needed to assess the longitudinal CV safety of pioglitazone in patients undergoing PCI.
In summary, the present study demonstrated that pioglitazone treatment prior to elective PCI improved glycemic control without significant body weight gain, and it may be safe for CV function in Japanese patients with T2DM. Furthermore, pioglitazone treatment increased HMW-adiponectin levels more than conventional therapy, irrespective of glycemic control at baseline.
References
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors Collaboration (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841
Li N, Yang YG, Chen MH (2016) Comparing the adverse clinical outcomes in patients with non-insulin treated type 2 diabetes mellitus and patients without type 2 diabetes mellitus following percutaneous coronary intervention: a systematic review and meta-analysis. BMC Cardiovasc Disord 16:238
Park KW, Lee JM, Kang SH, Ahn HS, Kang HJ, Koo BK, Rhew JY, Hwang SH, Lee SY, Kang TS, Kwak CH, Hong BK, Yu CW, Seong IW, Ahn T, Lee HC, Lim SW, Kim HS (2014) Everolimus-eluting Xience v/Promus versus zotarolimus-eluting resolute stents in patients with diabetes mellitus. JACC Cardiovasc Interv 7:471–481
Tada T, Kimura T, Morimoto T, Ono K, Furukawa Y, Nakagawa Y, Nakashima H, Ito A, Siode N, Namura M, Inoue N, Nishikawa H, Nakao K, Mitsudo K, j-Cypher Registry Investigators (2011) Comparison of three-year clinical outcomes after sirolimus-eluting stent implantation among insulin-treated diabetic, non-insulin-treated diabetic, and non-diabetic patients from j-Cypher registry. Am J Cardiol 107:1155–1162
Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, Midwall J, Simonton CA, Keim E, Wang P, Kuntz RE, Moses JW (2004) Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation 109:2273–2278
Komatsu T, Komatsu S, Nakamura H, Kuroyanagi T, Fujikake A, Hisauchi I, Sakuma M, Nakahara S, Sakai Y, Taguchi I (2016) Insulin resistance as a predictor of the late catch-up phenomenon after drug-eluting stent implantation. Circ J 80:657–662
Zhao LP, Xu WT, Wang L, Li H, Shao CL, Gu HB, Chan SP, Xu HF, Yang XJ (2015) Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron Artery Dis 26:5–10
Uetani T, Amano T, Harada K, Kitagawa K, Kunimura A, Shimbo Y, Harada K, Yoshida T, Kato B, Kato M, Marui N, Nanki M, Hotta N, Ishii H, Matsubara T, Murohara T (2012) Impact of insulin resistance on post-procedural myocardial injury and clinical outcomes in patients who underwent elective coronary interventions with drug-eluting stents. JACC Cardiovasc Interv 5:1159–1167
Quinn CE, Hamilton PK, Lockhart CJ, McVeigh GE (2008) Thiazolidinediones: effects on insulin resistance and the cardiovascular system. Br J Pharmacol 153:636–645
Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, Cusi K, Mandarino LJ, DeFronzo RA (2001) Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care 24:710–719
Pfutzner A, Marx N, Lubben G, Langenfeld M, Walcher D, Konrad T, Forst T (2005) Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol 45:1925–1931
Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D’Agostino RB Sr, Perez A, Provost JC, Haffner SM (2006) Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296:2572–2581
Permana PA, Zhang W, Wabitsch M, Fischer-Posovszky P, Duckworth WC, Reaven PD (2009) Pioglitazone reduces inflammatory responses of human adipocytes to factors secreted by monocytes/macrophages. Am J Physiol Endocrinol Metab 296:E1076–E1084
Saremi A, Schwenke DC, Buchanan TA, Hodis HN, Mack WJ, Banerji M, Bray GA, Clement SC, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Tripathy D, DeFronzo RA, Reaven PD (2013) Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler Thromb Vasc Biol 33:393–399
DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD, ACT NOW Study (2011) Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 364:1104–1115
Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochellière R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM, PERISCOPE Investigators (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299:1561–1573
Nakayama T, Komiyama N, Yokoyama M, Namikawa S, Kuroda N, Kobayashi Y, Komuro I (2010) Pioglitazone induces regression of coronary atherosclerotic plaques in patients with type 2 diabetes mellitus or impaired glucose tolerance: a randomized prospective study using intravascular ultrasound. Int J Cardiol 138:157–165
Nitta Y, Tahara N, Tahara A, Honda A, Kodama N, Mizoguchi M, Kaida H, Ishibashi M, Hayabuchi N, Ikeda H, Yamagishi S, Imaizumi T (2013) Pioglitazone decreases coronary artery inflammation in impaired glucose tolerance and diabetes mellitus: evaluation by FDG-PET/CT imaging. JACC Cardiovasc Imaging 6:1172–1182
Takagi T, Okura H, Kobayashi Y, Kataoka T, Taguchi H, Toda I, Tamita K, Yamamuro A, Sakanoue Y, Ito A, Yanagi S, Shimeno K, Waseda K, Yamasaki M, Fitzgerald PJ, Ikeno F, Honda Y, Yoshiyama M, Yoshikawa J, POPPS Investigators (2009) A prospective, multicenter, randomized trial to assess efficacy of pioglitazone on in-stent neointimal suppression in type 2 diabetes: POPPS (Prevention of In-Stent Neointimal Proliferation by Pioglitazone Study). JACC Cardiovasc Interv 2:524–531
Zhao SJ, Zhong ZS, Qi GX, Shi LY, Chen L, Tian W (2016) Effect of pioglitazone in preventing in-stent restenosis after percutaneous coronary intervention in patients with type 2 diabetes: a meta-analysis. PLoS One 11:e0155273
Marx N, Wohrle J, Nusser T, Walcher D, Rinker A, Hombach V, Koenig W, Hoher M (2005) Pioglitazone reduces neointima volume after coronary stent implantation: a randomized, placebo-controlled, double-blind trial in nondiabetic patients. Circulation 112:2792–2798
Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A (2014) Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 100:153–159
Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, Schönberger JP, Buller N, Bonser R, Disco C, Backx B, Hugenholtz PG, Firth BG, Unger F (2005) Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 46:575–581
Patel D, Walitt B, Lindsay J, Wilensky RL (2011) Role of pioglitazone in the prevention of restenosis and need for revascularization after bare-metal stent implantation: a meta-analysis. JACC Cardiovasc Interv 4:353–360
Hong SJ, Kim ST, Kim TJ, Kim EO, Ahn CM, Park JH, Kim JS, Lee KM, Lim DS (2010) Cellular and molecular changes associated with inhibitory effect of pioglitazone on neointimal growth in patients with type 2 diabetes after zotarolimus-eluting stent implantation. Arterioscler Thromb Vasc Biol 30:2655–2665
Martens FM, Visseren FL, de Koning EJ, Rabelink TJ (2005) Short-term pioglitazone treatment improves vascular function irrespective of metabolic changes in patients with type 2 diabetes. J Cardiovasc Pharmacol 46:773–778
Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K (1994) Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 56:559–564
Igarashi M, Hirata A, Yamaguchi H, Jimbu Y, Tominaga M (2008) Pioglitazone reduces atherogenic outcomes in type 2 diabetic patients. J Atheroscler Thromb 15:34–40
Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y (2002) Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106:2767–2770
Kudoh A, Satoh H, Hirai H, Watanabe T (2011) Pioglitazone upregulates adiponectin receptor 2 in 3T3–L1 adipocytes. Life Sci 88:1055–1062
Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M, Madiraju M, Jenkinson CP, Cersosimo E, Musi N, Defronzo RA (2009) Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 52:723–732
Kanatani Y, Usui I, Ishizuka K, Bukhari A, Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T, Kobayashi M (2007) Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes 56:795–803
Pereira RI, Leitner JW, Erickson C, Draznin B (2008) Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3′-kinase. Life Sci 83:638–643
Otto C, Otto B, Goke B, Pfeiffer AF, Lehrke M, Vogeser M, Spranger J, Parhofer KG (2006) Increase in adiponectin levels during pioglitazone therapy in relation to glucose control, insulin resistance as well as ghrelin and resistin levels. J Endocrinol Investig 29:231–236
Kubota T, Kubota N, Sato H, Inoue M, Kumagai H, Iwamura T, Takamoto I, Kobayashi T, Moroi M, Terauchi Y, Tobe K, Ueki K, Kadowaki T (2016) Pioglitazone ameliorates smooth muscle cell proliferation in cuff-induced neointimal formation by both adiponectin-dependent and -independent pathways. Sci Rep 6:34707
Hong SJ, Choi SC, Cho JY, Joo HJ, Park JH, Yu CW, Lim DS (2015) Pioglitazone increases circulating microRNA-24 with decrease in coronary neointimal hyperplasia in type 2 diabetic patients- optical coherence tomography analysis. Circ J 79:880–888
Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P (2005) Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 54:2206–2211
Strongman H, Korhonen P, Williams R, Bahmanyar S, Hoti F, Christopher S, Majak M, Kool-Houweling L, Linder M, Dolin P, Heintjes EM (2017) Pioglitazone and risk of mortality in patients with type 2 diabetes: results from a European multidatabase cohort study. BMJ Open Diabetes Res Care 5:e000364
Yokoyama H, Araki S, Kawai K, Hirao K, Oishi M, Sugimoto K, Sone H, Maegawa H, Kashiwagi A (2015) Pioglitazone treatment and cardiovascular event and death in subjects with type 2 diabetes without established cardiovascular disease (JDDM 36). Diabetes Res Clin Pract 109:485–492
Hippisley-Cox J, Coupland C (2016) Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ 354:i3477
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J, PROactive Investigators (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR, Trial Investigators IRIS (2016) Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 374:1321–1331
de Jong M, van der Worp HB, van der Graaf Y, Visseren FLJ, Westerink J (2017) Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc Diabetol 16:134
Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B (2017) Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open 7:e013927
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188
Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B (2017) Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis. Stroke 48:388–393
Hernandez AV, Usmani A, Rajamanickam A, Moheet A (2011) Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs 11:115–128
Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R (2004) Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 27:256–263
Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD (2005) Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med 11:861–866
Khodeer DM, Zaitone SA, Farag NE, Moustafa YM (2016) Cardioprotective effect of pioglitazone in diabetic and non-diabetic rats subjected to acute myocardial infarction involves suppression of AGE–RAGE axis and inhibition of apoptosis. Can J Physiol Pharmacol 94:463–476
Birnbaum Y, Long B, Qian J, Perez-Polo JR, Ye Y (2011) Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-gamma-independent manner. Basic Res Cardiol 106:431–446
Sakamoto A, Hongo M, Furuta K, Saito K, Nagai R, Ishizaka N (2013) Pioglitazone ameliorates systolic and diastolic cardiac dysfunction in rat model of angiotensin II-induced hypertension. Int J Cardiol 167:409–415
Ozawa T, Oda H, Oda M, Hosaka Y, Kashimura T, Ozaki K, Tsuchida K, Takahashi K, Miida T, Aizawa Y (2009) Improved cardiac function after sirolimus-eluting stent placement in diabetic patients by pioglitazone: combination therapy with statin. J Cardiol 53:402–409
Terui G, Goto T, Katsuta M, Aoki I, Ito H (2009) Effect of pioglitazone on left ventricular diastolic function and fibrosis of type III collagen in type 2 diabetic patients. J Cardiol 54:52–58
Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A (2000) Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 102:1014–1019
Lu CJ, Sun Y, Muo CH, Chen RC, Chen PC, Hsu CY (2013) Risk of stroke with thiazolidinediones: a ten-year nationwide population-based cohort study. Cerebrovasc Dis 36:145–151
Acknowledgements
This study was partly supported by JSPS KAKENHI Grant Number 17K09510. The authors thank Sae Katafuchi and Aya Yamada for their excellent secretarial assistance.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
HY has received honoraria from Takeda. TU has received honoraria from Daiichi Sankyo, Bayer, Mochida, Boehringer Ingelheim; research funding from Daiichi Sankyo. TI has received honoraria from Mochida and Bayer; and scholarships from Abbott, KAATHU JAPAN, GOODMAN, CLINICO, Shionogi, St. Jude Medical, Daiichi Sankyo, Takeda, Mitsubishi Tanabe, Teijin, Boehringer Ingelheim, Boston Scientific Japan, and UNION TOOL. KNo has received honoraria from Daiichi Sankyo, Merck, Pfizer, Eli Lilly, Amgen, Boehringer Ingelheim, Mitsubishi Tanabe, and Astellas; research funding from Bayer, Teijin, Mitsubishi Tanabe, Astellas, Boehringer Ingelheim, and Asahi Kasei; and scholarships from Astellas, Daiichi Sankyo, Sumitomo Dainippon, Takeda, Mitsubishi Tanabe, and Boehringer Ingelheim. The remaining authors have no financial interests to disclose related to this manuscript.
Rights and permissions
About this article
Cite this article
Tanaka, A., Komukai, S., Shibata, Y. et al. Effect of pioglitazone on cardiometabolic profiles and safety in patients with type 2 diabetes undergoing percutaneous coronary artery intervention: a prospective, multicenter, randomized trial. Heart Vessels 33, 965–977 (2018). https://doi.org/10.1007/s00380-018-1143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1143-3