Abstract
The present study investigates the hepatoprotective effect of a novel zinc-containing drug acyzol in comparison with silymarin, a medicinal extract of milk thistle (Silybum marianum). The hepatoprotective effect was studied in 40 albino nonlinear male rats in a model of toxic liver injury induced by intragastric administration of carbon tetrachloride. Both drugs were diluted in water and administered intragastrically at doses 10 mg/kg (acyzol) and 100 mg/kg (silymarin) for 10 days twice daily, after development of clinical toxic hepatitis. Biochemical and functional indicators of the liver parenchyma demonstrated that both drugs reduced mortality, normalized the body and relative liver weight, reduced intensity of cytolytic, cholestatic, and mesenchymal inflammatory syndromes, and restored liver function. The study demonstrates that acyzol and silymarin have comparable hepatoprotective effect, thus, providing a rationale for the use of acyzol in complex therapy of toxic hepatitis and hepatosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Liver disease is a worldwide health problem and one of the leading causes of mortality. Oxidative stress plays a major role in the development of this disease [1, 2]. Activation of pro-oxidant system and inhibition of antioxidant system in hepatocytes lead to accumulation of free radicals, enhancement of membrane lipid peroxidation (LPO) with further disruption of membrane structures, and violation of cellular self-regulation mechanisms. There are no effective and safe drugs for prevention or treatment of liver disorders [3].
Zinc is an essential trace element that plays a key role in more than 300 enzyme systems and demonstrates protective effect in different animal toxicity models [4, 5]. Zinc is known to reduce hepatotoxic influence of many damaging factors [6]. Acyzol is a novel zinc-containing drug with high bioavailability and excellent safety profile. It has been described as a powerful antidote to acute poisoning with lethal doses of carbon monoxide (carbon monoxide, CO) and other combustion products [7, 8]. Previous experimental and clinical studies also demonstrate a wide range of therapeutic effects of acyzol. In particular, this drug provides anti-inflammatory, reparative, detoxifying, immunomodulative, bacteriostatic, adaptogenic, antioxidant, cardioprotective, and other effects [9, 10]. Powerful antioxidant properties of acyzol provide a rationale for studying its hepatoprotective effects.
The objective of the present study was to investigate the protective effect of zinc-containing drug acyzol using the model of CCl4-induced toxic liver injury in comparison with hepatoprotector silymarin, a medicinal extract of milk thistle (Silybum marianum).
1 Materials and Methods
Our study has been performed in 40 albino nonlinear male rats weighed 200–220 g according to 11th World Medical Association Declaration of Helsinki (1964). The housing, feeding, and handling of animals and their exclusion from the experiment were performed according to the requirements of a national standard of Russian Federation, GOST R-53434-2009 “Principles of Good Laboratory Practice (GLP).” The experiments were performed after 20 days of animals’ adaptation at vivarium. Rats were housed in accordance with standards of Cage Space Guidelines for Animals in ventilated cages at an ambient temperature of 18–20 °C, 60–70 % humidity, and under 12-h light/dark cycle. Food and water were provided ad libitum.
We used an experimental model of toxic liver injury induced by intragastric administration (I/G) of 50 % solution of carbon tetrachloride (CCl4) in olive oil at a dose of 1 ml/kg for 6 days. In 10 days after starting of CCl4 administration, the rats were randomly divided into four groups (10 rats per group): group 1 intact, group 2 received equivalent amount of distilled water, group 3 received acyzol at a dose of 10 mg/kg diluted in water, group 4 received silymarin at a dose of 100 mg/kg. The drugs and water were administered I/G during 10 days twice daily via nontraumatic metal probe.
The evaluation of intensity of cytolytic, cholestatic, and mesenchymal-inflammatory syndromes was performed using Lachema Diagnostika reagents (Czech Republic) according to standard biochemical techniques.
For cytolytic syndrome evaluation, the activity of indicator enzymes, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDG), acid phosphatase, and ceruloplasmin was evaluated. Alkaline phosphatase (ALP) was measured as a cholestasis marker. The levels of total protein, cholesterol, and total lipids were determined. The synthesizing function of liver was estimated by thymol turbidity test, excretory function by measuring bromsulphalein retention (BSP), and total bilirubin in blood. LPO and activity of liver antioxidant system were estimated by the level of reduced glutathione (iodometric titration) and the level of thiol groups in whole blood.
Liver antitoxic function was investigated by quantitative determination of cytochrome P450 in liver microsome suspension using a two-beam spectrophotometer “Perkin Elmer.” The absorbance value of CO complex of reduced hemoproteins at 450 nm was used to assess the amount of cytochrome P450. The amount of cytochrome B5 was calculated from the absorbance difference between its oxidized and reduced forms.
The relative liver weight (absolute liver weight (mg) divided by body weight (g)) was measured on electronic scales 1602 MP, “Sartorius.” This indicator characterizes the liver inflammation intensity.
During the hexenal testing, the rats were intraperitoneally injected with a single dose of hexenal 80 mg/kg. The animal anesthesia duration allowed us to evaluate the rate of hexenal metabolism by cytochrome P450-dependent monooxygenase system of hepatocytes and characterized the intensity of antitoxic protection. In addition to the mentioned indicators, the treatment efficacy was assessed by clinical pattern and animal survival.
The animals were euthanized with Nembutal at a dose of 70 mg/kg, and then the blood and liver samples were taken.
Statistical analysis of results was performed using Student’s t test or Fisher’s test, where appropriate. A p value of less than 0.05 was considered to indicate significant differences.
2 Results and Discussion
About 40 % of rats died in three experimental groups at the start of treatment (day 10). After CCl4 administration, all the experimental animals had clinical features of toxic hepatitis.
Animals from group 2, which received CCl4 without treatment, were characterized by slight dynamics of weight gain, decreased appetite, hypodynamia, lethargy, ruffled fur, dirtiness, and nosebleed. Yellowness of mucous membranes and sclera was also observed. Slight increase of liver size was detected with palpation. To the 15th day of investigation, 70 % of animals from this group died. To the end of treatment (day 20), the rate of survived animals in the third and fourth groups was 60 %, respectively.
The administration of acyzol and silymarin significantly decreased the hepatotoxicity symptoms. Table 1 demonstrates that CCl4 intoxication was accompanied by body weight loss and increase in relative liver weight. The administration of both drugs normalized these indicators.
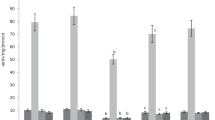
The key hepatic biochemical and functional indicators are shown in Table 2. It is observed that CCl4 intoxication causes disturbance of key liver functions and systems responsible for protein synthesis, detoxification, and lipoprotein synthesis, and these changes are accompanied by biochemical signs of cytolysis. Increased activity of transaminases and phosphatases (Fig. 1), decreased levels of serum total protein and lipids (Fig. 2), and increased levels of bilirubin and ceruloplasmin are also observed. Hepatic levels of reduced glutathione and metabolizing cytochromes were decreased. Liver injury was associated with a decreased functional activity: the hexenal sleep duration was significantly increased, and BSP excretion was slowed down.
The serum levels of total protein, lipids, and cholesterol in CCl4-intoxicated rats without treatment (group 2) and in animals treated with acyzol (group 3) and silymarin (group 4). Significant differences compared to intact animals at p < 0.05 (asterisk). Data are presented as % relative to the intact group (100 %)
The use of acyzol and silymarin significantly improved the overall health of animals, reduced mortality, and significantly normalized liver function, as evidenced by biochemical and functional indicators of liver parenchyma.
3 Conclusions
The results of the present study demonstrate that acyzol and silymarin have comparable hepatoprotective effect, thus, providing a rationale for the use of acyzol in complex therapy of toxic hepatitis and hepatosis.
References
Blachier, M., Leleu, H., Peck-Radosavljevic, M., Valla, D. C., Roudot-Thoraval, F. (2013). The burden of liver disease in Europe: a review of available epidemiological data. Journal of Hepatology, 58, 593–608.
Zhu, R., Wang, Y., Zhang, L., Guo, Q. (2012). Oxidative stress and liver disease. Hepatology Research, 42, 741–749.
Vladimir-Knežević, S., Cvijanović, O., Blažeković, B., Kindl, M., Bival, Š. M., Domitrović, R. (2015). Hepatoprotective effects of Micromeria croatica ethanolic extract against CCl4–induced liver injury in mice. BMC Complementary and Alternative Medicine, 15, 233.
Kelleher, S. L., McCormick, N. H., Velasquez, V., Lopez, V. (2011). Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Advances in Nutrition, 2(2), 101–111.
Malhotra, A., & Dhawan, D. K. (2014). Current view of zinc as a hepatoprotective agent in conditions of chlorpyrifos induced toxicity. Pesticide Biochemistry and Physiology, 112, 1–6.
Liu, J., Zhou, Z. X., Zhang, W., Bell, M. W., Waalkes, M. P. (2009). Changes in hepatic gene expression in response to hepatoprotective levels of zinc. Liver International, 29(8), 1222–1229.
Radionov, I. A., Shantyr, I. I., Barinov, V. A. (2012). The effect of Acyzol on the kinetics of carboxyhemoglobin in firemen. Medical and biological, social and psychological security problems in emergency situations, 2, 11–13.
Barinov A.V., Nechiporenko S.P. (2006). The antidote for carbon monoxide poisoning has been developed. UNIFOR RASHA. 116–117.
Babaniyazova, Z. H., Babaniyazov, H. H., Radionov, I. A., Skalniy, A. V., Bobr, I. S. (2010). Acyzol addressing zinc deficiency states. Trace Elements in Medicine, 11(1), 25–30.
Lebedeva, S. A., Babaniyazova, Z. H., Radionov, I. A., Skalniy, A. A. (2013). Zinc and cobalt metal complexes in regenerative treatment of hypoxic conditions. Journal of Restore Medicine Rehabilitation, 2, 67–69.
Acknowledgments
The work is performed according to the Russian Government Program of Competitive Growth of I.M. Sechenov First Moscow State University and Kazan Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shakhmardanova, S.A., Babaniyazova, Z.H., Tarasov, V.V. et al. Protective Effect of Acyzol in a Model of Carbon Tetrachloride-Induced Hepatotoxicity. BioNanoSci. 7, 329–332 (2017). https://doi.org/10.1007/s12668-016-0352-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0352-4