Abstract
The aim of the present study was to evaluate the hepatoprotective, antioxidant, and anti-inflammatory potential of chebulagic acid on carbon tetrachloride–induced hepatic fibrosis in rats. The liver was damaged in the rats using CCL4 (2 ml/kg b.w dissolved in 20% corn oil) intragastrically twice/week for 8 weeks. After 8 weeks, activities of serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and lactate dehydrogenase, lipid peroxidation markers, and enzymic and non-enzymic antioxidant enzymes, inflammatory and anti-inflammatory cytokines were analyzed and all these parameters were altered. Chebulagic acid administration reversed all the altered parameters to near-normal levels. Improved histological and immunohistochemical observations liver supported the biochemical investigated. Hence, chebulagic acid exhibits hepatoprotective effects against CCl4-induced liver damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is one of the most important organs that play crucial roles in the physiological functions of our body. In the human body, the liver is the site of the regulation of glycogen storage, decomposition of RBCs, hormone and plasma protein production, and detoxification. Since the liver also plays a central role in detoxifying and transforming chemicals, it is in a way exposed to their harmful effects increasing its susceptibility to diseases. Therefore, it may not be surprising that over 10% of the world’s population suffers from liver diseases. The most common of these conditions are hepatitis, hepatic steatosis (fatty liver), fibrosis, cirrhosis, alcoholic, and drug-induced diseases (Latief and Ahmad 2018).
Hepatic fibrosis is a common pathological process resulting from various chronic hepatic injuries, which is characterized by an increase of extracellular matrix deposition in Disse’s space and the imbalance between synthesis and degradation of the extracellular matrix. Accumulating evidence suggests that hepatic fibrosis is a reversible disease; therefore, an effective treatment would probably prevent or reverse the hepatic fibrotic process (Iredale 2008). In recent years, considerably clinical and experimental pieces of evidence show that oxidative stress caused by an imbalance between the oxidant and antioxidant systems of the body in favor of the oxidants should be a major apoptotic stimulus in different types of acute and chronic liver injury and hepatic fibrosis (Ghatak et al. 2011). Although early-phase liver fibrosis is considered, a reversible pathological process, late-stage liver fibrosis will deteriorate to cirrhosis, portal hypertension, or even hepatocellular carcinoma which leads to increased morbidity and mortality.
CCl4 is a potent hepatoxin producing centrilobular hepatic necrosis which is widely used for animal models of liver fibrosis. Hepatic fibrosis induced by CCl4 is associated with the exacerbation of lipid peroxidation and the depletion of antioxidant status (Fu et al. 2008). Accordingly, a number of herbal derivatives show protective effects of their natural antioxidants against hepatic fibrosis and hepatotoxicity either experimentally in cell culture (in vitro), in animals models (in vivo) or even in clinical trials (Frei and Higdon 2003).
Fruits of Terminalia chebula have been used in various Ayurvedic preparations for the treatment of various disorders and its fruit powder is one of the main constituents of Triphala, a well-known Ayurvedic medicine used to treat allergies and common health disorders. Chebulagic acid is one of the main bio-active constituents of Terminalia chebula fruit powder. Chebulagic acid has been shown to inhibit α-glucosidase activity (Gao et al. 2007). ROS generation from PMA (Phorbol 12-myristate 13-acetate)-stimulated leukocytes (Kinoshita et al. 2007) and CTL-mediated cytotoxicity (Hamada et al. 1997). In addition, it has been reported to suppress arthritis in mice (Lee et al. 2005) and LPS-induced nitric oxide (NO) generation in RAW 264.7 mouse macrophage cells (Murakami et al. 2005). However, the detailed molecular anti-inflammatory mechanism has not yet been studied. In the present study, we show for the first time that chebulagic acid inhibits NF-kB activity and phosphorylation of MAP kinases in LPS-stimulated RAW 264.7 macrophages. Recently, chebulagic acid has been reported to have a gastroprotective effect on ethanol-induced gastric injury in rats (Liu et al. 2017). Scientific reports on the effect of chebulagic acid on CCl4-induced hepatic injury in rats are scarce. Therefore, the present study was designed to investigate the hepatoprotective and anti-inflammatory potential of chebulagic acid on carbon tetrachloride–induced hepatic fibrosis by antioxidative activities in rats.
Materials and methods
Chemicals
Carbon tetrachloride (CCL4) and silymarin were purchased from Sigma Chemical Company (St. Louis, MO, USA). Chebulagic acid was procured from Chem Faces, China. All other chemicals are analytical grade procured from SRL.
Experimental animals
Adult Male albino Wistar rats weighing about 200–220 g were obtained from Sri Muthukumaran Medical College Hospital & Research Institute, Mangadu, Chennai, Tamil Nadu, India. Rats were housed in clean, sterile, and polypropylene cages under standard vivarium conditions 12-h light/12-h dark cycle and constant temperature (25 ± 2 °C) with free access to standard commercial rat chow (Pranav Agro Industries Ltd., Pune, Maharashtra, India) and water. The experimental protocol was approved by the Ministry of Social Justices and Empowerment, Government of India, and Institutional Animal Ethics Committee Guidelines (IAEC No: No. 12/09/2016).
Experimental design
A total of 42 rats (30 CCL4-induced hepatic injured rats and 12 normal rats) were used and experimental animals were divided into 7 groups, each group consists of a minimum of six rats (n = 6). Before the start of the experiment, randomly selected animals were tested for liver function. Animals with a normal range of liver function test (LFT) parameters were selected for experimentation and details are given below.
Group I | Normal control rats received 1 ml of double-distilled water |
Group II | Drug control (normal healthy control rats received intragastrically chebulagic acid (80 mg/kg b.wt/day) dissolved in 1 ml of corn oil for 8 weeks |
Group III | CCL4-induced liver injured control rats (rats received intragastrically 2 ml/kg b.wt of CCL4 dissolved in 20% corn oil twice/week for 8 weeks |
Group IV | CCL4-induced liver injured rats received intragastrically chebulagic acid (20 mg/kg b.wt/day) dissolved in 1 ml of corn oil for 8 weeks |
Group V | CCL4-induced liver injured rats received intragastrically chebulagic acid 40 mg/kg b.wt/day) dissolved in 1 ml of corn oil for 8 weeks |
Group VI | CCL4-induced liver injured rats received intragastrically chebulagic acid (80 mg/kg b.wt/day) dissolved in 1 ml of corn oil for 8 weeks |
Group VII | CCL4-induced liver injured rats received intragastrically silymarin (200 mg/kg b.wt/day) dissolved in 1 ml of corn oil for 8 weeks |
Sample collection
After experimental periods, the animals were deprived of food overnight and sacrificed by cervical decapitation. The blood was collected in two different tubes, i.e., one without ethylenediaminetetraacetic acid (EDTA) for estimation of serum liver function test and another with ethylenediaminetetraacetic acid (EDTA) for the estimation of interleukins. Liver tissues were excised immediately and rinsed in ice-chilled normal saline to remove the blood. The known weights of the tissues were minced and homogenized in 5 ml of 0.1 M Tris–HCl buffer (pH 7.4) in ice-cold condition. The homogenate was centrifuged and the supernatant was used for the estimation of various biochemical parameters.
Biochemical analysis
The collected blood was allowed to clot and serum was separated by centrifugation at 3000 rpm for 15 min and the serum enzymes, namely aspartate aminotransferase (AST), serum glutamate pyruvate transaminase (ALT), serum alkaline phosphatase (ALP), and lactate dehydrogenase (LDH), were assessed by commercial kit methods. Lipid peroxidation and hydroperoxides were estimated in liver tissues by the method of Niehius and Samuelsson (1968) and Jiang et al. (1992) respectively. Catalase (CAT) superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferase (GST), ascorbic acid (vitamin C), α-tocopherol (vitamin E), GSH, and protein were determined by the method of Sinha (1972). Stringer et al.(1989), Rotruck et al. (1973), Habig et al. (1974), Omaye et al. (1979), Baker et al. (1980), Ellman (1959), and Lowry et al. (1951) respectively.
Histopathological studies
A portion of liver tissue in each group of rats was selected and fixed in 10% formalin. The liver tissues were fixed in paraffin embedding. Thin Sects. (5 μm) were cut and stained with hematoxylin and eosin and observed the changes in the liver architecture under a microscope.
Determination of inflammatory cytokines
Plasma concentrations of interleukin 1β, interleukin 6 (IL6), interleukin 10 (IL10), tumor necrosis factor-alpha (TNF-α), and NF-kB p65 were determined using commercial rat ELISA kits (Ray Biotech, Inc., Norcross, GA, USA), following the instructions in respective kit manuals. The concentration of proinflammatory cytokines was determined spectrophotometrically at 450 nm. Standard plots were constructed using standard cytokines and the concentrations for unknown samples were calculated from the standard plot.
Immunohistochemical observation of NF-κB and TNF-α in hepatic tissue
Immunohistochemical analysis was carried out according to the method of Sambrook et al. (1989) with slight modification. The hepatic tissue sections were deparaffinized in xylene and rehydrated in ethanol series (100, 90, 70, 50, and 30%). After washing with phosphate-buffered saline (PBS: 50 mM mono- and diphosphate, 100 mM NaCl, pH 7.4), the slides were incubated with 3% H2O2 at room temperature for 15 min to quench endogenous peroxidase activity. The slides were incubated with citrate buffer at 60 °C for 15 min for antigen retrieval. Then, the slides were incubated with blocking buffer 3% BSA for 3 h at room temperature, and then, the slides were incubated overnight at 4 °C with monoclonal anti-NF-kB and TNF-α (dilution 1:200 and 1:200, Santa Cruz, CA, USA). Subsequently, the sections were incubated with secondary antibodies (anti-rabbit and anti-mouse) for 2 h at 4 °C and then washed with PBS (phosphate-buffered saline). The slides were incubated with DAB solution (3,3-diaminobenzidine tetrahydrochloride 0.05%, 1X PBS-10 ml, and H2O2 0.01%). Then, the sections were dehydrated with ethanol series (30, 50, 70, 90, and 100%). Finally, the sections were counter-stained with hematoxylin and mounted with DPX (distyrene, a plasticizer, and xylene) and photographs were taken using a Nikon microscope. Histological changes in the stained sections were viewed by a pathologist.
Statistical analysis
The results are expressed as mean ± SD. Differences between groups were assessed by ANOVA using the SPSS software package for windows. Post hoc testing was performed for inter-group comparisons using the least significant difference (LSD). P-values < 0.05 were considered as significantly altered.
Results
Liver function and liver cell necrosis
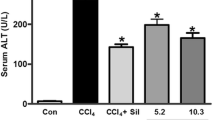
The activities of liver function indices such as aspartate transaminase, alanine transaminase alkaline phosphatase, and cell necrosis indicator lactate dehydrogenase were significantly elevated in ccl4 induced rats compared to control rats. However, administration of different doses of ( 20, 40, and 80 mg/kg b.w) chebulagic acid to ccl4 induced rats significantly reduced the abovementioned parameters to near normal compared to untreated ccl4 induced rats. Among the three doses, a pronounced effect was found at 80 mg/kg b.w and that effect was comparable to that of silymarin—a hepatoprotective agent, used as a reference drug. Therefore, 80 mg/kg body weight was fixed as an effective dose and used for further analysis. Normal rats treated with chebulagic acid at a dose of 80 mg/kg b.w did not show any significant changes in the above-tested parameters (Table 1).
Measurement of lipid peroxidation markers
Table 2 shows the levels of TBARS and HP in the liver of control and experimental rats. CCl4 induced rats showed increased levels of TBARS and HP when compared to normal control rats. Oral administration of chebulagic acid and silymarin to ccl4 induced rats significantly decreased lipid and hydroperoxides in the liver. In addition, no significant alternation in chebulagic acid–treated normal rats was found with respect to these lipid peroxidation markers.
Enzymic and non-enzymic antioxidant enzymes
Figure 1 and Table 3 illustrate the activities of enzymatic antioxidants (SOD, CAT, GPx, GST, and GR) and non-enzymatic antioxidants (vitamin C, vitamin E, and GSH) in the liver of control and ccl4 induced rats. The levels of enzymatic and non-enzymatic antioxidant were significantly decreased in ccl4 induced diabetic rats when compared to control rats. However, chebulagic acid and silymarin supplements restore the level of these enzymes to near-normal levels. These results indicate that chebulagic acid possesses antioxidant potential against ccl4-induced oxidative stress. No significant differences were observed in normal rats treated with chebulagic acid alone.
Effect of chebulagic acid on the levels of hepatic antioxidant enzyme activities in control and experimental animals. Notes: Values are given as mean ± SD for six animals in each group. Values are considered significantly different at P < 0.05 with the post hoc LSD test *P < 0.05. (a) Control vs. drug control (chebulagic acid alone treated control rats). (b) Control rats vs. CCL4 induced rats. (c) CCL4 induced rats vs. chebulagic acid. (d) chebulagic acid–treated CCL4 rats vs. silymarin-treated diabetic rats. The activities of enzymes are expressed as follows: SOD, one unit of activity was taken as the enzyme quantity which gave 50% inhibition of nitroblue tetrazolium reduction in 1 min/mg protein; CAT, μmoles of H2O2 consumed/minute; GPx, μg of glutathione consumed/minute/mg protein; GST, μmoles of 1-chloro 2,4-dinitrobenzene-GSH conjugate formed/minute/mg protein
The levels of inflammatory cytokines
The levels of inflammatory cytokines such as TNF-a, IL-1β, IL-6, and NF-kB p65 were significantly increased, whereas the levels of anti-inflammatory cytokine IL-10 were significantly decreased in ccl4 induced rats when compared with control rats. However, oral administration of chebulagic acid and silymarin to ccl4 induced rats significantly declined these levels to near normalcy. No significant deviation was observed in the control rats treated with chebulagic acid alone (Fig. 2).
Effect of chebulagic acid on the levels of IL-1β, IL-6, TNF-α, p65 subunit of NF-κB, and IL-10 in the plasma of control and experimental animals. Notes: Values are given as mean ± SD for six animals in each group. Values are considered significantly different at P < 0.05 with the post hoc LSD test *P < 0.05. Units: TNF-α pg/ml, NF-KB p65–ng/ml, IL1 β IL-6, and IL10–pg/ml. (a) Control vs. drug control (chebulagic acid alone treated control rats). (b) Control rats vs. CCL4 induced rats. (c) CCL4 induced rats vs. chebulagic acid. (d) Chebulagic acid–treated CCL4 rats vs. silymarin-treated diabetic rats
Immunohistochemical expression of NF-κB in the liver
The hepatic immunohistochemical expression of NF-κB in control and experimental rats is shown in Figure 3. There was no nuclear signal or immune-reactivity for NF-κB in the liver of control rats (Fig. 3A) and those treated with chebulagic acid alone (Fig. 3B), while the liver of rats administered with ccl4 showed higher immunostaining of NF-κB, especially around the portal area, hepatocytes, and non-parenchymal cells (Fig. 3C). Treatment with chebulagic acid and silymarin showed no nuclear signal or no immunostaining of NF-κB in the liver of ccl4 induced rats (Fig. 3D and E).
Immunohistochemical expression of TNF-α in the liver
The hepatic immunohistochemical expression of TNF-α in control and experimental rats showed in Fig. 4. The proinflammatory cytokine TNF-α expression was increased in the liver of ccl4 induced rats (Fig. 4C) when compared to control (Fig. 4A) and those treated with chebulagic acid alone (Fig. 4B). Chebulagic acid and silymarin-treated ccl4 induced rats showed diminished expression of TNF-α (Fig. 4D and E).
Liver histopathological examination
Changes in liver architecture revealed by H&E staining are illustrated in Fig. 1. The control and drug control rats confirmed perfect lobular architecture with intact liver parenchyma and absence of inflammation or any damage (Fig. 5A and B). However, ccl4 induced rats showed hepatocellular necrosis, massive fatty infiltration, and inflammatory cellular infiltration along with disrupted hepatic cords (Fig. 5C). Histopathological disruptions observed in ccl4 induced rats were significantly reduced by the supplementation of chebulagic acid and silymarin (Fig. 5D and E).
Effect of chebulagic acid on the changes of histological morphology of rat liver in control and experimental rats by hematoxylin and eosin (H&E) staining, magnification 40 × . Control (A), normal + chebulagic acid (B), CCL4 induced (C), CCL4 induced + chebulagic acid (D), CCL4 induced + silymarin (E)
Discussion
CCl4 is a widely used industrial solvent and it is the best-characterized animal model of xenobiotic-induced and oxidative stress-mediated hepatotoxicity (Ogeturk et al. 2005; Jaramillo-Juarez et al. 2008). The degree of hepatic damage is assessed by histological and biochemical parameters in circulation. In the present study, the levels of aspartate transaminase, alanine transaminase, alkaline phosphatase, and lactate dehydrogenase were significantly increased in ccl4 induced rats and these elevated enzymes levels confirm the hepatic damage/injury and hepatic cells necrosis caused by ccl4. However, those enzymes levels were found to be decreased to near normal in ccl4 induced rats upon treatment with chebulagic acid and silymarin.
Lipid peroxidation is an important parameter of oxidative stress. This process may cause peroxidative tissue damage in inflammation, cancer and toxicity of xenobiotics, and aging (Jalali Ghassam et al. 2014). Malondialdehyde is a cytotoxic product that is a hallmark of lipid peroxidation. Elevated lipid peroxidation is responsible for the formation of lipid hydroperoxides. In our study, the levels of TBARS and HP are found to be higher in ccl4 induced rats which indicate membrane damage, alterations in structure and function of cellular membranes and failure of antioxidant defense mechanisms to prevent the formation of excessive free radicals. These results are in agreement with the previous study (Elgawish et al. 2015). Oral administration of chebulagic acid and silymarin to ccl4 induced rats significantly decreased levels of TBARS and HP to near normal. These results indicate the antioxidant potential of chebulagic acid which protects the liver against lipid peroxidation.
Contrary to the levels of lipid peroxide markers, the levels of enzymic antioxidant (SOD, CAT, GPx, GST, and GR) and non-enzymic antioxidant (vitamin C, vitamin E, and GSH) were significantly declined. These results are consistent with a previous study that demonstrated that ccl4 treatment enhanced hepatic fibrosis which is associated with the exacerbation of lipid peroxidation and the depletion of antioxidant status (Chen et al. 2013). These changes were reversed upon treatment with chebulagic acid and silymarin.
CCl4 induces the production of reactive oxygen species (ROS), thereby causing liver injury which is confirmed by elevated levels of lipid peroxidation markers (lipid peroxidation and hydroperoxides) in this study. The reactive oxygen species induce inflammation by upregulating the expression of inflammatory mediators. Among those, the inflammatory response is mediated by the action of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, which are involved in the chemical-induced hepatotoxic process causes infiltration of neutrophils and monocytes into the damaged organ (Zhang et al. 2004; Novo and Parola 2008; Weber et al. 2003).In our study, we observed that oral administration of chebulagic acid and silymarin to ccl4 induced rats prevented the increased plasma level of inflammatory cytokines (IL-1β, IL-6, TNF-α, and NF-κB) with an elevation of anti-inflammatory cytokine (IL-10).In addition, the immunohistochemical studies show that the elevated levels of TNF-α and NF-κB were diminished in the liver of CCL4 induced rats after treatment with chebulagic acid and silymarin.
These results indicated that chebulagic acid was able to improve CCl4-induced hepatotoxicity by suppressing inflammatory response in rats. The anti-inflammatory properties of chebulagic acid are attributed to inhibition of NF-κB, therefore, blocking the release of proinflammatory cytokines and amplification of inflammation via inhibition of downstream inflammatory mediators.
Similarly, a remarkable improvement was observed in the CCL4-induced histopathology of the liver after treatment with chebulagic acid and silymarin. These results are in agreement with the findings of previous studies (Elgawish et al. 2015; Liu et al. 2010). Altogether, our biochemical, histological, and immunohistochemistry data clearly demonstrate that chebulagic acid exerts hepatoprotection against ccl4-induced hepatic damage in rats by its antioxidant and anti-inflammatory potential.
Conclusion
Results of the present study suggested that chebulagic acid has the hepatoprotective potential against CCl4-induced hepatic injury through preventing oxidative stress and elevations of the inflammatory cytokines, keeping them at near control values.
References
Baker H, Frank O, Angelis B, Feingold S (1980) Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Rep Int 21:531–536
Jalali Ghassam B, Ghaffari H, Prakash HS, Kini KR (2014) Antioxidant and hepatoprotective effects of Solanum xanthocarpum leaf extracts against CCl4-induced liver injury in rats. Pharm Biol 52(8):1060–1068
Chen X, Ying X, Zhang W, Chen Y, Shi C, Hou Y, Zhang Y (2013) The hepatoprotective effect of fraxetin on carbon tetrachloride induced hepatic fibrosis by antioxidative activities in rats. Int Immunopharmacol 17:543–547
Elgawish RAR, Rahman HGA, Abdelrazek HMA (2015) Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicol Rep 2:1149–1156
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Frei B, Higdon JV (2003) Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr 133(10):3275–3284
Fu Y, Zheng S, Lin J, Ryerse J, Chen A (2008) Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol 73:399–409
Gao H, Huang YN, Xu P, Kawabata YJ (2007) Inhibitory effect on a-glucosidase by the fruits of Terminalia chebula Retz. Food Chem 105:628–634
Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A (2011) Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis inmice. Toxicol Appl Pharmacol 251:59–69
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferase: the first step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamada S, Kataoka T, Woo JT, Yamada A, Yoshida T, Nishimura T, Otake N, Nagai K (1997) Immunosuppressive effects of gallic acid and chebulagic acid on CTLmediated cytotoxicity. Biol Pharm Bull 20:1017–1019
Iredale J (2008) Defining therapeutic targets for liver fibrosis: exploiting the biology of inflammation and repair. Pharmacol Res 58:129–136
Jaramillo-Juarez F, Rodriguez-Vazquez ML, Rincon-Sanchez AR, Consolacion M, Martinez GG, Ortiz J, Reyes JL (2008) Caffeic acid phenethyl esteragainst carbon tetrachloride toxicity in rats. Ann Hepatol 7:331–338
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–387
Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y (2007) Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 14:755–762
Latief U, Ahmad R (2018) Herbal remedies for liver fibrosis: a review on the mode of action of fifty herbs. J Tradit Complement Med 8:352–360
Lee SI, Hyun PM, Kim SH, Kim KS, Lee SK, Kim BS, Maeng PJ, Lim JS (2005) Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a natural product library. Arthritis Rheum 52:345–353
Liu C, Tao Q, Sun M, Wu JZ, Yang P, Jian Peng J, Hu Y, Liu C, Liu P (2010) Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest 90:1805–1816
Liu W, Shang P, Liu T, Xu H, Ren D, Zhou W, Wen A, Ding Y (2017) Gastroprotective effects of chebulagic acid against ethanol-induced gastric injury in rats. Chem Biol Int 278:1–8
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folinphenol reagent. J Biol Chem 193:265–275
Murakami A, Ishida HK, Furukawa K, Ikeda I, Yonaha Y, Aniya M, Ohigashi YH (2005) Suppressive effects of Okinawan food items on free radical generation from stimulated leukocytes and identification of some active constituents: implications for the prevention of inflammation-associated carcinogenesis. Asian Pac J Cancer Prev 6:437–448
Niehius WG, Samuelsson D (1968) Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:26–130
Novo E, Parola M (2008) Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair 1(44):247–256
Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M (2005) Caffeic acidphenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol 97:273–280
Omaye ST, Turnbull TD (1979) Sauberlich. H.E. Selected method for the determination of ascorbic acid in animal cells, tissues and fluid. In: McCormic DB, Wright DL (eds) Methods Enzymol, vol 62. Academic Press, New York, pp 3–11
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press
Sinha KA (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Stringer MD, Gorog PG, Freeman A, Kakkar VV (1989) Lipid peroxides and atherosclerosis. Br Med J 298:281–284
Weber LWD, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33:105–136
Zhang LJ, Yu JP, Li D, Huang YH (2004) Effects of cytokines on carbon tetrachlorideinduced hepatic fibrogenesis in rats. World J Gastroenterol 10:77–81
Funding
The authors are thankful to the DST-SERB ECR project, New Delhi, India, for providing financial assistance to purchase chebulagic acid for performing this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The experimental protocol was approved by the Ministry of Social Justices and Empowerment, Government of India, and Institutional Animal Ethics Committee Guidelines (IAEC No: No. 12/09/2016).
Informed consent
All authors are consented to publish this manuscript in this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sundaram, R., Ganesh, V., Nandhakumar, E. et al. Hepatoprotective and anti-inflammatory potential of chebulagic acid on carbon tetrachloride–induced hepatic fibrosis by antioxidative activities in rats. Comp Clin Pathol 30, 961–971 (2021). https://doi.org/10.1007/s00580-021-03295-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-021-03295-0