Abstract
The high temperature phase stability characteristics of U–xCr (x = 0, 1, 2, 3, 4, 5, 7, 9, 15 wt%) alloys have been investigated by dynamic calorimetry under slow heating (1 K min−1) in pure flowing Ar-atmosphere. The temperatures of various eutectic, eutectoid solid state phase transformations and that of melting have been measured as a function of Cr-content. These data are used to construct a partial U–Cr phase diagram on U-rich side, which showed good agreement with the recent assessment. The liquidus temperatures for higher Cr-contents, however exhibited a small upward shift as compared to equilibrium phase diagram estimates. The measured transformation enthalpies for the on-heating eutectoid and eutectic transformations exhibited an increase with Cr content of the alloys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With emerging interest on metal-fuelled fast reactors in India, it becomes necessary to develop a comprehensive understanding of high temperature thermo-kinetic stability and thermophysical properties of U-Transition metal M (M=Zr, Fe, Cr, Rh, Ru, Nb, Mo, V, Ti, Pd, Re etc.) alloy systems, since they relate directly to the long term in-pile metallurgical compatibility of metal fuel with either austenitic or ferritic steel clads. Some of these transition elements, though not present as a part of nascent fuel, may however form as fission induced products under the influence of intense neutron irradiation [1]. On the contrary, the binary U–Cr system assumes special importance for a different reason, as Cr constitutes the second major element in nuclear grade 9Cr-based ferritic-martensitic steels [2]. A survey of extant literature on U–Cr binary system reveals that bcc-Cr does not dissolve appreciably even in the high temperature γ-bcc phase of uranium, owing probably to size effect [3–12]. In addition, uranium is also insoluble in chromium, due to disparity in electronic structure characteristics [7, 8]. Interestingly, chromium is one of the few transition elements that form a simple eutectic phase diagram with uranium, without any intermediate phase [3, 4, 7]. Further, the presence of temperature induced polymorphic transitions in uranium, namely α(orthorhombic) → β(tetragonal) → γ(bcc) yields two more additional eutectoid reactions [3]. It is also interesting to note that small additions of Cr to U–Pu–Zr based metal fuel results in a decrease of liquidus temperature of fuel matrix, which is not desirable from reactor operational safety point of view [13]. Therefore, the possible formation of a low temperature eutectic due to Cr-admixture in nuclear fuel is also an issue that has nontrivial consequences over fuel design.
Although considerable experimental results have already been accrued towards elucidating U–Cr system in general, much of these classical data are at the least 40 years old as of now [7, 8]. As a result, some discrepancies as well as inadequacies are felt with regard to reconciling the limited experimental data against the requirement of a rigorous assessment, as done for example by Venkatraman et al. [4] and also an update by Okamoto in 2012 [3]. It appears that the liquidus temperature, especially for high Cr-alloys and the slightly higher solubility of Cr in γ-Uranium (exceeding 1.5 wt%), as recommended in an earlier report of Rough are somewhat suspect [7]. Further, there are no measured data on transformation enthalpies, including melting and various thermophysical properties on this important binary system [3, 4, 9]. The kinetics related aspects of various solid state transformations as influenced by Cr-content have not been addressed in adequate detail [4]. With a view to address some of these issues, it has been decided to reinvestigate U–Cr system, mainly through calorimetry, the results of which are supplemented by metallography. In this brief communication, some select results of our ongoing study are presented and briefly discussed.
2 Experimental Procedure
Using reactor grade uranium (316 mass ppm C, 615 ppm Si, 349 ppm Al) and highly pure Cr (99.99 % plus, Sigma Aldrich), small buttons of U–xCr(x = 0,1, 2, 3, 4, 7, 9, 15 wt%) alloys were melted in a graphite resistance furnace under flowing Ar-atmosphere in alumina crucible. The weight loss after melting is found to be less than 0.5 %. The alloys were subject to a homogenization anneal at 1473 K for 1 h, followed by slow cooling under Ar-inert gas cover. The density of alloys is determined by standard immersion technique, to cross check composition. The differential scanning calorimetry (DSC) experiments were performed in Setaram Setsys Evolution 1600®, heat flux type calorimeter, using small samples of 50 to 70 mg mass. Based on previous experience, Uranium has been used as an additional secondary calibration standard, both for temperature and heat flux [14]. The on-heating and cooling phase transformation sequence up to melting is determined by performing a slow scan experiment at 1 K min−1 under 50 mL per min Ar flow. Proper baseline compensation has also been carried out.
3 Results
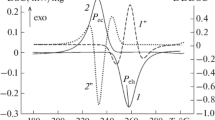
In Fig. 1, a typical base line compensated DSC profile obtained on U–15Cr alloy is shown. The four distinctly numbered on-heating thermal arrests are due to following reactions.
(1) α-U + Cr → β-U + Cr; (2) β-U + Cr → γ-U + Cr; (3) γ-U + Cr → Liq. + Cr; (4) Liq. + Cr → Liquid.
The transformation sequence is fully reversible upon cooling. Similar transformation thermal arrests are observed for other alloy compositions. It may be noted that for hypoeutectic alloys (x Cr ≤ 5 wt%) the appropriate eutectic reaction would be γ-U + Liq. → L. Further, except for the final melting reaction, namely Liq. + Cr → Liquid (hypereutectic) or Liq. + γ-U → Liq (hypoeutectic) the other thermal arrests due to solid state phase changes were fairly sharp. Since completion of melting spans an extended temperature domain, which increases with chromium content and also in view of the slow heating rate adopted in present study, the melting thermal arrest is rather diffuse in Fig. 1. This results in some uncertainty in fixing properly the melting finish or liquidus temperature. Although slow cooling at 1 K min−1 has been adopted with a view to maintain near equilibrium conditions, considerable undercooling to the tune of 50 K was still noticed. The extent of undercooling increased with Cr content of alloys, although in a nonsystematic manner. It is because of this reason that in the present study, the on-heating thermal arrest peak temperatures are used for constructing partial U–Cr phase diagram. This is illustrated in Fig. 2, wherein the measured transformation temperatures for various alloy compositions are superposed over the recent updated equilibrium diagram by Okamoto [3]. In general a good agreement to within ±15 K is noticed between present data and that of Okamoto for hypoeutectic alloys. However, for alloys of higher Cr-content, the measured liquidus temperatures exceed the equilibrium diagram estimates by about +30 K. Notwithstanding the inherent uncertainty in experimental liquidus temperature measurement, this discrepancy derives partly from kinetic factors associated with γ-U + Liq → Liquid or Cr + Liq → Liquid transformation. An indirect support for this is readily obtained from the nature of thermal arrest profile associated with the beginning of formation of liquid or γ-U + Cr → Liquid transformation. This is shown in Fig. 3. It is clear that progressive or time (temperature in this case) dependent liquid formation affects the endothermic signal in such a way that the smooth regular decay of DSC signal which would otherwise be observed in a normal phase change event is significantly localized over a temperature zone near peak of transformation. Such smearing of melting thermal arrest profiles is more pronouncedly seen in the case of high Cr-alloys. Since mutual solubility is very less in U–Cr binary, it takes considerable thermal activation (due to slow diffusion) for the proeutectic constituent to dissolve in liquid.
U–Cr binary phase diagram adopted from the assessment of Okamoto [3]. The circles represent the measured on-heating transformation arrest temperatures in this study
In Fig. 4, a graphical summary of measured transformation temperatures for different alloy compositions is presented. It may be noted that for alloys with x Cr = 1 wt% or nearby composition, the eutectic reaction at 1133 K will be absent (see, Fig. 1). For all other alloys, a fair degree of agreement between measured eutectic and eutectoid temperatures with those recommended by Okamoto’s assessment is noticed.
Graphical collage of measured transformation temperature data for various alloy compositions. The dash-dotted horizontal lines correspond to Okamoto’s assessed equilibrium values [3]
In Fig. 5, the measured enthalpies for L → γ-U + Cr eutectic reaction are compared for different alloy compositions. At present there are no measured data available on phase transformation enthalpies in U–Cr system, so that a comparison can be made. However, it is clear that with regard to eutectic composition (~5 wt%), the measured eutectic transformation enthalpy decreases on either side, which implies that it is directly related to the amount of liquid remaining at the eutectic temperature, for each alloy composition. In a similar fashion, the transformation enthalpies of other two eutectoid invariant reactions have also been obtained in this study; but these data are not reported here in deference to brevity.
4 Discussion
In the present study, which is still ongoing, an attempt has been made to gather some important thermodynamic and related phase diagram information on a restricted range of composition on the Uranium rich side, which is of interest to Indian fast reactor materials program. However, some more experimentation on both dilute and slightly more concentrated alloys (x Cr ≥ 15 wt%) are needed to make a definite comparison and theoretical assessment of phase equilibria in U–Cr alloys. Nevertheless, the data obtained in this study are in broad agreement with Okamoto’s assessment, except for the liquidus temperature of concentrated alloys. To the best of author’s knowledge, the measured values of transformation enthalpy constitute the first open-source experimental data on U–Cr system. It may be added that further research on measurement of thermal properties and phase transformation enthalpies are currently underway.
5 Conclusions
Using differential scanning calorimetry, accurate determination of Uranium rich side of U–Cr phase diagram has been constructed. The constructed phase diagram is found to be in good agreement with the recent theoretically assessed U–Cr equilibrium diagram. In addition, the transformation enthalpies for all solid state phase changes and melting and their variation with Cr-content have also been estimated.
References
Konings R J M in Comprehensive Nuclear Materials, (ed) Allen T R, Stoller R E, and Yamanaka S, vol. 3, Elsevier, Oxford (2012) p 29
Kaity S, Kutty T R G, Agarwal R, Laik A, and Kumar A, Nucl Eng Des, 250 (2012) 267.
Okamoto H, J Phase Equilib Diffus 33 (2012) 495.
Venkatraman M, Neumann J P, and Peterson D E, Bull Alloy Phase Diagr, 6 (1985) 425.
Ivanov O S, Badaeva T A, Sofronova R M, Kishenevskii V B, and Kushnir N P, Phase Diagrams of Uranium Alloys, Amerind Publishing Co. Pvt. Ltd., New Delhi (1983), p 59
Chapman L R, J Nucl Mater 116 (1983) 328.
Rough F A, and Bauer A A, Constitution of Uranium and Thorium Alloys, USAEC Report No. BMI-1300, Battelle Memorial Institute, Ohio (1958) p 21.
Klepfer H H, and Chiotti P, Characteristics of the Solid State Transformations in Uranium, USAEC Report No. ISC-893, Ames Laboratory, Iowa (1957).
Berche A, Dupin N, Gueneau C, Rado C, Sundman B, and Dumas J C, J Nucl Mater 411 (2011) 131.
Eckelmeyer K H, Eatough M O, and McAllaster M E, J Nucl Mater 170 (1990) 157.
McAllaster M E, and Eckelmeyer K H, J Nucl Mater 154 (1988) 342.
Rothman S J, The Diffusion of Chromium in Gamma Uranium, USAEC Report No. ANL-6320, Argonne National Laboratory, Illinois (1961).
Fackelmann J M, Bauer A A, and Moak D P, Literature Survey on Dilute Uranium Alloys, BMI Report X-10264, Battelle Memorial Institute, Ohio (1969) p D1.
Rai A K, Raju S, Jeyaganesh B, Mohandas E, Sudha R, and Ganesan V, J Nucl Mater 383 (2009) 215.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santhosh, R., Raju, S., Rai, A.K. et al. Differential Scanning Calorimetry Study of U–Cr Alloys. Trans Indian Inst Met 68 (Suppl 2), 305–308 (2015). https://doi.org/10.1007/s12666-015-0601-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0601-1