Abstract
Differential scanning calorimetry is used to determine the thermodynamic parameters (enthalpy, entropy, activation energy) of the forward and reverse processes solidification and melting of Pb–Cd, Pb–Sn, Pb–Bi, and Cd–Sn eutectic alloys for the first time. As the melting temperature of eutectic decreases, a trend toward an increase in the configuration entropy of phase transformation is observed. When describing the solidification of eutectic melts, it is possible, based on the obtained experimental data, to use the existing concept of the formation of numerous clusters, which are close to eutectic solidification nuclei in concentration and morphology, on approaching solidus; at a slight undercooling, the rapid growth of the nuclei leads to the spontaneous transition of a melt into a crystalline state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The phase diagrams of some binary metallic systems exhibit the presence of alloys characterized by the minimum melting temperature, namely, the presence of eutectics. These alloys differ from the other alloys of these systems in the fact that, according to the Gibbs rule, the melting and solidification of eutectics corresponds to the nonvariant equilibrium similar to that observed upon melting and solidification of pure metals (first-order phase transition). It is natural that, at all times, such alloys were a subject of study of their nature, contact melting mechanism, solidification kinetics, and morphology of solidified products [1, 2]. In this case, studies mainly focused on phenomena occurred upon melt cooling and focused less on the melting of eutectic alloys. Historically, data on the solidification of organic eutectics and results of X-ray diffraction studies of liquids, which were obtained by A.A. Bochvar and V.I. Danilov (see [3]), probably should be noted. Along with the results, the book of W. Kurz and D.J. Fisher [4], which was republished several times, should be noted. In subsequent monographs (see [5, 6]), fairly traditional concepts of eutectic reactions were improved and developed.
It is possible to list methods used for the study of eutectic alloys in the above mentioned works; these are X-ray diffraction analysis, electron and proton diffraction, sedimentation, electrical resistivity measurements, quenching from the melt, and some others.
The early 21st century was marked by the appearance of a wide range of equipment for measuring calorimetric effects of phase transformations; these are installations for high-resolution differential scanning calorimetry (DSC), the operation principles of which were developed by N.S. Kurnakov in the early 20th century [7].
The appearance of the equipment gave fresh impetus to the interest in differential thermal analysis of melting and solidification processes. However, in this case, the majority of investigations were related to the study of melting and solidification processes of industrial magnesium- and aluminum-based alloys in order to solve concrete production problems. To a much lesser extent, the studies were related to fundamental aspects of melting and solidification, in particular, to the study of eutectic reactions (see, for example, [8, 9]). Studies of the Al–Si [10] and some low-melting systems [11, 12] should be considered as a certain exception to above mentioned investigations.
In present study, the calorimetric effects of melting and solidification of the binary eutectics of a number of low-melting elements (Pb, Sn, Bi, Cd) are determined for the first time.
EXPERIMENTAL
Pb–Sn, Pb–Bi, Pb–Cd, and Cd–Sn eutectic alloys were a subject of the study; they were prepared by melting of starting charge under a borax flux using a muffle electric furnace. As the starting components, pure metals (the fraction of base component was no less than 99.9 wt % (STP TU KOMP 3-290–10) were used. The alloy composition was determined with an Inca X-Act (Oxford) analyzer. The alloy compositions were chosen with test melts method using data on the compositions of eutectic alloys, which are available in the literature. In the present study, the maximum accuracy of the preparation of the eutectic alloy compositions was within 1 wt %.
DSC data were obtained using a STA 449 Jupiter (Netzsch, Germany) installation. Samples 230 ± 20 mg in mass were heated and cooled in an argon atmosphere (99.999% Ar) at rates of 5, 10, 20, and 40 K/min; the gas flow rate was 25–30 mL/min. Experimental data were processed using Proteus Analysis software. The structure of exo- and endothermic peaks was analyzed using Fityk software.
To exclude the appearance of artefacts during investigation of alloys, which are due to special peculiarities of the experiment, experimental data were smoothed using sixth- to eighth-degree polynomial. The same approach was used in analyzing data on both the first (DDSC) and second (DDDSC) derivatives of DSC signal for temperature. The reported exemplifying data correspond to heating and cooling rates of 10 K/min.
To compare measurements performed at different heating and cooling rates (β) and for samples differing in masses (ms), the measured heat flow was normalized to so-called excess specific heat capacity \({{C}_{{{{p}_{{{\text{excess}}}}}}}}\) (J/(g K)) in accordance with the equation [13]
where \({{\dot {Q}}_{{\text{s}}}}\) and \({{\dot {Q}}_{{{\text{st}}}}}\) are the heat flow upon heating with a sample and base (standard) heat flow, respectively.
In order to determine the activation energy upon heating at different rates, the Kissinger equation [14] was used,
where Q is the activation energy (kJ/mol); R is the gas constant; T1 and T2 are temperatures corresponding to maxima in DSC curves; and β1 and β2 are the heating rates corresponding to T1 and T2, respectively.
RESULTS AND DISCUSSION
The temperature dependences of a DSC signal measured upon heating at a constant rate and subsequent cooling exhibit certain endo- and exothermic effects during structural and phase transformations in condensed matters in the temperature range corresponding to the transformations. In the case of first-order phase transitions (PTI), such as solidification, melting, polymorphous transformations, and eutectic and eutectoid reactions, corresponding maxima and minima are recorded in DSC curves; the areas under the minima and maxima contain information about heat effects of the transformations. This is an almost unique method, which gives direct information on the enthalpy and entropy of the associated phase transformation. Moreover, in contrast to other methods used for the study of phase transformations upon heating and cooling, this method allows one to relate such transformations to first-order transitions (see [15–18]) based on the coincidence of minimum/maximum temperatures of in the temperature dependence of the DSC signal with the corresponding maximum/minimum temperature in the second derivative of DSC signal for temperature (DDDSC).
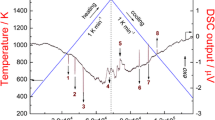
Figure 1 shows an example of DSC signal upon thermal cycling of the Pb–Cd eutectic alloy (72.0 at % Pb) at a rate of 10 K/min; Table 1 gives data for this and other eutectics.
The following characteristics were obtained for the dependence shown in Fig. 1: for the endothermic peak Peh, Tmax = 258.1/258.0°C are the temperatures of the endothermic peak maximum and the second derivative maximum in the temperature range, Ts = 246.9°C, Tf = 270.2°C, and heat effect is 25 J/g. As is seen, the disagreement between the extreme temperatures is 0.1°C.
For the exothermic peak Pec: Tmax = 232.8/232.5°C, Ts = 246.9°C, Tf = 220.5°C, and the heat effect is 25.5 J/g. The disagreement between the extreme temperatures (the maximum of the exothermic peak and the minimum of the second derivative in this temperature range) is 0.3°C.
When the molar mass of eutectic and the heat effect of transformation (J/g) are available, the enthalpy (ΔH) and configuration entropy (ΔS = ΔH/Tmax) of the eutectic reaction can be calculated. Since the values of thermal effect and temperature corresponding to the calorimetric peak maximum upon melting and solidification are different, the enthalpy and entropy for each of the processes were calculated individually (see Table 1). Their average values (see row Effective value) are also given.
Figure 2 shows the structures of the endo- and exothermic peaks. It follows from the reported data that the peak cannot be approximated only by a single function. In the course of eutectic reaction, at least two phase-transformation mechanisms are operative within close temperature ranges.
Structure of (a) endothermic Peh and (b) exothermic Pec peaks in Fig. 1. Symbols correspond to experimental data and Papp curves indicate approximation results; P1 and P2 are subpeaks.
One of the subpeaks observed both upon heating and cooling results from the process occurs over the entire temperature range of the eutectic reaction; the other subpeak characterizes its starting stage upon heating of the eutectic or solidification of the melt having the eutectic composition.
Figure 3 shows the effect of the heating rate on the DSC signal upon melting of the Pb–Cd eutectic. The following results are obtained: for a heating rate of 5 K/min, the temperature is 255.5/255.5°C (the disagreement is 0.0°C); for a heating rate of 10 K/min, it is 258.1/258.0°C (0.1°C); for a heating rate of 20 K/min, it is 264.1/264.1°C (0.0°C); and for a heating rate of 40 K/min, it is 275.1/274.6°C (0.5°C). As the heating rate increases, the absorbed heat almost does not increase and is ~25 J/g.
Within this heating rate range, the heating rate does not affect the temperature of the start of the eutectic melting, but the maximum rate of the process shifts toward the high-temperature range.
The average activation energy of eutectic melting was calculated using the difference method based on the Kissinger equation (see Table 1).
When, for example, the results obtained for the Pd–Cu eutectic are plotted on the ln(β/\(T_{{\max }}^{2}\))–(1/Tmax) coordinates, where β is the heating rate and Tmax is the endothermic peak maximum, the activation energy can be determined as the slope of the curves plotted on these coordinates (Fig. 4). In this case, two values of activation energy of the transition are obtained; the highest and lowest values are typical of heating at low and high heating rates, respectively (see Table 1). This circumstance indicates the fact that the transition mechanisms or their relation depend on the heating rate.
Figure 5 shows the effect of the cooling rate on the DSC signal during solidification of the Pb–Cd eutectic. The hardware capacities do not allow us to uniformly cool at rates of more than 20 K/min within this temperature range.
The following results were obtained: for a cooling rate of 5 K/min, the temperature is 235.5/235.2°C (the disagreement is 0.3°C) and the temperature range of the transformation realization is ΔT = 23°C; for a cooling rate of 10 K/min, the temperature is 232.2/233.0°C (0.8°C) and ΔT = 41°C; and for a cooling rate of 20 K/min, the temperature is 229.5/229.6°C (0.1°C) and ΔT = 53°C. As the cooling rate increases, the released heat does not increase and is equal to ~25 J/g.
As on heating, the temperature of the start of solidification of eutectic is independent of the cooling rate for this cooling rate range. Qualitatively, analogous dependences were also obtained in studying the calorimetric effects upon melting and solidification of the eutectics in the Pb–Sn, Bi–Sn, and Pb–Bi systems (see Table 1).
It follows from the data given in Table 1 that the enthalpy of melting of eutectic and the configuration entropy are always slightly lower than those upon solidification of eutectic composition. Therefore, for the generated values of the enthalpy and entropy of the transformation, it was suggested to use their averaged value (see data given in the row Effective value).
The trend toward an increase in the entropy as the melting temperature of the eutectic alloy in the considered binary systems decreases is noteworthy. The obtained values of the enthalpy of melting and solidification of the eutectics are substantially lower than the additive sum of the partial contributions of the enthalpies of melting of the components formed a eutectic.
Thus, according to the data of calorimetric measurements upon melting of a two-phase eutectic mixture and upon its solidification from the melt, the behavior of the eutectic is similar to that of a single-phase system, the typical feature of which is the closeness of the extreme temperatures in the DSC curves to the corresponding extreme temperatures in the DDDSC curves.
The structure of the endothermic peaks formed upon heating allows us to consider that the transition of a eutectic into the melt of the same composition occurs when a liquid forms at the contact boundary of different crystals (contact melting) and the components comprising the eutectic then dissolve [1]. This is likely to be the cause for the fact that, after eutectic melting, a melt retains a quasi-inhomogeneous structure to a certain heating temperature.
When the solidus temperature is approached, a eutectic melt separates into numerous areas differing in the concentration of one of the components. Clusters, which are close to eutectic solidification nuclei in concentration and morphology, form. When passing the solidus point, eutectic nuclei form in such microareas, which initiate a two-phase solidification process. At a great number of growing nuclei of eutectic solidification, the volume that earlier corresponded to the eutectic melt is rapidly filled up. A spontaneous character of this process explains the weak dependence of the temperature of the start of eutectic solidification on the cooling rate.
In considering the data on the structure of the exothermic peaks corresponding to eutectic solidification, we can assume that, as in the case of solidification of a pure component, processes of the appearance of eutectic solidification nuclei and their growth develop. This idea was stated earlier [19] in the course of a theoretical analysis of nucleation upon solidification of eutectic alloys. The concept of a “diffuse dipole” as a two-phase object, which is in a melt or solid solution of eutectic composition, was introduced; within a diffuse dipole, two nuclei differing in composition are united with a common diffuse field.
The formation of pearlite and austenite grains also was analogously explained [20].
CONCLUSIONS
(1) Our calorimetric studies showed that both eutectic solidification and eutectic melting processes (coexistence of three phases such as liquid, A and B phase crystals, and α and β solid solutions) can be considered as a first-order phase transition with inherent changes in the phase transformation parameters.
(2) The enthalpy and entropy of the eutectic reaction upon heating are always slightly lower than those upon solidification.
(3) As the eutectic melting temperature decreases, a trend toward an increase in the configuration entropy of such a transition is observed.
(4) To describe the solidification of eutectic melts, we suggested using the concept of the appearance of numerous clusters, which are close to eutectic solidification nuclei in composition and morphology, in a melt when its solidus is approached. The spontaneous formation of eutectic solidification nuclei from such clusters upon reaching the solidification temperature leads to a rapid transition of the melt into a crystalline state.
(5) The enthalpy and entropy of melting and solidification of eutectics are substantially lower than those of the phase transitions in the pure components comprising these eutectics.
REFERENCES
V. M. Zalkind, Nature of Eutectic Alloys and Contact Melting Effect (Metallurgiya, Moscow, 1982).
D. M. Stefanescu, G. J. Abbaschian, and R. J. Bayuzick, in Proceedings of Symposium on Solidification Processing of Eutectic Alloys (Warrendale, PA, Metallurgical Soc., 1988).
Ya. S. Umanskii, B. I. Finkel’shtein, and M. E. Blanter, Physical Materials Science (Metallurgiya, Moscow, 1958).
W. Kurz and D. J. Fisher, Fundamentals of Solidification, 4th ed. (Trans. Tech. Publication, Switzerland, 1998).
M. E. Glicksman, Principles of Solidification: An Introduction to Modern Casting and Crystal Growth Concepts (Springer, New York, 2011).
D. E. Andrusevich and A. A. Shchetinin, Physical Foundations of Solidification of Metals and Alloys (VGTU, Voronezh, 2008).
N. S. Kurnakov, Introduction to Physicochemical Analysis, 4th ed. (Izd. AN SSSR, Moscow, 1940).
L. Heusler and W. Schneider, “Influence of alloying on the thermal analysis results of Al–Si cast alloys,” J. Light Metals 2, 17–26 (2002).
J. Piatkowski, V. Przeliorz, and V. Szymszal, “The application of ATD and DSC methods to study of the EN AC-48000 alloy phase transformations,” Arch. Found. Eng. 17 (2), 207–211 (2017).
J. Piatkowski and B. Gajdzik, “Testing phase changes in Al–Si alloys with application of thermal analysis and differential calorimetric analysis,” Metalurgija 52 (4), 469–472 (2013).
L. V. Spivak and N. E. Shchepina, “Peculiarities and regularities of melting and solidification processes of binary metallic alloys,” Fiz. Tverd. Tela 61 (8), 1407–1413 (2019).
L. V. Spivak and N. E. Shchepina, “Thermal effects upon solidification of alloys characterized by continuous and terminal solubility of components,” Zh. Tekhn. Fiz. 89 (9), 1395–1399 (2019).
S. M. Sarge, G. W. H. Höhne, and W. F. Hemminger, Calorimetry. Fundamentals Instrumentation and Applications (Wiley, Weinheim, 2014).
H. E. Kissinger, “Reaction kinetics in differential thermal analysis” Analyt. Chem. 29, 1702–1706 (1957).
Handbook of Thermal Analysis and Calorimetry. Principles and Practice, Ed. by P. J. Van Ekeren and M. E. Brown (Elsevier Science, 1998), Vol. 1, pp. 75–114.
Introduction to Thermal Analysis Ed. by M. E. Brown (Kliwer Acad. Publ., New York, 2001).
V. A. Aleshkevich, Molecular Physics (Fizmatlit, Moscow, 2016).
N. V. Karyakin, Fundamentals of Chemical Thermodynamics (Akademiya, Moscow, 2003).
S. A. Kukushkin and A. V. Osipov, “Growth, structure, and morphological stability of nuclei grown in eutectic melts,” Fiz. Tverd. Tela 39 (8), 1464–1469 (1997).
A. S. Pandit and H. K. D. H. Bhadeshia, “Divorced pearlite in steels,” Proc. Royal Soc. A 468 (2145), 2767–2778 (2012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Kolchugina
Rights and permissions
About this article
Cite this article
Spivak, L.V., Shchepina, N.E. Calorimetry of the Melting and Solidification Processes in the Eutectics of Pb–Cd, Pb–Sn, Pb–Bi, and Cd–Sn Binary Alloys. Russ. Metall. 2021, 1471–1476 (2021). https://doi.org/10.1134/S0036029522010116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522010116