Abstract
Argania spinosa press cake, kernels and hulls extracts are excellent corrosion inhibitors for steel. In this work electrochemical methods are used to study the inhibition of corrosion on steel in 1 M HCl. It has shown that Argan press cake, kernels and hulls extracts suppress both the anodic and cathodic process and they behave as mixed-type inhibitors. The inhibition efficiency was found to increase with inhibitors content. Data obtained from EIS measurements, were analyzed to model the corrosion inhibition process through appropriate equivalent circuit model; a constant phase element has been used. The values of the inhibition efficiency calculated from these techniques are in reasonably good agreement. The adsorption isotherm of natural compounds on the steel surface was found to obey Langmuir’s adsorption isotherm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrochloric acid is widely used for the removal of rust and scale in several industrial processes. The corrosion of steel and ferrous alloys in such environments and its inhibition constitute a complex problem of process. HCl is one of the most important pickling acids. Various types of organic compounds are widely used as corrosion inhibitors for protection materials against deterioration from corrosion. These compounds adsorb on the metal surface, block the active sites on the surface and thereby reduce the corrosion process [1–7]. However, study on the inhibition efficiency of natural products for mild steel in acidic medium is still lacking. Aiming to find cheap, environmentally benign, non-toxic natural compound that could be used for acidization and acid pickling of metals in acid medium. Numerous studies had been conducted to examine extracts from naturally materials as corrosion inhibitors: Aloe vera extract [8], Gossipium hirsutum [9], Cocos nucifera [10], lawsonia [11], Azadirachta indica [12], Salvia aucheri mesatlantica [13], olive leaves extract [14], Pennyroyal Mint oil [15], Argan oil [16], Argania plant extract [17], Argania spinosa Kernels Extract and Cosmetic Oil [18], Argan Hulls Extract [19], henna extract [20], Eucalyptus oil [21], Jojoba oil [22], Rosemary oil [23–25], Artemisia oil [25–27], Lavender oil [28], Menthol derivatives [29], Eugenol and Acetyleugenol [30], Pulegone [31] and Limonene [32]. It has been found to be very efficient corrosion inhibitors for steel in acid media. However, the constituents that provide inhibitive action, the mechanisms and the best condition for inhibition are still unclear.
The Argan tree, called Argania spinosa (L.) Skeels, is a tropical plant, which belongs to the Sapotaceae family. Populations of Morocco traditionally use the fruits of A. spinosa to prepare edible oil [33]. It represents the only endemic species of the genus Argania. As an important traditional alimentary medicine, A. spinosa is a valuable potential for Moroccan. Traditionally, the Argan tree is used for many purposes. In cosmetics, Argan oil is advocated as moisturizing oil, against acne juvenile and flaking of the skin as well as for nourishing the hair [34].
The aim of the present work is to compare the effect of A. spinosa extracts: press cake, kernels and hulls bean as inhibitors for the acidic corrosion of steel by electrochemical techniques and to discuss their inhibition mechanism.
2 Experimental Setup
2.1 Electrochemical Tests
The electrochemical study was carried out using a potentiostat PGZ100 piloted by Voltamaster software. This potentiostat is connected to a cell with three electrode thermostats with double wall (Tacussel Standard CEC/TH). A saturated calomel electrode (SCE) and platinum electrode were used as reference and auxiliary electrodes, respectively. The material used for constructing the working electrode was C38 steel that had the following chemical composition (wt%): 0.179 % C, 0.165 % Si, 0.439 % Mn, 0.203 % Cu, 0.034 % S and Fe balance.For electrochemical tests, the commercial C38 steel samples are in the form of a surface parallelepiped of 0.056 cm2 used as a work electrode that is mechanically abraded with 180, 320, 800 grades of emery papers, rinsed with distilled water then with ethanol before plunging the electrode in the solution. Anodic and cathodic potentiodynamic polarization curves were plotted separately at a polarization scan rate of 0.5 mV/s. Before all experiments, the potential was stabilized at free potential during 30 min. The polarisation curves are obtained from −800 to −400 mV at 298 K. The solution test is there after de-aerated by bubbling nitrogen. Gas pebbling is maintained prior and through the experiments. The electrochemical impedance spectroscopy (EIS) measurements are carried out with the electrochemical system (Tacussel), which included a digital potentiostat model Voltalab PGZ100 computer at Ecorr after immersion in solution without bubbling. After the determination of steady-state current at a corrosion potential, sine wave voltage (10 mV) peak to peak, at frequencies between 100 kHz and 10 mHz are superimposed on the rest potential. Computer programs automatically controlled the measurements performed at rest potentials after 0.5 h of exposure at 298 K. The impedance diagrams are given in the Nyquist representation. Experiments are repeated three times to ensure the reproducibility.
2.2 Solutions Preparation
Sample of leaves and fruits of A. spinosa plant was collected from the area of Biougra located at Chtouka Ait Baha (Morocco) at December month. Dried and pulping fruits were crushed. Stock solution of the extracts was prepared by stirring cold weighed amounts of the ground press cake, leaves or hulls of Argania spinosa plant for 24 h in 1 M HCl solution (The solution 1 M HCl was prepared by dilution of analytical grade 37 % HCl with double distilled water). The resulting solution was filtered. These extracts were used to study the corrosion inhibition properties and to prepare the required concentrations of as. The solution tests are freshly prepared before each experiment.
3 Result and Discussion
3.1 Polarization Curves
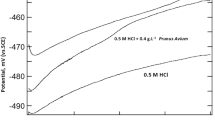
Typical potentiodynamic polarization curves for steel in 1 M HCl in the presence and absence of the tested inhibitors are shown in Figs. 1, 2 and 3. The corrosion parameters including corrosion current densities (Icorr), corrosion potential (Ecorr), cathodic Tafel slope (βc), anodic slop (βa) and inhibition efficiency (E %) are listed in Table 1.
The percentage of inhibition efficiency (E %) was calculated from polarization measurements using the relation:
where Icorr and I′corr are, respectively, the uninhibited and inhibited current density respectively.
The corrosion current density was calculated from the intersection of cathodic and anodic Tafel line.
Inspection of the Figs. 1, 2 and 3 reveals that the polarization curves are shifted toward less current density upon addition of Argan extracts. This result confirms the presence of the Argan extracts inhibits the acid corrosion of steel [35]. Addition of AKE and AHE affects both anodic dissolution of steel and cathodic reduction reactions indicating that the two compounds could be classified as mixed-type inhibitors. Moreover, the presence of APCE affects both the anodic and cathodic reactions with a huge displacement of Ecorr towards cathodic potentials; the curves must be compared with E−Ecorr. The nature of chemical composition of this kind of extract reveals that it is different of the two others. APCE may be also classified as a mixed type inhibitor with predominance in cathodic branche.
The anodic and cathodic Tafel slopes (βa and βc) are affected in the presence of extracts suggesting that the inhibiting action occurred by modification of the mechanism of this process [36].
From electrochemical polarisation measurements, it is clear that the addition of the different inhibitors leads to a decrease in the cathodic current densities. Examination of the IE values given in Table 1 reveals that inhibition efficiency increases with an increase in the concentration of additives. The increase in inhibition efficiency observed at higher inhibitors concentration indicates that more inhibitors molecules are adsorbed on the metal surface thus providing wider surface coverage and these compounds are acting as adsorption inhibitors. We remark that the corrosion current densities were more significantly reduced in the presence of different extracts in the following order : AKE then APCE and finally AHE, and became only 10, 18 and 29 μA/cm2 at 2 g/L respectively. The maximum of inhibition (96.8 %) was obtained at 3 g/L AKE extract.
3.2 Electrochemical Impedance Spectroscopy Measurements
The Nyquist impedance plots for the steel in 1 M HCl solution in the presence and absence of the different inhibitors are shown in Figs.4, 5 and 6. The impedance parameters derived from these investigations are mentioned in Table 2.
The inhibition efficiencies obtained from the EIS measurements are calculated by the relation:
where R t and \( R_{t}^{0} \) are the charge transfer resistances in inhibited and uninhibited solutions respectively.
The charge transfer resistance (R t ) values are calculated from the difference in impedance at lower and higher frequencies, as suggested by Tsuru et al. [37]. The double layer capacitance (C dl) and the frequency at which the imaginary component of the impedance is maximal (−Zmax) are found as represented in equation:
where ω = 2π fmaxWith C dl : Double layer capacitance (μF cm−2); fmax: maximum frequency (Hz) and Rt: Charge transfer resistance (Ω cm2)
As we notice, Figs. 4, 5 and 6, impedance diagrams almost a semi-circular appearance, indicating a charge transfer process mainly controls the corrosion of steel [38]. Deviations of perfect circular shape are often referred to the frequency dispersion of interfacial impedance. This anomalous phenomenon may be attributed to the inhomogeneity of the electrode surface arising from surface roughness or interfacial phenomena [39, 40].
When a non-ideal frequency response is present, it is commonly accepted to employ distributed circuit elements in an equivalent circuit. The most widely used is the constant phase element (CPE), which has a non-integer power dependence on the frequency [41]. Often a CPE is used in a model in place of a capacitor to compensate for non-homogeneity in the system. Fig. 7 shows the electrical equivalent circuit employed to analyze the impedance spectra with one capacitive loop [42, 43].
Excellent fit with this model was obtained with our experimental data. It is observed that the fitted data match the experimental, with an average error inferior to 0.1 %. R t values were simultaneously determined by analysis of the complex-plane impedance plots and the equivalent circuit model and the result are very similar with insignificant changes. According to the equivalent circuit, it was found that R t values increased with increasing inhibitors concentration, this result indicated that charge transfer process mainly controls the corrosion process.
The general shape of the curves is very similar for all samples; the shape is maintained throughout the whole concentrations, indicating that almost no change in the corrosion mechanism occurred due to the inhibitors addition [44]. From the impedance data given in Table 2, we note that the value of R t increases with increase in the concentration of the different inhibitors. Values of double layer capacitance are also brought down to the maximum extent in the presence of inhibitors and the decrease in the values of C dl follows the order similar to that obtained for Icorr in this study. It has been reported that the adsorption process on the metal surface is characterized by a decrease in C dl [37]. The best efficiencies obtained in the presence of APCE, AKE and AHE respectively are 81.7 % at 3 g/L, 92.5 % at 3 g/L and 86.5 % at 5 g/L. The results obtained from the polarization technique were in good agreement with those obtained from the EIS for the tree compounds.
3.3 Adsorption Isotherm
Additional information about the properties of the tested compounds may be provided from the kind of adsorption isotherm. Several adsorption isotherms were tested and the Langmuir adsorption isotherm was found to provide best description of the adsorption behaviour of the investigated inhibitor. The Langmuir isotherm is given by the equation [45]:
where C is the inhibitor concentration, θ the fraction of the surface covered determined by E/100, k the equilibrium constant.
Fig. 8 shows the dependence of the ratio C/θ as function of C for different extracts.
The data fit straight line, with slope nearly equal unity, indicating that these compounds adsorbed according to Langmuir adsorption isotherm.
4 Conclusion
The following results can be drawn from this study:
-
APCE, AKE and AHE act as mixed type inhibitors.
-
The inhibition efficiency of APCE, AKE and AHE extracts increases with the rise of inhibitions concentration.
-
The data obtained from the different methods: potentiodynamic polarization and EIS are in good agreement.
-
APCE, AKE and AHE extracts are excellent inhibitors for steel in acidic medium at 298 K.
-
APCE, AKE and AHE extracts being natural and environmentally benign products they can be used as an alternative for toxic chemical inhibitors in acidization and acid pickling of mild steel.
References
Zarrok H, Oudda H, Zarrouk A, Salghi R, Hammouti B and Bouachrine M, Der Pharma Chemica 3 (6) (2011) 576.
El Issami S, Bazzi L, Mihit M, Hammouti B, Kertit S, Ait Addi E, and Salghi R, Pigment Resin Technol 36 (3) (2007) 161.
Barouni K, Bazzi L, Salghi R, Mihit M, Hammouti B, Albourine A, and El Issami S, Mater Lett 62 (2008) 3325.
El Issami S, Bazzi L, Hilali M, Salghi R and Kertit S, Ann Chim Sci Mat 27 (2002) 63.
Mihit M, El Issami S, Bouklah M, Bazzi L, Hammouti B, Ait Addi E, Salghi R, and Kertit S, Applied Suf Sci 252 (2006) 2389.
Hammouti B, Salghi R, and Kertit S, J Electrochem Soc India 47 (1998) 31.
El Issami S, Bazzi L, Mihit M, Hilali M, Salghi R, and Ait Addi E, J Phys IV 123 (2005) 307.
Olusegun K, Abiola A O, and James A, Corros Sci 52 (2010) 661.
Abiola O K, Otaigbe J O E, and Kio O J, Corros Sci 51 (2009) 1879.
Abiola O K, and Oforka N C, Scientia Africana 2 (2003) 82.
El-Etre A Y, Abdallah M, and El-Tantawy Z E, Corros Sci 47 (2005) 385.
Valek L, and Martinez S, Mater Lett 61 (2007) 148.
Znini M, Majidi L, Bouyanzer A, Paolini J, Desjobert J M, Costa J, and Hammouti B, Arabian J Chem 2010. http://www.sciencedirect.com/science/journal/18785352.
El-Etre A Y, J Colloid Interface Sci 314 (2007) 578.
Bouyanzer A, Hammouti B, and Majidi L, Mater Lett 60 (2006) 2840.
Afia L, Salghi R, Bammou L, Bazzi L h, Hammoutin B, Bazzi L, and Bouyanzer A, J Saudi Chem Soc 2011. http://www.sciencedirect.com/science/journal/13196103.
Afia L, Salghi R, Bammou L, Bazzi L h, Hammouti B, and Bazzi L, Acta Metalurgica Sinica 25(1) (2012) 10.
Afia L, Salghi R, Bazzi E l, Bazzi L, Errami M, Jbara O, Al-Deyab S S, and Hammouti B, Int J Electrochem Sci 6 (2011) 5918.
Afia L, Salghi R, Bazzi E l, Zarrouk A, Hammouti B, Bouri M, Zarrouk H, Bazzi L, Bammou L, Research on Chemical Intermediates (in press).
Ostovari A, Hoseinieh S M, Peikari M, Shadizadeh S R, and Hashemi S J, Corros Sci 51 (2009) 193521.
Bouyanzer A, Majidi L, and Hammouti B, Bull Electrochem 22 (2006) 321.
Chetouani A, Hammouti B, and Benkaddourm M, Pigment Resin Technol 33 (2004) 26.
Bendahou M, Benabdellah M, and Hammouti B, Pigment Resin Technol, 35 (2006) 95.
Chaieb E, Bouyanzer A, Hammouti B, Benkaddour M, and Berrabah M, Trans SAEST 39 (2004) 58.
El-Ouariachi E, Paolini J, Bouklah M, Elidrissi A, Bouyanzer A, Hammouti B, Desjobert J.M, and Costa J, Acta Metall Sin 23 (2010) 13.
Ouachikh O, Bouyanzer A, Bouklah M, Desjobert J M, Costa J, Hammouti B, and Majidi L, Surf Rev Lett 16 (2009) 49.
Benabdellah M, Benkaddour M, Hammouti B, Bendahou M, Aouiti A, Appl Surf Sci 252 (2006) 6212.
Bouyanzer A, and Hammouti B, Pigment Resin Technol 33 (2004) 287.
Zerga B, Sfaira M, Rais Z, Ebn Touhami M, Taleb M, Hammouti B, Imelouane B, and Elbachiri A, Mater Tech 97 (2009) 297.
Faska Z, Majidi L, Fihi R, Bouyanzer A, and Hamouti B, Pigment Resin Technol 36 (2007) 293.
Chaieb E, Bouyanzer A, Hammouti B, and Benkaddour M, Appl Surf Sci 246 (2005) 199.
Faska Z, Bellioua A, Bouklah M, Majidi L, Fihi R, Bouyanzer A, and Hammouti B, Monatsh Chem 139 (2008) 1417.
Khallouki F, Spiegelhalder B, Bartsch H, and Owen R W, Afr J Biotechnol 4 (2005) 381.
Henry F, Danoux L, Pauly G, and Charrouf Z, Patent EP 1430900 (2004).
El-Etre A Y, Mater Chem Phys 108 (2008) 278.
Bammou L, Mihit M, Salghi R, Bazzi L, Bouyanzer A, and Hammouti B, Int J Electrochem Sci 6 (2011) 1454.
Tsuru T, Haruyama S, and Gijutsu B, J Jpn Soc Corros Eng 27 (1978) 573.
Dahmani M, Et-Touhami A, Al-Deyab S S, Hammouti B, and Bouyanzer A, Int J Electrochem Sci 5 (2010) 1060.
Shih H, and Mansfeld H, Corros Sci 29 (1989) 1235.
Martinez S, and Metikos-Hukovic M, J Appl Electrochem 33 (2003) 1137.
Macdonald J R, Impedance, A Wiley-Interscience Publication ,New York(1987).
McCafferty E, Corros Sci 39 (1997) 243.
Morad M S, Corros Sci 42 (2000) 1313.
Chaieb E L, Bouyanzer A, Hammouti B, and Benkaddour M, Appl Surf Sci 249 (2005) 183.
Langmuir I, J Amer Chem Soc 39 (1947) 1848.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afia, L., Salghi, R., Zarrouk, A. et al. Comparative Study of Corrosion Inhibition on Mild Steel in HCl Medium by Three Green Compounds: Argania spinosa Press Cake, Kernels and Hulls Extracts. Trans Indian Inst Met 66, 43–49 (2013). https://doi.org/10.1007/s12666-012-0168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-012-0168-z