Abstract
The corrosion inhibition behavior of Ruta graveolens extract (RGE) for mild steel in 1 M HCl is studied using gravimetric and electrochemical techniques. 1–4 V/V% RGE is added to the corrosive media and analyzed. Gravimetric measurements conducted at different time intervals reveal the potential activity of RGE in the system investigated. RGE exhibits 95 % inhibition efficiency in the investigated system for 7 days of exposure time. Electrochemical impedance spectroscopic studies show that mild steel is protected 98 % from corrosion in 1 M HCl in the presence of RGE. Potentiodynamic polarization study agrees this observation showing 99 % inhibition efficiency and also reveals the mixed type inhibition behavior of RGE. One of the active constituents of Ruta graveolens, Rutin is also monitored for its inhibition behavior and is found to be active. The theoretical parameters obtained for rutin also stands for the good inhibition potential of the extract. Both RGE and Rutin show a decrease in inhibition efficiency as the temperature raised. Adsorption behavior of RGE is according to Langmuir isotherm and that of Rutin obeys Temkin isotherm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Study of metal corrosion has always been a topic of great interest among corrosion engineers. Mild steel and hydrochloric acid being materials of great industrial importance, study on the inhibition behavior of a corrosion inhibitor for such a system also becomes important. The major use of inhibitors in acid solutions is in pickling process, industrial acid cleaning, oil, and gas well acidizing and for removal of rust, scale, and corrosion products [1–4]. The corrosion protection by inhibitors is based mostly on the modification of metal surfaces by the adsorption of the inhibitor molecules and the subsequent formation of a protective (blocking) mono layer [5]. The effectiveness of organic inhibitors depends on their adsorption rates and covering capabilities on metal surfaces. It has been realized from many sources that adsorption depends on the molecular structure, surface charge of the metal, and type of the electrolytes. In an aqueous solution, inhibitors are adsorbed by replacing water molecules that have already been adsorbed on the surface. For this, the electrostatic interaction between an inhibitor molecule and metal should be more dominant over that between metal and water molecules. The electron densities on different functional groups and their polarizability and electro negativity in an inhibitor are the main factors for such interactions [6]. Most of the organic inhibitors investigated are environmental hazardous. Recently researchers have reported so many natural inhibitors which are eco-friendly [7–10].
A study of the inhibitive characteristics of Ruta graveolens leaf extract (RGE) for the corrosion of mild steel in 1 M HCl was taken up during the present investigation. In the current scenario, the study of a natural corrosion inhibitor becomes relevant only if it is applicable at higher temperatures and for a longer time. Taking these aspects into account, the effect of varying temperature and extended time of exposure has been studied in detail. Electrochemical measurements such as EIS and potentiodynamic polarization techniques and theoretical calculations have been employed to determine the inhibition efficiency of the inhibitor.
Ruta graveolens is an aromatic perennial shrub belonging to the family of Rutaceae. The active components in Ruta graveolens are coumarins, alkaloids, terpenoids, flavonoids and essential oil with methyl ketones, alcohols, and a few compounds [11, 12]. 2-nonanone, 2-undecanone, 2-nonanol, 2-octylacetate, and 2-undecanol are the major components in Ruta graveolens leaves [13, 14]. To understand the potential ability of the leaf extract more, one of the main constituents was also intended to monitor. But the constituents that met the structural criteria of an electron donor were only a few of them. Considering the structural aspects and availability, Rutin (Fig. 1), one of the main active flavonoids of the leaves, [11, 15, 16] was monitored for its activity as an inhibitor.
2 Experimental
2.1 Materials and Medium
Mild steel coupons cut into 2 × 1.8 cm2 dimension were used for weight loss technique. Mild steel specimen of 1 cm2 exposed surface areas were used for electrochemical studies. Prior to the experiments, the metal samples were abraded with emery papers (600–1200 grade). Then they were degreased with acetone, washed with double distilled water, and dried in air before immersion in a corrosive medium. The corrosive medium was prepared using analytical grade HCl (E. Merck).Rutin (Rutin trihydrate) was supplied by SRL chemicals.
Preparation of the extract: Ruta graveolens leaves were collected from residential area near nilgiris. The cleaned leaves were shade dried and ground to powder. 9 g of the powdered leaves were then refluxed with 300 ml methanol for 2 and half hours. It is then kept overnight and filtered. Ruta graveolensleaf extract (RGE) thus obtained was used to study the inhibitive behavior for mild steel corrosion.
2.2 UV–Visible Spectroscopy
UV–Visible absorption spectra of Ruta graveolens leaf extract (RGE) and rutin were measured with JASCO-V-550 spectrophotometer.
2.3 Weight Loss Technique
The effect of addition of different concentrations of RGE on the corrosion of mild steel in 1 M HCl was studied by weight loss measurements at 303 K. The pre-weighed mild steel samples of area 1.8 × 2 cm2 were exposed in1 M HCl without and with varying concentrations of RGE (1, 2, 3, 4 V/V%) for 1, 3, 5 and 7 days. After the immersion period, the steel specimens were withdrawn, carefully rinsed with bi-distilled water, cleaned with acetone, dried at room temperature, and then weighed.
2.4 Electrochemical Measurements
Electrochemical experiments were conducted in a three-electrode cell using ACM instrument Gill AC model no: 1475 potentiostat. A platinum sheet was used as the counter electrode, and a saturated calomel electrode (SCE) was used as the reference electrode. Working electrodes were made of mild steel samples machined into circular specimens and then embedded in polytetrafluoroethylene (PTFE) with an exposed surface area of 1 cm2. All the experiments were done under atmospheric pressure at three different temperatures (303, 308, and 313 K). At each temperature, the inhibition efficiency corresponding to an increase in inhibitor concentration has been observed.
For an organic coating to serve its intended purpose, surface pretreatment of the metal substrate is very much important. This pretreatment could be either mechanical or chemical in nature. The chemical pretreatment comprises of use of primers or coupling agents, solvent degreasing, acid pickling, etc., which will form a chemical bridge b/w the steel substrate and the organic coating [17]. Electrochemical impedance spectroscopy (EIS) is a very powerful tool in coating analysis. EIS data can predict the quality of corrosion protection, film porosity, and solution absorption into the coating and film characteristics [18, 19]. EIS spectra were recorded in the frequency range 10000–0.1 Hz at an amplitude 10 mV.
After measuring the open circuit potential, potentiodynamic polarization curves were obtained with a 60 mV/min scan rate in +250 to −250 mV potential range. The polarization resistance R p was determined from the slope of the obtained potential—current figures.
2.5 DFT Study
DFT method was used to perform quantum chemical calculations for rutin molecule. Geometry optimization of the structural parameters were done using B3LYP functional hybrid with the basis set 6-31G*. All of the calculations were performed using Gaussian 09 program to obtain the total energy, energies of the frontier molecular orbitals, energy gap ∆E, and dipole moment.
3 Results and Discussion
3.1 UV–Visible Spectroscopy
UV–Visible absorption of RGE and Rutin has been examined. Figure 2 represents the absorption spectra obtained for RGE and the component rutin. The absorption peak at 352 nm wavelength of rutin is observed in the spectra corresponding to RGE which suggests the presence of rutin in the extract. The absorption band of RGE also shows peaks in the region 420 and 630 nm, which may be attributed to the presence of other phytochemicals in the extract.
3.2 Weight Loss Studies
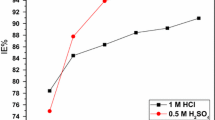
The weight loss after exposing the mild steel specimen in 1 M HCl with and without RGE was analyzed. It was observed that with increasing inhibitor concentration the percentage inhibition efficiency (IE %) also increased. Also the exposure time affected the performance of the extract in such a way that 87 % inhibition efficiency of RGE, after 1 day, reached 96 % after 3 days exposure. For an exposure period of 5 days, the efficiency reached 97 %.And even after 7 days of exposure time in 1 M HCl, the mild steel sample was seemed protected 95 %. The effect of increasing concentration of RGE on inhibition efficiency is pictorially represented in Fig. 3. The IE (%) was obtained as
where W 0 and W are the weight loss in the absence and presence of RGE, respectively.
3.3 Electrochemical Impedance Spectroscopy
EIS technique is widely used to determine the metal/solution and interface behavior and kinetics of the electrochemical processes in the absence and presence of an inhibitor [20]. The corrosion at the metal/solution interface is controlled by charge transfer resistance [21–23]. While dc methods such as polarization curves do not provide sufficient information on poorly conducting layers, alternate current methods such as electrochemical impedance spectroscopy have proved useful in studying the properties of polymer-coated metals and their changes during exposure to a corrosive medium [24, 25]. In EIS, the complex impedance system is measured over a wide range of frequency. The data are then interpreted using an equivalent circuit and evaluated in order to give the best agreement between the experimental and computed spectra [24, 26]. By understanding the change in the coating capacitance, the effect of changing temperature and inhibitor concentration may be understood [27, 28]. The equivalent circuit used in the present investigation is Randle’s circuit (Fig. 4). It consists of solution resistance (R s) which is in series with a parallel combination of charge transfer resistance (R ct) and double-layer capacitance (C dl) [29]. In the semicircular Nyquist plots obtained for the Randle’s circuit, the high frequency intercept with the real axis is ascribed to the solution resistance (R s) and that with the low-frequency region is assigned as the charge transfer resistance.
To compensate for the deviations from ideal dielectric behavior arising from the inhomogeneous nature of the electrode surfaces, a constant phase element (CPE) is used instead of C dl.
where Q and n represent the magnitude and exponent of the CPE, respectively. j is an imaginary number and ω is the angular frequency in rad/s.
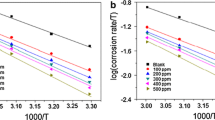
The parameters obtained in EIS technique are summarized in Table 1. RGE showed fairly good inhibition efficiency increasing the charge transfer resistance with increasing concentration. The IE (%) obtained for the highest concentration studied (4 V/V%) is 98 %. But as the temperature got raised by 5 K, the efficiency decreased to 93 % and then lowered to 90 % for a further temperature rise of 5 K. The impedance spectra and bode plots obtained for the inhibitive interaction of RGE (only the highest concentration) on mild steel surface in 1 M HCl at different temperatures are represented in Fig. 5a–d. The inhibition efficiency was obtained as
where R ct* and R ct are the charge transfer resistances with and without the inhibitor, respectively.
The increase charge transfer resistance with RGE concentration in the corrosive medium is depicted in Fig. 6. Evaluation of the protective layer formation of RGE on mild steel surface may be otherwise explained from C dl values. The double-layer capacitance value got decreased as the inhibitor concentration increased. This may be attributed to the formation of dielectric constant by the adsorptive layer. It may be otherwise explained as lower C dl values corresponding to reduced double-layer capacitance, which according to the Helmoltz plane results from a decrease in the dielectric constant (ɛ) or an increase in the interfacial layer thickness (δ).
where ɛ is the dielectric constant of the medium, ɛ 0 is the vacuum permittivity, A is the electrode surface area, and δ is the thickness of the interfacial layer. An organic molecule gets adsorbed onto a metal surface by replacing the adsorbed water molecules. Thus the smaller dielectric constant of the organic molecule (than water) and the increased interfacial thickness together reduce the double-layer capacitance. Thus C dl values summarized in Table 1 also stand for the inhibition capacity of RGE. The corrosion current I corr observed for mild steel in 1 M HCl was 1.28 mA cm−2 which got decreased to 0.02 mA cm−2 in the presence of 4 V/V% RGE. Even at elevated temperatures, the corrosion current values showed a noticeable decrease.
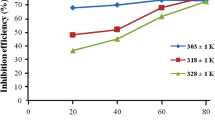
The component rutin was also found to exhibit a protective interaction on the mild steel surface in 1 M HCl. Rutin showed a higher IE (%) at elevated temperatures. This may be attributed to the increased solubility of rutin at higher temperature. The highest inhibition efficiency obtained at 303 K is 46 %. At 308 K, the highest inhibitor concentration gave 70 % efficiency and then decreased to 63 % at 313 K.
When come to the bode plots, the single peak obtained in the phase angle bode plots (Fig. 5c, d) for RGE and rutin indicates that the electrochemical impedance measurements fit well in a one-time constant equivalent model.
3.4 Potentiodynamic Polarization Study
Tafel plots obtained using the polarization technique for the system under investigation is represented in Fig. 7. It is seen that both cathodic and anodic branches of the polarization curves are shifted toward lower current density. This indicates the mixed type inhibition behavior of RGE which affects both anodic and cathodic reactions. The tafel constants β a and β c (Table 2) also reveals the mixed inhibition mechanism of the inhibitor.
The protection offered by RGE for mild steel corrosion in 1 M HCl reached a higher extent of 99 % as the concentration of RGE increased from 1 to 4 V/V% but for a rise in temperature by 5 K up to 313 K, lowered the inhibition efficiency to 94 %.This may be attributed to the desorption of the inhibitor molecules from metal surface at elevated temperatures. Thus it may also be assumed that the adsorption is predominantly by physisorption. The corrosion kinetic parameters are summarized in Table 2. Using the current density (I corr) values the inhibition efficiencies were calculated as
where I corr and I corr* represents the corrosion current in the absence and presence of the inhibitor. As RGE is added to the corrosive medium the corrosion current density lowered to a greater extent which reveals the inhibition capacity of RGE.
The potential ability of RGE as an effective inhibitor results from the intermolecular interaction among the components. The main active constituent of RGE, rutin was also monitored for its corrosion inhibition behavior. The polarization parameters summarized in Table 3 reveal the ability of rutin to act as an electron donor. The polarization curves suggest that rutin affects both anodic and cathodicreactions.
3.5 Theoretical Study
The optimized geometry, HOMO, and LUMO of rutin are represented in Fig. 8. The quantum chemical parameters corresponding to the optimization have been determined and analyzed and are summarized in Table 4. The energy gap between HOMO and LUMO (ΔE) is considered an important factor in determining the molecular activity. As the gap energy ΔE decreases, inhibition efficiency increases [30]. The low ΔE value 3.8901 eV obtained for rutin indicates an appreciable electron donating ability of rutin. The dipole moment (µ) provides information regarding the polarity of the whole molecule. Higher value of dipole moment has found to be a key factor that facilitates adsorption by influencing the transport process through the inhibitor layer adsorbed [31]. 5.2478 D seems to be a higher value for dipole moment that adds to the fact that the component rutin shows a potential ability to act against corrosion. This in turn confirms the inhibition activity of RGE.
3.6 Adsorption Studies
Metal-inhibitor interaction resulting from the adsorption of an organic molecule on a metal surface may be considered as a replacement reaction. This interaction is well understood by analyzing the adsorption process using adsorption isotherms [32].
The surface interaction of RGE on mild steel surface was found obeying Langmuir adsorption isotherm. Langmuir isotherm assumes that there would be a finite number of uniform adsorption sites, and there would be hardly any lateral interaction. The Langmuir adsorption isotherm can be described as
where C inh is the inhibitor concentration, K ads is the adsorption equilibrium constant, and θ is the surface coverage.
The adsorption of the component rutin on mild steel surface obeyed Temkin adsorption isotherm. According to Temkin, the energy of adsorption is a linear function of the surface coverage θ [33]. This isotherm ignores very low and high concentrations. Also Temkin isotherm takes into account the adsorbent–adsorbate interactions [34]. The characteristics of the isotherm is given as
where ‘a’ is molecules interaction parameter,’θ’ is the degree of surface coverage, ‘K’ is equilibrium constant of adsorption process, and ‘C’ is the concentration of the inhibitor. Figure 9a and b represents the Langmuir and Temkin isotherms for the adsorption of RGE and rutin, respectively.
RGE extract is composed of numerous organic compounds in it. The adsorption of these components on the metal surface may be the reason for the inhibitive action of the extract. The experimental and theoretical investigation of the constituent rutin reveals the potential ability to act against corrosion. The lower inhibition efficiencies observed for rutin experimentally may be attributed to the effect of the trihydrate form of the same. Theoretical parameters show that rutin is a very good electron donor hence an inhibitor. The inhibitory action of rutin would be enhanced by the synergistic interaction among the other constituents.
4 Conclusions
-
Ruta graveolens leaf extract (RGE) act effectively against mild steel corrosion 1 M HCl.
-
Weight loss studies strongly support the protective barrier formation by RGE on mild steel surface that remained even after 1-week exposure time.
-
The results obtained from electrochemical impedance and polarization techniques are in good agreement producing comparable inhibition efficiencies for the interaction of RGE and the component rutin on mild steel surface.
-
Polarization technique reveals the mixed type inhibition characteristics of RGE and rutin.
-
Computational calculations reveal the potential inhibition activity of rutin which in turn supports the inhibition capacity of RGE
-
The adsorption pattern of RGE was according to Langmuir and that of rutin was found obeying Temkin adsorption isotherm.
-
The main limitation of the study is the disability to explain how the inhibitor behaves at very high temperatures.
-
It is evident that rutin acts as an effective inhibitor. But in order to explain the exceptionally good inhibition capacity of RGE, the study of a single component would not be sufficient.
References
Krishnegowda PM, Venkatesha VT, Kumar MKP, Shivayogiraju SB (2013) Acalyphatorta leaf extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Ind Eng Chem Res 52:722–728
Ji G, Anjum S, Sundaram S, Prakash R (2015) Musa paradisica peel extract as green corrosion inhibitor for mild steel inHCl solution. Corros Sci 90:107–117
Mourya P, Banerjee S, Singh MM (2014) Corrosion inhibition of mild steel in acidic solution by Tageteserecta (Marigold flower) extract as a green inhibitor. Corros Sci 85:352–363
Raja PB, Fadaeinasab M, Qureshi AK, Rahim AA, Osman H, Litaudon M, Awang K (2013) Evaluation of green corrosion inhibition by alkaloid extracts of Ochrosiaoppositifolia and Isoreserpiline against mild steel in 1 M HCl medium. Ind Eng Chem Res 52:10582–10593
Ghareba S, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: towards the development of ‘green’ corrosion inhibitors. Corros Sci 52:2104–2113
Mourya P, Banerjee S, Rastogi RB, Singh MM (2013) Inhibition of mild steel corrosion in hydrochloric and sulfuric acid media using a thiosemicarbazone derivative. Ind Eng Chem Res 52:12733–12747
Asan A, Soylu S, Kıyak T, Yıldırım F, Oztas SG, Ancın N, Kabasakaloglu M (2006) Investigation on some Schiff bases as corrosion inhibitors for mild steel. Corros Sci 48:3933
Bentiss F, Lagrenée M, Traisnel M (2000) 2,5-Bis(n-pyridyl)-1,3,4-oxadiazoles as corrosion inhibitors for mild steel in acidic media. Corrosion 56:733
Lebrini M, Traisnel M, Lagrene M, Mernari B, Bentiss F (2007) Inhibitive properties, adsorption and a theoretical study of 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles as corrosion inhibitors for mild steel in perchloric acid. Corros Sci 50:473
Morad MS, Kamal AM (2006) El-Dean, 2,2′-Dithiobis(3-cyano-4,6-dimethylpyridine): a new class of acid corrosion inhibitors for mild steel. Corros Sci 48:3398
Pathak S, Multani AS, Banergy P, Banergy P (2003) Ruta 6 selectively induces cell death in brain cancer cells but proliferation in normal peripheral blood lymphocytes, a novel treatment for human brain cancer. Int J Oncol 23:975–982
Kostova I, Ivanova A, Mikhova B, Klaiber I (1999) Alkaloids and coumarins from RutaGraveolens. Montash Chem 130:703–707
Fredj MB, Marzouk B, Chraief I, Boukef K, Marzouk Z (2007) Analysis of Tunisian Rutagraveolens L. oils from Jemmel. J Food Agric Environ 5:52–55
De Feoa V, De Simonea F, Senatore F (2002) Potential allelochemicals from the essential oil of Rutagraveolens. Phytochemistry 61:573–578
Asgarpanah J, Khoshkam R (2012) Phytochemistry and pharmacological properties of Rutagraveolens L. J Med Plants Res 6:3942–3949
Parray SA, Bhat JU, Ahmad G, Jahan N, Sofi G, Faisal Iqbal SM (2012) Rutagraveolens: from traditional system of medicine to modern pharmacology: an overview. Am J Pharm Tech Res 2:239–252
Hare CH (1986) Adhesive and cohesive failure in applied coating system composites. J Prot Coat Linings 3(9):38–48
Mansfeld F, Jeanjaquet SL, Kendig MW (1987) An electrochemical impedance spectroscopy study of reactions at the metal/coating interface, in corrosion protection by organic coatings. Electrochem Soc Proc 87–2:217
Scully JR (1989) Electrochemical impedance of organic-coated steel: correlation of impedance parameters with long-term coating deterioration. J Electrochem Soc 136:979–990
Oguzie EE, Li Y, Wang SG, Wang F (2011) Understanding corrosion inhibition mechanisms—experimental and theoretical approach. RSC Adv 1:866–873
Zhang QB, Hua YX (2009) Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim Acta 54:1881–1887
Touhami F, Aouniti A, Abed Y, Hammouti B, Kertit S, Ramdani A, Elkacemi K (2000) Corrosion inhibition of armco iron in 1 M HCl media by new bipyrazolic derivatives. Corros Sci 42:929–940
El Achouri M, Kertit S, Gouttaya HM, Nciri B, Bensouda Y, Perez L, Infante MR, Elkacemi K (2001) Corrosion inhibition of iron in 1 M HCI by some Gemini surfactants in the series of alkanediyl- a, x-bis-(dimethyl tetradecyl) ammonium bromide). Prog Org Coat 43:267–273
Mansfeld F (1995) Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J Appl Elecrochem 25(1995):187
Bonora PL, Deflorian F, Fedrizzi L (1996) Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim Acta 41:1073
J. Hollaender, R. Brandsch, Application of Impedance Methods for Corrosion Protecting Properties of Coated Sub-strates in Metal Packaging,International Meeting on Corrosion Science and Control T ectnologies IMC ORR, Rio de Janeiro, Brazil, Conference paper, 1995
do Nascinento GG, dos Santos JLC, Margarit ICP, Mattos OK (1996) Lacquered tinplate: corrosion resistance in the function of lacquering conditions. EIectrochim Acta 41:1099
Kern P, Baner AL, Lange J (1999) Electrochemical impedance spectroscopy as a tool for investigating the quality and performance of coaled food cans. J Coat Technol 71:68–74
Yadav DK, Maiti B, Quraishi MA (2010) Electrochemical and quantum chemical studies of 3,4-dihydropyrimidin-2(1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 52:3586–3598
Musa AY, Mohamad AB, Kadhum AAH, Takriff MS, Ahmoda W (2012) Quantum chemical studies on corrosion inhibition for series of thio compounds on mild steel in hydrochloric acid. J Indus Eng Chem 18:551–555
Popova A, Christov M, Deligeorigiev T (2003) Influence of molecular structure on the inhibition properties of benzimidazole derivatives on mild steel corrosion in 1 M HCl. Corrosion 59:756–764
Li X, Deng S, Fu H (2011) Triazolyl blue tetrazolium bromide as a novel corrosion inhibitor for steel in HCl and H2SO4 solutions. Corros Sci 53:302–309
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327–356
Aharoni C, Ungarish M (1977) Kinetics of activated chemisorption. Part 2. Theoretical models. J Chem Soc Faraday Trans 73:456–464
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anupama, K.K., Shainy, K.M. & Joseph, A. Excellent Anticorrosion Behavior of Ruta Graveolens Extract (RGE) for Mild Steel in Hydrochloric Acid: Electro Analytical Studies on the Effect of Time, Temperature, and Inhibitor Concentration. J Bio Tribo Corros 2, 2 (2016). https://doi.org/10.1007/s40735-016-0032-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-016-0032-5